Abstract
Prostate cancer is a major public health problem throughout the developed world. For patients with clinically localised prostate cancer, the diagnosis is typically established by histopathological examination of prostate needle biopsy samples. Major and minor criteria are used to establish the diagnosis, based on the microscopic appearance of slides stained using haematoxylin and eosin. Major criteria include an infiltrative glandular growth pattern, an absence of basal cells and nuclear atypia in the form of nucleomegaly and nucleolomegaly. In difficult cases, basal cell absence may be confirmed by immunohistochemical stains for high‐molecular‐weight cytokeratins (marked with antibody 34βE12) or p63, which are basal cell markers. Minor criteria include intraluminal wispy blue mucin, pink amorphous secretions, mitotic figures, intraluminal crystalloids, adjacent high‐grade prostatic intraepithelial neoplasia, amphophilic cytoplasm and nuclear hyperchromasia. Another useful diagnostic marker detectable by immunohistochemistry is α‐methylacyl coenzyme A racemase (AMACR), an enzyme selectively expressed in neoplastic glandular epithelium. Cocktails of antibodies directed against basal cell markers and AMACR are particularly useful in evaluating small foci of atypical glands, and in substantiating a diagnosis of a minimal adenocarcinoma. Reporting of adenocarcinoma in needle biopsy specimens should always include the Gleason grade and measures of tumour extent in the needle core tissue. Measures of tumour extent are (1) number of cores positive for cancer in the number of cores examined, (2) percentage of needle core tissue affected by carcinoma and (3) linear millimetres of carcinoma present.
In 2002, prostate cancer was the fifth most common cancer in the world and the second most common cancer in men, with 679 000 new cases.1 This represents 19% of all cancers diagnosed in developed countries and 5.3% in developing countries.1 Incidence rates are high in North America, northern and western Europe, and Australia and New Zealand.1 As a primary approach to the establishment of a definitive diagnosis of prostate cancer is the histopathological interpretation of transrectal 18‐gauge needle core biopsy specimens, it is critical for diagnostic pathologists to appreciate the histomorphological features of prostatic carcinoma in needle biopsy tissue, and to have an organised approach to the establishment of the diagnosis.
The histopathological diagnosis of adenocarcinoma of the prostate in needle core biopsy specimens presents a unique set of challenges. Firstly, early detection efforts, including screening with the prostate‐specific antigen (PSA) and digital rectal examination, have resulted in identification of lower‐stage and smaller‐volume carcinomas of the prostate.2,3,4,5 As a result, many PSA‐detected carcinomas comprise <5% of needle core tissue. Secondly, it can be difficult to appreciate an infiltrative architectural pattern of growth in thin 18‐gauge needle core biopsy specimens. Finally, the needle cores can fragment, which can also generate problems in interpretation.
The focus of this review is an approach to the histopathological diagnosis of carcinoma in prostate needle biopsy specimens, especially limited or minimal adenocarcinoma. We define minimal carcinoma in needle biopsy tissue as a tumour with size <1 mm in the greatest dimension.6 Another definition of minimal adenocarcinoma is cancer involving <5% of needle core tissue.7 The first four sections include discussion on major and minor criteria for the diagnosis of prostate carcinoma on the basis of sections stained with haematoxylin and eosin (H&E), features considered specific for carcinoma and minimal carcinoma. Next, entities in the differential diagnosis of prostatic adenocarcinoma are briefly presented, followed by information on the use of ancillary diagnostic studies, particularly the use of immunohistochemical staining. The final section discusses the reporting of prostatic carcinoma in prostate needle biopsy tissue.
Major criteria for diagnosis of adenocarcinoma in prostate needle biopsy tissue sections
Diagnosis of prostatic carcinoma requires a synthesis of a constellation of histological attributes that allows for a definitive diagnosis. A conceptual framework for a rationale approach to this diagnosis entails application of major and minor criteria (box 1).8,9
Box 1: Criteria for the diagnosis of prostatic adenocarcinoma9
-
Major criteria
-
-
Architectural: infiltrative small glands or cribriform glands too large or irregular to represent high‐grade prostatic intraepithelial neoplasia (PIN)
-
-
Single cell layer (absence of basal cells)
-
-
Nuclear atypia: nuclear and nucleolar enlargement
-
-
-
Minor criteria
-
-
Intraluminal wispy blue mucin (blue‐tinged mucinous secretions)
-
-
Pink amorphous secretions
-
-
Mitotic figures
-
-
Intraluminal crystalloids
-
-
Adjacent high‐grade PIN
-
-
Amphophilic cytoplasm
-
-
Nuclear hyperchromasia
-
-
Before searching for these criteria, it is important to scan sections of the needle core tissue, at both low‐power and high‐power magnification, in order to appreciate the architecture and cytological features of benign glands (if present) in the tissue. The arrangement of the benign glands and the nuclear appearances of the lining cells (both basal and luminal secretory) serve as important points of reference for comparison when evaluating atypical glands because there can be substantial variability between individual cases in histological characteristics because of differences in fixation, section thickness and H&E staining.
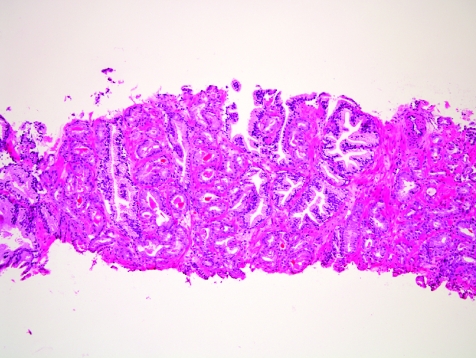
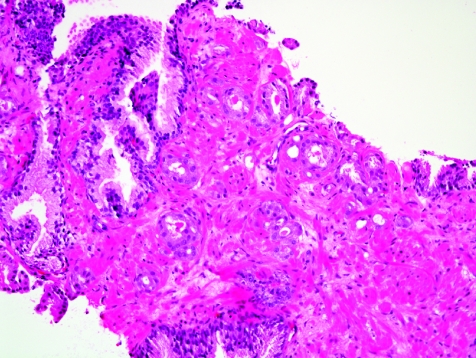
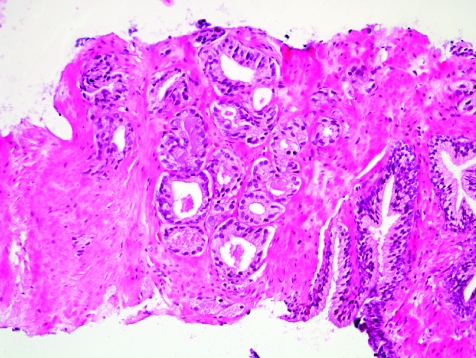
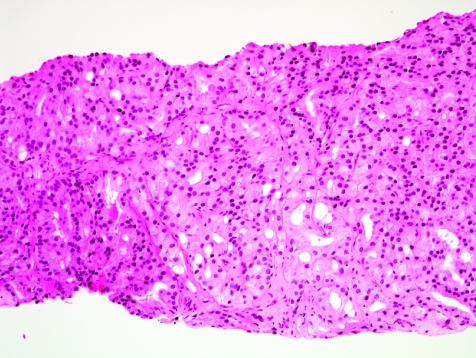
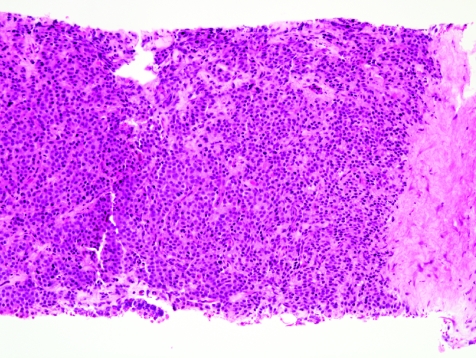
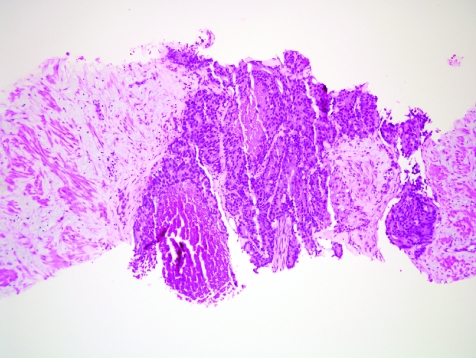
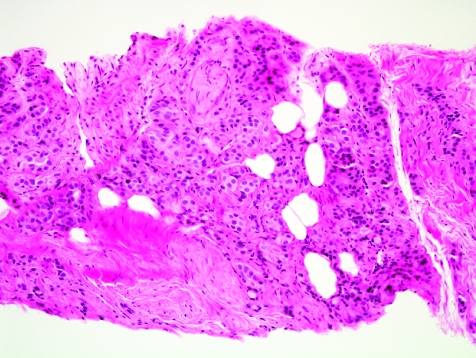
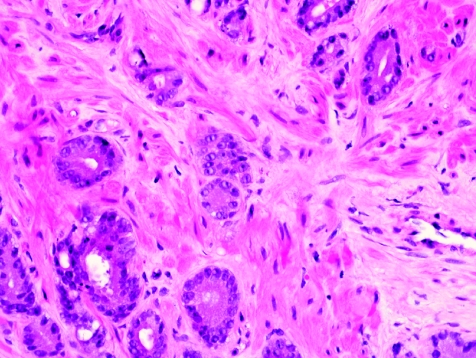
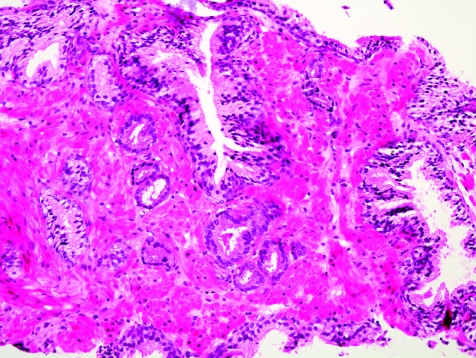
The initial search using light microscopy should be an assessment for the first of the major criteria, an infiltrative growth pattern, which often presents as small malignant glands extending between or around larger, more complex (and often paler) benign glands (fig 1). These Gleason pattern 3 adenocarcinomas are currently the most common pattern recognised in needle biopsy specimens.10,11,12,13,14,15,16 They exhibit variably sized individual and discrete glands.13,14 In carcinomas that are minimal (<1 mm)6 or limited10 in extent in needle biopsy specimens, the presence of a few malignant acini between the benign glands is indicative of invasion although the glands appear only embedded within the stroma (fig 2). This deceptive appearance is because the invading glands do not usually elicit a desmoplastic or inflammatory response that characterises many invasive carcinomas at other anatomical sites. A distinctive pattern of infiltration is the formation of a column of malignant glands spanning the width of the needle core (fig 3). The infiltrative character of high‐grade Gleason pattern 4 prostatic carcinoma in needle biopsy specimens is typified by ragged invasion of fused microacinar (fig 4), cribriform or papillary masses. Linkage of carcinoma cells into chains and cords also indicates poorly differentiated Gleason pattern 4 adenocarcinoma.11,12,14 Another descriptor of Gleason pattern 4 is ill‐defined glands with poorly formed glandular lumina.13 High‐grade carcinoma can also invade as sheets (fig 5), with destruction and effacement of benign prostatic tissue, or be manifested as comedocarcinoma (fig 6). These arrangements represent Gleason pattern 5, which is uncommon in needle biopsy specimens.12,15,16
Figure 1 Crowded small glands of adenocarcinoma, Gleason grade 3+3 = score 6, with invasion between and around larger, more complex benign glands.
Figure 2 Haphazard distribution of small acini indicative of infiltrative growth. The lack of fibrogenic or inflammatory response is evident.
Figure 3 Column‐like growth of minimal adenocarcinoma across the width of the needle core tissue, a sign of invasion.
Figure 4 Fused acinar adenocarcinoma, Gleason grade 4+4 = score 8.
Figure 5 Sheet of prostatic adenocarcinoma, Gleason grade 5+5 = score 10.
Figure 6 Comedocarcinoma, high‐grade Gleason pattern 5, with comedonecrosis.
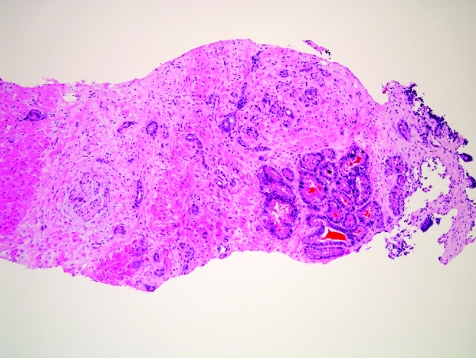
In some cases, benign prostate glands are not present to serve as landmarks to evaluate for invasion. This can happen as a result of destructive growth of the tumour, due to invasion into an area of pure fibromuscular stroma, and due to invasion into extraprostatic tissues, such as periprostatic adipose tissue or seminal vesicle tissue. Destructive growth can occur with any Gleason grade carcinoma that is extensive. Malignant prostatic glands in thick smooth‐muscle bundles in a needle biopsy specimen can represent invasion of inadvertantly sampled extraprostatic bladder neck, but it is difficult to be certain in a needle biopsy specimen that this represents extraprostatic spread and not prostatic stroma. Also, benign or malignant prostatic glands can be observed in skeletal muscle tissue at the apex, where skeletal muscle of the urogenital diaphragm interdigitates into the prostate gland. The detection of infiltrating carcinoma into the skeletal muscle in a needle biopsy specimen does not necessarily equate with extraprostatic spread. As incidental findings, periprostatic tissue can be found in as many as 77% of needle biopsy specimens, 17 and seminal vesicle or ejaculatory duct tissue can be seen in about 20% of specimens.18 Periprostatic adipose tissue is most often seen at the tips of the needle cores, and the presence of carcinoma in this fat can be a useful diagnostic finding (fig 7). However, this is rarely an isolated finding and is generally associated with extensive carcinoma in the rest of the needle biopsy tissue. Prostatic carcinoma infiltration into the seminal vesicle or ejaculatory duct tissue is an uncommon, incidental discovery (fig 8), which tends to be more common in more extensive, higher‐grade carcinoma in needle biopsy tissue. Thus, in prostatic needle biopsy, carcinoma of the prostate can present a range of images of infiltration, with invasion into prostatic as well as extraprostatic tissues.
Figure 7 Extraprostatic extension by prostatic carcinoma into periprostatic adipose tissue sampled by needle biopsy.
Figure 8 Penetration of small‐gland prostatic adenocarcinoma into the seminal vesicle or ejaculatory duct wall. It is difficult to tell, on needle biopsy, whether this is definitely a seminal vesicle or an ejaculatory duct. Seminal vesicle or ejaculatory duct glands are crowded larger glands at lower right.
An infiltrative pattern is not evident for carcinomas with a partially nodular configuration. In rare cases, we may see well‐circumscribed small‐gland proliferations, with smooth, pushing edges. These would be, by definition, well‐differentiated adenocarcinomas, with Gleason score 3–4.11,12,13 However, an entire Gleason score 3–4 nodule is not captured by needle biopsy and, also, well‐differentiated Gleason score 3 and 4 nodules are characteristically found in the transition zone of the prostate, whereas it is the peripheral zone that is typically targeted for needle biopsy. Most apparently, well‐differentiated adenocarcinomas in needle biopsy tissue actually represent intermediate‐grade carcinomas at follow‐up radical prostatectomy,6 such that it has been recommended that carcinomas with Gleason score 3–4 should be rarely, if ever, assigned to adenocarcinoma in needle biopsy tissue.13
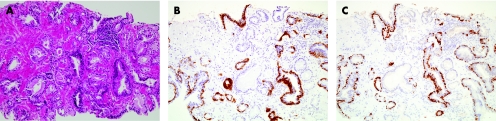
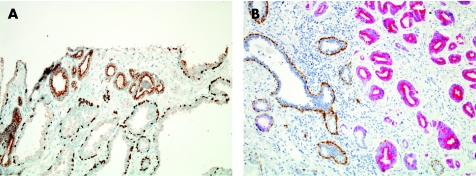
Absence of basal cells in the atypical glands is the second of the major criteria. Basal cells may assume a range of appearances,19,20 and so careful study of basal cells in glands that are clearly benign is a vital exercise before scrutinising abnormal glands of concern to rule out their presence in malignant glands. Thin, well‐fixed sections with quality H&E staining are crucial for the appreciation of a single cell‐lining layer in malignant glands. A historic challenge for the surgical pathologist has been the distinction of periglandular stromal fibroblasts from basal cells. Another common difficulty is that distorted, crushed or poorly preserved carcinoma cells in minimal cancer foci can be mistaken for basal cells. In such cases, application of a basal cell‐specific immunohistochemical stain for high‐molecular‐weight cytokeratin (fig 9B) or p63 (fig 9C) can be diagnostically advantageous (also described in Ancillary Diagnostic Studies). However, these immunostains are not a “malignant stain”, and Totten et al8 put basal cells in their proper context when they noted the following based on examination of H&E‐stained sections:
Figure 9 Minimal adenocarcinoma. Compared with staining by haematoxylin and eosin (A), the 34βE12 (B) and p63 (C) immunohistochemical stains highlight the invasion of the malignant glands, which lack basal cells (brown signal).
This basal cell layer is not always present in benign small glands, so that its absence is not an absolute criterion of carcinoma. Conversely, however, we have not seen it in any case in which the diagnosis was cancer.8
Basal cells may be completely absent in scattered benign and especially atrophic glands,21,22 and a fragmented basal cell layer is characteristic of atypical adenomatous hyperplasia (adenosis)23,24 where, on average, 50% of glands lack a basal cell layer. Additional but rare benign mimickers of prostatic carcinoma that can lack basal cells include mesonephric hyperplasia25 and nephrogenic adenoma.26,27 Loss of basal cells in a few glands of partial atrophy or crowded benign glands is the most common problem that leads to diagnostic difficulty.28 To emphasise, absence of basal cells is a central finding in an atypical small acinar proliferation, but by itself, it is not fully diagnostic of adenocarcinoma.29
Nuclear atypia in the form of nuclear enlargement and nucleolar enlargement is the third of the major criteria for diagnosis of adenocarcinoma. Nuclear atypia in malignant glands most often manifests itself as nuclear enlargement and prominent nucleoli (fig 10). Failure to detect prominent nucleoli in prostatic carcinoma nuclei is probably multifactorial; large nucleoli might be present but undetectable owing to poor preservation, poor fixation, overstaining or section thickness. This last factor of overly thick sections is an extremely common problem. In addition, lack of chromatin clearing might contribute to the inability to detect nucleoli. Finally, some prostate cancers do not harbour macronucleoli. Examples include foamy gland carcinoma that can have shrunken nuclei,30 prostatic carcinoma with androgen deprivation treatment effect31 and some well‐differentiated adenocarcinomas (so‐called nucleolus‐poor adenocarcinomas).32 The last two examples are usually not seen in needle biopsy tissue. One essential point is that when macronucleoli are absent, there should be significant nucleomegaly with or without nuclear hyperchromasia to definitively diagnose carcinoma.
Figure 10 Nuclear atypia in adenocarcinoma: appearance of nuclei, some with prominent nucleoli, 400×.
Minor diagnostic criteria
Minor diagnostic criteria (box 1) are found on an individual basis in a smaller proportion of cases than those with major diagnostic criteria, with the exception of intraluminal amorphous pink material. These minor or “soft” diagnostic attributes are not specific for carcinoma but are useful for prompting in‐depth study of the glands harbouring these changes, with a view towards assessment of the aforementioned major diagnostic criteria. With the possible exception of high‐grade prostatic intraepithelial neoplasia (PIN), none of the listed minor criteria should, by itself, be considered a sufficiently atypical finding to warrant rebiopsy. (A recent recommendation is that men do not need routine repeat needle biopsy within the first year after the diagnosis of high‐grade PIN.33)
Diagnostic features considered specific for prostatic carcinoma
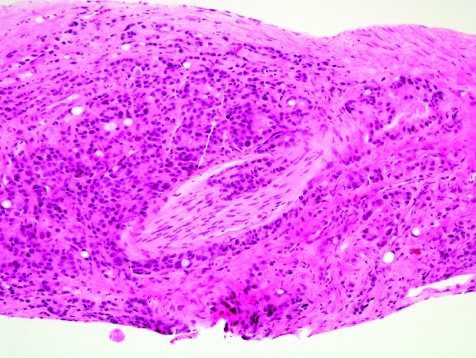
After assessment for major and minor criteria, the pathologist should look for diagnostic features that have been forwarded as specific for malignancy,34 including perineural invasion (fig 11), collagenous micronodules (also known as mucinous fibroplasia; fig 12),35 and glomeruloid intraglandular projections.36 These findings are present less often in prostate needle biopsy specimens compared with whole glands with carcinoma.37 They are rare in minimal prostatic adenocarcinoma,34 and this obviously diminishes their diagnostic usefulness in this setting where they are needed most. Perineural invasion by carcinoma can be found in 11–37% of needle biopsy specimens with carcinoma,37 but in only 0–3% of tissue with minimal or limited prostate cancer.6,7,10,34 True perineural invasion, which is characteristic of adenocarcinoma of the prostate, needs to be distinguished from benign glands abutting the prostatic peripheral nerve.38,39,40 Collagenous micronodules are seen in 1–5% of all needle biopsy cases with carcinoma,37 and in as low as 0.1% of cases with minimal carcinoma.6,7,34 Glomerations are discovered in 3–15% of carcinomas on needle biopsy,37 but are extremely rare in minimal carcinoma in needle biopsy tissues; we saw only one case with a suggestion of glomeruloid intraluminal tufting.6 In another series,34 not a single case had glomerulations as a key feature in diagnosing very limited cancer. Another finding specific for carcinoma—lymphovascular space invasion—is vanishingly rare in prostate needle biopsy tissue.
Figure 11 Perineural invasion, with focal intraneural invasion, by prostatic adenocarcinoma.
Figure 12 Collagenous micronodules, a feature thought to be specific for adenocarcinoma in the prostate.
Minimal adenocarcinoma
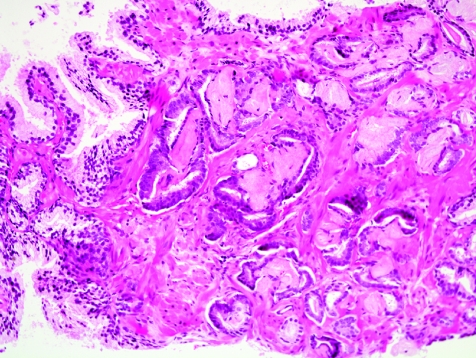
One of the major diagnostic challenges confronting the histopathologist in interpretation of a prostate needle biopsy specimen is the establishment of a malignant diagnosis based on a minimal or limited amount of carcinoma in the needle biopsy tissue. The major and minor diagnostic criteria should be put into use here, just as for more extensive carcinoma. The presence of a few small malignant glands located between larger, more complex, paler benign glands is the most common pattern of invasion in minimal carcinoma (fig 13), accounting for 80% of cases in one series.10 The second most common pattern of infiltration of limited carcinoma was a haphazard growth in stroma, without adjacent benign glands. Uncommon patterns of growth that typically accompanied the common patterns were cords of cells, single cells and cribriform glands.10
Figure 13 Minimal adenocarcinoma, Gleason grade 3+3 = score of 6.
No definite quantitative threshold exists for the number of glands required to establish a diagnosis of malignancy, although most authorities require at least 2–3 glands (I prefer three glands). Clearly though, each case must be interpreted on it own merits such that a very few glands may be definitely diagnostic in one case but not in another. Many cases of minimal carcinoma harbour well over 2–3 glands. In our series,6 80% of minimal carcinoma foci contained >10 glands, and in a second series,10 the median number of malignant glands was 20. In a third series, the mean (standard deviation (SD)) number of acini to diagnose carcinoma was 17 (14).7 In our experience, the smallest number of atypical glands that formed the basis for a definitive diagnosis of malignancy was 3–4. In these cases, there should be excellent preservation of cellular detail, without tangential sectioning, such that it was clear on the H&E sections that basal cells were absent and that considerable nuclear atypia (with numerous macronucleoli) was present. Both of these major criteria are of immense importance for these most minimal of the minimal carcinomas, where discerning architectural abnormalities is difficult to impossible. In another series on minimal (limited) carcinoma, the lowest number of diagnostic glands was two.10 At a consensus conference,9 most urological pathologists thought that three glands constituted the typical lowest numerical cut‐off. Finally, in this era, there is a trend to confirm the diagnosis of minimal adenocarcinoma with immunohistochemical stains for basal cells and α‐methylacyl coenzyme A racemase (AMACR)29 (described later in Ancillary Diagnostic Studies).
Differential diagnosis of adenocarcinoma in needle biopsy tissue
Numerous entities, both benign and malignant, should be considered in the differential diagnosis of prostatic adenocarcinoma.37 In‐depth discussion of these entities is beyond the scope of this review.
Recent reviews have highlighted the benign lesions or pseudoneoplasms (box 2) that may mimic prostatic adenocarcinoma.41,42 Atypical adenomatous hyperplasia (adenosis) and atrophy are the benign conditions that are most likely to be misdiagnosed as prostatic carcinoma.28,41,42 Crowded benign glands may also be mistaken for prostatic adenocarcinoma.28 The pathologist should always consider the possibility of a benign mimicker of prostatic carcinoma, particularly atrophy, but also all other entities (box 2), before making a diagnosis of adenocarcinoma of the prostate.
A descriptive diagnosis which may be rendered if the histological or immunohistochemical findings are thought to be worrisome but not fully diagnostic of carcinoma, is atypia,33,43 also known as atypical suspicious for carcinoma or atypical small acinar proliferation. Such a diagnosis is given in about 4–5% of all prostate needle biopsy specimens.33,43
The main non‐prostatic, secondary malignancy to think about before diagnosing prostatic carcinoma in needle biopsy of the prostate is urothelial (transitional cell) carcinoma involving the prostate.37,44
Ancillary diagnostic studies
The most valuable adjunctive study for the diagnosis of adenocarcinoma of the prostate, particularly minimal adenocarcinoma, is immunohistochemistry with antibodies directed against basal cells (34βE12 and p63) and AMACR (also known as P504S or racemase). In the past, basal cell immunostains were used by themselves. Currently, many laboratories combine use of basal cell markers with an immunostain for AMACR, sometimes in a cocktail.
Historically, the most widely used immunostain is monoclonal antibody 34βE12 (fig 9B), which binds to high‐molecular‐weight cytokeratin proteins expressed in the cytoplasm of basal cells and not in luminal cells.21,29 This antibody, which also has been identified by its commercial catalogue number CK903, is useful for documenting the absence of basal cells in focal atypical small acinar proliferations, yet there are caveats in the use and interpretation of this immunohistochemical staining reaction. Firstly, absence of basal cells is an important criterion for the histological diagnosis of prostatic carcinoma, but is only one of several of the major criteria. Thus, this immunostain, as other adjunctive studies, should be interpreted in the context of all histological findings in the particular case. Also, not all benign glands have basal cells. Finally, this immunohistochemical stain is based on a negative result to give a positive diagnosis of malignant neoplasm. Many factors can cause failure of immunohistochemical staining, and absence of proof is not proof of absence. So, it is absolutely critical to study the immunostained cores for a positive internal control—benign glands with a strong, positive basal cell signal. Preferably, these benign glands are located near the adjacent carcinoma (fig 9B) or are at least in the same core.
The 34βE12 immunostain can be useful as a confirmatory measure in specific circumstances. It should not be used as a screening test in all prostate needle biopsy specimens but rather should be applied specifically in selected cases with selection and the differential diagnosis driven by the histological appearance of the H&E‐stained slides. In the past 10 years, the use of basal cell immunostains has increased: in a large series from 1995, only 2.8% of all prostate needle biopsy specimens were immunostained with the antibody 34βE12,45 compared with a recent series from 2004, where immunostains were used in 22% of prostate needle biopsy specimens.46 Basal cell stains are most often ordered to evaluate a focus of atypical glands. They have also been ordered in selected cases in which the differential diagnosis encompassed atypical adenomatous hyperplasia (adenosis), PIN, basal cell hyperplasia and atrophy.45 The immunostain also can be used to distinguish cribriform high‐grade PIN from cribriform invasive carcinoma.47 In one series of needle biopsy specimens, it was concluded that the 34βE12 immunostain substantially reduced the percentage of prostate needle biopsy cases diagnosed as atypical.48
One setting where basal cell markers, including the 34βE12 immunostain, can be particularly valuable is in biopsies after radiation therapy for prostatic carcinoma, when the differential diagnostic consideration based on H&E slides is radiation‐induced atypia in benign glands versus minimal residual carcinoma.49,50 In this setting, reactive basal cells can mimic cancer cells cytologically in nuclear changes and, here, the 34βE12 immunostain can provide important diagnostic information. Also, minimal residual carcinoma after hormonal therapy can pose diagnostic challenges, and positive immunostains for PSA and pan‐cytokeratin, with a negative 34βE12 immunostain, can sometimes be helpful.50
Additional basal cell markers for the diagnosis of adenocarcinoma include p63 (fig 9C),51,52 cytokeratins (CK) 5/6,53 cystatin A54 and calcyclin.55 The p63 immunostain, which detects p63 protein in the nucleus of basal cells, seems to be more sensitive in basal cell detection than the immunostain using the 34βE12 antibody.29,56 Some have combined p63 and 34βE12 antibodies in a cocktail.46,56,57 Although in one study this cocktail increased the sensitivity of basal cell detection and reduced the staining variability,57 data from a second study on atypical glandular proliferations showed that there was little, if any, advantage in using the cocktail compared with a p63 immunostain alone.56
It would be desirable to diagnose adenocarcinoma and to have a positive marker that is relatively specific for malignant prostatic epithelial cells, in addition to the negative basal cell markers. From immunohistochemical studies,29,58,59,60,61,62,63,64,65,66,67,68,69 one such marker, AMACR, is selective and very sensitive for carcinoma. About 80–100% of prostatic adenocarcinomas stain with AMACR. As many as 21% of benign prostatic glands also stain, including atrophy,29,67 but the staining signal is usually more focal and weaker than with carcinoma. A misleading result can be obtained with partial atrophy, where up to 79% of cases have been reported to be AMACR positive.28 This finding, combined with focal basal loss and nuclear atypia70 that may be seen in atrophy, could result in a misdiagnosis of partial atrophy as adenocarcinoma. Most high‐grade PIN foci stain,64 a minority of atypical adenomatous hyperplasia cases stain,58 and nephrogenic adenoma, which rarely involves the prostate, can also be positive.26,27 This immunostain can be quite helpful, as a confirmatory tool, in cases of minimal carcinoma.61,62 It may have utility in diagnosing carcinoma in patients after radiotherapy and after hormonal therapy, although its value here is less certain.29,49 It is best interpreted only in a histological context and in conjunction with 34βE12 or p63 immunostaining.
Cocktails comprised of AMACR and a basal cell marker such as p63,65,66,67,68 or AMACR/p63/34βE12,69 with single or two chromogens, are useful for simultaneous assessment of these markers in the same glands (fig 14). Application of such a cocktail is decidedly advantageous if only a small atypical focus is present, and especially if the focus is present only on a single section or slide that is available for immunohistochemical evaluation.67 Such cocktails can also be used on destained H&E‐stained sections, if unstained sections are unavailable.68
Figure 14 Basal cell markers/α‐methylacyl‐–coenzyme A racemase (AMACR) cocktails with one (A) and two (B) chromogens. (A) p63/AMACR cocktail immunohistochemical stain: p63 is detected in the nuclei of basal cells in benign glands (below), compared with granular cytoplasmic AMACR stain, with apical luminal accentuation (above) in glands of adenocarcinoma, which lack p63. (B) 34βE12/p63/AMACR cocktail: 34βE12 and p63 antibody binding to basal cells is indicated by brown stain, whereas red stain corresponds to AMACR detection. AMACR‐positive, basal‐cell‐negative adenocarcinoma is on the right, whereas benign, AMACR‐negative, basal‐cell‐positive glands are on the left.
In certain cases, additional immunohistochemical studies are indicated.29 For example, in the differential diagnosis of focal granulomatous prostatitis versus focal poorly differentiated prostatic adenocarcinoma, a panel of antibodies to cytokeratins (AE1/AE3), PSA, prostatic acid phosphatase (PSAP), lysozyme, antimacrophage M and leucocyte common antigen (CD45) reliably distinguished the disorders.71 The differential diagnostic investigation of prostatic carcinoma versus urothelial (transitional cell) carcinoma in prostate needle biopsy tissue likewise can be aided by using a targeted panel of antibodies,29 including PSA, PSAP, 34βE12 and thrombomodulin. PSA and PSAP are the best prostatic markers in general, but they are not absolutely specific and can stain benign and malignant non‐prostatic cells and tissue. Although usually resolved by examination of H&E‐stained slides, the distinction of small seminal vesicle glands and minimal prostatic adenocarcinoma also can be accomplished by PSA, PSAP and 34βE12 immunohistochemical studies, in which seminal vesicle glands should be negative for the first two immunostains and positive for the last one.
Genetic abnormalities such RNA overexpression or underexpression, DNA ploidy, chromosomal anomalies, gain or loss of specific DNA sequences, DNA methylation and specific gene mutations are not used in the diagnosis of carcinoma in prostate needle biopsy specimens.
Box 2: Differential diagnosis of prostatic adenocarcinoma: pseudoneoplasms of the prostate
Atypical adenomatous hyperplasia (adenosis)
Atrophy
Crowded benign glands
Basal cell hyperplasia
Sclerosing adenosis
Cribriform hyperplasia
Mesonephric hyperplasia
Nephrogenic metaplasia (adenoma)
Verumontanum mucosal gland hyperplasia
Squamous metaplasia
Transitional cell metaplasia
Radiation atypia
Prostatitis
Malacoplakia
Endometriosis
Postoperative spindle cell nodule
Atypical stromal cells
Extramedullary haematopoiesis
Cowper glands
Paraganglia in prostate
Benign glands adjacent to nerves and skeletal muscle
Reporting
The most clinically important attributes of adenocarcinoma in prostate needle biopsy specimens that merit reporting are Gleason grade and amount of tumour in the needle biopsy tissue.11,12,13,72,73 Measures of tumour extent in the needle biopsy tissue include (1) number of positive needle cores in the total number of cores, (2) percentage of positive cores, (3) percentage of needle core tissue affected by carcinoma as determined by visual inspection and (4) linear millimetres of tumour extent. All these measures provide nearly equivalent information, such that there is not a standard method of appraising the tumour extent in a needle biopsy specimen. Indeed, several measures should be given for the same set of needle biopsies.72,73,74 It is also worthwhile to report, for needle core biopsies, perineural invasion by carcinoma, the presence of extraprostatic carcinoma in adipose tissue or seminal vesicle, and lymphvascular space invasion by carcinoma.73
Abbreviations
AMACR - α‐methylacyl coenzyme A racemase
H&E - haematoxylin and eosin
PIN - prostatic intraepithelial neoplasia
PSA - prostate‐specific antigen
PSAP - prostatic acid phosphatase
Footnotes
Competing interests: None declared.
References
- 1.Parkin D M, Bray F, Ferlay J.et al Global cancer statistics, 2002. CA Cancer J Clin 20055574–108. [DOI] [PubMed] [Google Scholar]
- 2.Humphrey P A. Clinical aspects of prostatic carcinoma, with histopathologic correlations. In: Prostate pathology. Chicago: ASCP Press, 2003226–257.
- 3.Epstein J I, Walsh P C, Carmichael M.et al Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA 1994271368–374. [PubMed] [Google Scholar]
- 4.Berger A P, Spranger R, Kofler K.et al Early detection of prostate cancer with low PSA cut‐off values leads to significant stage migration in radical prostatectomy specimens. Prostate 20035793–98. [DOI] [PubMed] [Google Scholar]
- 5.van der Cruijsen‐Koeter I W, Vis A N, Roobol M J.et al Comparison of screen detected and clinically diagnosed prostate cancer in the European randomized study of screening for prostate cancer, section Rotterdam. J Urol 2005174121–125. [DOI] [PubMed] [Google Scholar]
- 6.Thorson P, Vollmer R T, Arcangeli C.et al Minimal carcinoma in prostate needle biopsy specimens: diagnostic features and radical prostatectomy follow‐up. Mod Pathol 199811543–551. [PubMed] [Google Scholar]
- 7.Iczkowski K A, Bostwick D G. Criteria for biopsy diagnosis of minimal volume prostatic adenocarcinoma: analytic comparison with nondiagnostic but suspicious atypical small acinar proliferation. Arch Pathol Lab Med 200012498–107. [DOI] [PubMed] [Google Scholar]
- 8.Totten R S, Heinemann M W, Hudson P B.et al Microscopic differential diagnosis of latent carcinoma of prostate. Arch Pathol 195355131–141. [PubMed] [Google Scholar]
- 9.Algaba F, Epstein J I, Aldape H C.et al Assessment of prostate carcinoma in core needle biopsy—definition of minimal criteria for the diagnosis of cancer in biopsy material. Cancer 199678376–381. [DOI] [PubMed] [Google Scholar]
- 10.Epstein J I. Diagnostic criteria of limited adenocarcinoma of the prostate on needle biopsy. Hum Pathol 199526223–229. [DOI] [PubMed] [Google Scholar]
- 11.Humphrey P A. Gleason grading and prognostic factors in carcinoma of the prostate. Mod Pathol 200417292–306. [DOI] [PubMed] [Google Scholar]
- 12.Amin M B, Grignon D J, Humphrey P A.et al In: Gleason grading of prostate cancer. A contemporary approach. Philadelphia: Lippincott Williams & Wilkins, 2004
- 13.Epstein J I, Allsbrook W C, Jr, Amin M B.et al The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol 2005291228–1242. [DOI] [PubMed] [Google Scholar]
- 14.Gleason D F. Histologic grading of prostatic carcinoma. In: Bostwick DG, ed. Pathology of the prostate. New York: Churchill Livingstone, 199083–93.
- 15.Bostwick D G. Gleason grading of prostatic needle biopsies. Correlation with grade in 316 matched prostatectomies. Am J Surg Pathol 199418796–803. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg D M, Sauvageot J, Piantodosi S.et al Correlation of prostate needle biopsy and radical prostatectomy Gleason grade in academic and community settings. Am J Surg Pathol 199721566–576. [DOI] [PubMed] [Google Scholar]
- 17.Ravery V, Boccon‐Gibod L A, Dauge‐Geffroy M C.et al Systematic biopsies accurately predict extracapsular extension of prostate cancer and persistent/recurrent detectable PSA after radical prostatectomy. Urology 199444371–376. [DOI] [PubMed] [Google Scholar]
- 18.Bostwick D G, Dundore P A. Normal anatomy and histology. In: Biopsy pathology of the prostate. London: Chapman and Hall Medical, 199712
- 19.Cleary K R, Choi H Y, Ayala A G. Basal cell hyperplasia of the prostate. Am J Clin Pathol 198380850–854. [DOI] [PubMed] [Google Scholar]
- 20.Devaraj L T, Bostwick D G. Atypical basal cell hyperplasia of the prostate. Immunophenotypic profile and proposed classification of basal cell proliferations. Am J Surg Pathol 199317645–659. [DOI] [PubMed] [Google Scholar]
- 21.Hedrick L, Epstein J I. Use of keratin 903 as an adjunct in the diagnosis of prostate carcinoma. Am J Surg Pathol 198913389–396. [DOI] [PubMed] [Google Scholar]
- 22.Varma M, Amin M B, Linden M D.et al Discriminant staining patterns of small glandular and preneoplastic lesions of the prostate using high molecular weight cytokeratin (HMCK): a study of 301 consecutive needle biopsies [abstract]. Mod Pathol 19971093A [Google Scholar]
- 23.Gaudin P B, Epstein J I. Adenosis of the prostate. Histologic features in needle biopsy specimens. Am J Surg Pathol 199519737–747. [DOI] [PubMed] [Google Scholar]
- 24.Bostwick D G, Srigley J, Grignon D.et al Atypical adenomatous hyperplasia of the prostate: morphologic criteria for its distinction from well‐differentiated carcinoma. Hum Pathol 199324819–832. [DOI] [PubMed] [Google Scholar]
- 25.Bostwick D G, Qian J, Ma J.et al Mesonephric remnants of the prostate: incidence and histologic spectrum. Mod Pathol 200316630–635. [DOI] [PubMed] [Google Scholar]
- 26.Skinnider B F, Oliva E, Young R H.et al Expression of alpha‐methylacyl‐CoA racemase (P504S) in nephrogenic adenoma: a significant immunohistochemical pitfall compounding the differential diagnosis with prostatic adenocarcinoma. Am J Surg Pathol 200428701–705. [DOI] [PubMed] [Google Scholar]
- 27.Gupta A, Wang H L, Policarpio‐Nicolas M L.et al Expression of alpha‐methylacyl coenzyme A racemase in nephrogenic adenoma. Am J Surg Pathol 2004281224–1229. [DOI] [PubMed] [Google Scholar]
- 28.Herawi M, Parwani A V, Irie J.et al Small glandular proliferations: most common benign mimickers of prostatic adenocarcinoma sent for expert second opinion. Am J Surg Pathol 200529874–880. [DOI] [PubMed] [Google Scholar]
- 29.Hameed O, Humphrey P A. Immunohistochemistry in diagnostic surgical pathology of the prostate. Semin Diagn Pathol 20052288–104. [DOI] [PubMed] [Google Scholar]
- 30.Nelson R S, Epstein J I. Prostatic carcinoma with abundant xanthomatous cytoplasm. Foamy gland carcinoma. Am J Surg Pathol 199620419–426. [DOI] [PubMed] [Google Scholar]
- 31.Reuter V E. Pathological changes in benign and malignant tissue following androgen deprivation therapy. Urology 199749(Suppl 3A)16–22. [DOI] [PubMed] [Google Scholar]
- 32.Kramer C E, Epstein J I. Nucleoli in low‐grade prostate adenocarcinoma and adenosis. Hum Pathol 199324618–623. [DOI] [PubMed] [Google Scholar]
- 33.Epstein J I, Herawi M. Prostate needle biopsies containing prostatic intraepithelial neoplasia or atypical foci suspicious for carcinoma: implications for patient care. J Urol 2006175820–834. [DOI] [PubMed] [Google Scholar]
- 34.Baisden B L, Kahane H, Epstein J I. Perineural invasion, mucinous fibroplasia, and glomerulations: diagnostic features of limited cancer on prostate needle biopsy. Am J Surg Pathol 199923918–924. [DOI] [PubMed] [Google Scholar]
- 35.Bostwick D G, Wollan P, Adlakha K. Collagenous micronodules in prostate cancer. A specific but infrequent diagnostic finding. Arch Pathol Lab Med 1995119444–447. [PubMed] [Google Scholar]
- 36.Pacelli A, Lopez‐Beltran A, Egan A J.et al Prostatic adenocarcinoma with glomeruloid features. Hum Pathol 199829543–546. [DOI] [PubMed] [Google Scholar]
- 37.Humphrey P A. Adenocarcinoma. In: Prostate pathology. Chicago: ASCP Press, 2003259–337.
- 38.Carstens P H B. Perineural glands in normal and hyperplastic prostate. J Urol 1989123686–688. [DOI] [PubMed] [Google Scholar]
- 39.McIntire T L, Franzini D A. The presence of benign prostatic glands in perineural spaces. J Urol 1986135507–509. [DOI] [PubMed] [Google Scholar]
- 40.Ali T Z, Epstein J I. Perineural involvement by benign prostatic glands on needle biopsy. Am J Surg Pathol 2005291159–1163. [DOI] [PubMed] [Google Scholar]
- 41.Young R H. Pseudoneoplastic lesions of the urinary bladder, prostate gland, and urethra. In: Wick MR, Humphrey PA, Ritter JR, eds. Pathology of pseudoneoplastic lesions. New York: Lippincott‐Raven, 1997223–235.
- 42.Srigley J R. Benign mimickers of prostatic adenocarcinoma. Mod Pathol 200417328–348. [DOI] [PubMed] [Google Scholar]
- 43.Humphrey P A. Focal glandular atypia. In: Prostate pathology. Chicago: ASCP Press, 2003218–225.
- 44.Oliai B R, Kahane H, Epstein J I. A clinicopathologic analysis of urothelial carcinomas diagnosed on prostate needle biopsy. Am J Surg Pathol 200125794–801. [DOI] [PubMed] [Google Scholar]
- 45.Wojno K J, Epstein J I. The utility of basal cell‐specific anti‐cytokeratin antibody (34 beta E12) in the diagnosis of prostate cancer. A review of 228 cases. Am J Surg Pathol 199519251–260. [DOI] [PubMed] [Google Scholar]
- 46.Browne T J, Hirsch M S, Brodsky G.et al Prospective evaluation of AMACR (P504S) and basal cell markers in the assessment of routine needle biopsy specimens. Hum Pathol 2004351462–1468. [DOI] [PubMed] [Google Scholar]
- 47.Amin M B, Schultz D S, Zarbo R J. Analysis of cribriform morphology in prostatic neoplasia using antibody to high‐molecular‐weight cytokeratins. Arch Pathol Lab Med 1994118250–264. [PubMed] [Google Scholar]
- 48.Kahane H, Sharp J W, Shuman G B.et al Utilization of high molecular weight cytokeratin on prostate needle biopsies in an independent laboratory. Urology 199545981–986. [DOI] [PubMed] [Google Scholar]
- 49.Martens M B, Keller J H. Routine immunohistochemical staining for high‐molecular weight cytokeratin 34‐beta and alpha‐methylacyl CoA racemase (P504S) in postirradiation prostate biopsies. Mod Pathol 200619287–290. [DOI] [PubMed] [Google Scholar]
- 50.Gaudin P B. Histopathologic effects of radiation and hormonal therapies on benign and malignant prostate tissues. J Urol Pathol 1988855–67. [Google Scholar]
- 51.Parsons J K, Gage W R, Nelson W G.et al p63 protein expression is rare in prostate adenocarcinoma: implications for cancer diagnosis and carcinogenesis. Urology 200158619–624. [DOI] [PubMed] [Google Scholar]
- 52.Signoretti S, Waltregny D, Dilks J.et al p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol 20001571769–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abrahams N A, Ormsby A H, Brainard J. Validation of cytokeratin 5/6 as an effective substitute for keratin 903 in the differentiation of benign from malignant glands in prostate needle biopsies. Histopathology 20024135–41. [DOI] [PubMed] [Google Scholar]
- 54.Mirtti T, Alanen K, Kallajoki M.et al Expression of cystatins, high molecular weight cytokeratin, and proliferation markers in prostatic adenocarcinoma and hyperplasia. Prostate 200354290–298. [DOI] [PubMed] [Google Scholar]
- 55.Rehman I, Cross S S, Azzouzi A R.et al S100A6 (calcyclin) is a prostate basal cell marker absent in prostate cancer and its precursors. Br J Cancer 200491739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shah R B, Kunju L P, Shen R.et al Usefulness of basal cell cocktail (34betaE12+p63) in the diagnosis of atypical prostate glandular proliferations. Am J Clin Pathol 2004122517–523. [DOI] [PubMed] [Google Scholar]
- 57.Zhou M, Shah R, Shen R.et al Basal cell cocktail (34betaE12+p63) improves the detection of prostate basal cells. Am J Surg Pathol 200327365–371. [DOI] [PubMed] [Google Scholar]
- 58.Yang X J, Wu C L, Woda B A.et al Expression of alpha‐methylacyl‐CoA racemase (P504S) in atypical adenomatous hyperplasia of the prostate. Am J Surg Pathol 200226921–925. [DOI] [PubMed] [Google Scholar]
- 59.Rubin M A, Zhou M, Dhanasekaran S M.et al (Alpha)‐methylacyl coenzyme A racemase as a tissue biomarker for prostate cancer. JAMA 20022871662–1670. [DOI] [PubMed] [Google Scholar]
- 60.Beach R, Gown A M, de Peralta‐Venturina M N.et al P504S immunohistochemical detection in 405 prostatic specimens including 376 18‐gauge needle biopsies. Am J Surg Pathol 2002261588–1596. [DOI] [PubMed] [Google Scholar]
- 61.Jiang Z, Wu C ‐ L, Woda B A.et al P504S/alpha‐methylacyl‐CoA racemase. A useful marker for diagnosis of small foci of prostatic carcinoma on needle biopsy. Am J Surg Pathol 2002261169–1174. [DOI] [PubMed] [Google Scholar]
- 62.Magi‐Galluzzi C, Luo J, Issacs W B.et al Alpha‐methylacyl‐CoA racemase: a variably sensitive immunohistochemical marker for the diagnosis of small cancer foci on needle biopsy. Am J Surg Pathol 2003271128–1133. [DOI] [PubMed] [Google Scholar]
- 63.Jiang Z, Woda B, Rock K L.et al P504S: a new molecular marker for the detection of prostate carcinoma. Am J Surg Pathol 2001251397–1404. [DOI] [PubMed] [Google Scholar]
- 64.Wu C L, Yang X J, Tretiakova M.et al Analysis of alpha‐methylacyl‐CoA racemase (P504S) expression in high‐grade prostatic intraepithelial neoplasia. Hum Pathol 2004351008–1013. [DOI] [PubMed] [Google Scholar]
- 65.Sanderson S O, Sebo T J, Murphy L M.et al An analysis of the p63/alpha‐methylacyl coenzyme A racemase immunohistochemical cocktail stain in prostate needle biopsy specimens and tissue microarrays. Am J Clin Pathol 2004121220–225. [DOI] [PubMed] [Google Scholar]
- 66.Molinie V, Fromont G, Sibony M.et al Diagnostic utility of p63/alpha‐methyl‐CoA‐racemase (p504s) cocktail in atypical foci in the prostate. Mod Pathol 2004171180–1190. [DOI] [PubMed] [Google Scholar]
- 67.Hameed O, Sublett J, Humphrey P A. Immunohistochemical stains for p63, alpha‐methylacyl‐CoA racemase, versus a cocktail comprising both, in the diagnosis of prostatic carcinoma: a comparison of the immunohistochemical staining of 430 foci in radical prostatectomy and needle biopsy tissues. Am J Surg Pathol 200529579–587. [DOI] [PubMed] [Google Scholar]
- 68.Hameed O, Humphrey P A. P63/AMACR antibody cocktail restaining of prostate needle biopsy tissues after transfer to charged slides: a viable approach in the diagnosis of small atypical foci that are lost on block sectioning. Am J Clin Pathol 2005124708–715. [DOI] [PubMed] [Google Scholar]
- 69.Jiang Z, Li C, Fischer A.et al Using an AMACR (P504S)/34betaE12/p63 cocktail for the detection of small focal prostate carcinoma in needle biopsy specimens. Am J Clin Pathol 2005123231–236. [DOI] [PubMed] [Google Scholar]
- 70.Ruska K M, Sauvageot J, Epstein J I. Histology and cellular kinetics of prostatic atrophy. Am J Surg Pathol 1998221073–1077. [DOI] [PubMed] [Google Scholar]
- 71.Presti B, Weidner N. Granulomatous prostatitis and poorly differentiated prostate carcinoma. Their distinction with the use of immunohistochemical methods. Am J Clin Pathol 199195330–334. [DOI] [PubMed] [Google Scholar]
- 72.Epstein J I, Potter S R. The pathologic interpretation and significance of prostate biopsy findings: implications and current controversies. J Urol 2001166402–410. [PubMed] [Google Scholar]
- 73.Amin M B, Boccon‐Gibod L, Egevad L.et al Prognostic and predictive factors and reporting of prostate carcinoma in prostate needle biopsy specimens. Scand J Urol Nephrol 2005216(Suppl)20–33. [DOI] [PubMed] [Google Scholar]
- 74.Bismar T A, Lewis J S, Jr, Vollmer R T.et al Multiple measures of carcinoma extent versus perineural invasion in prostate needle biopsy tissue in prediction of pathologic stage in a screening population. Am J Surg Pathol 200327432–440. [DOI] [PubMed] [Google Scholar]