Abstract
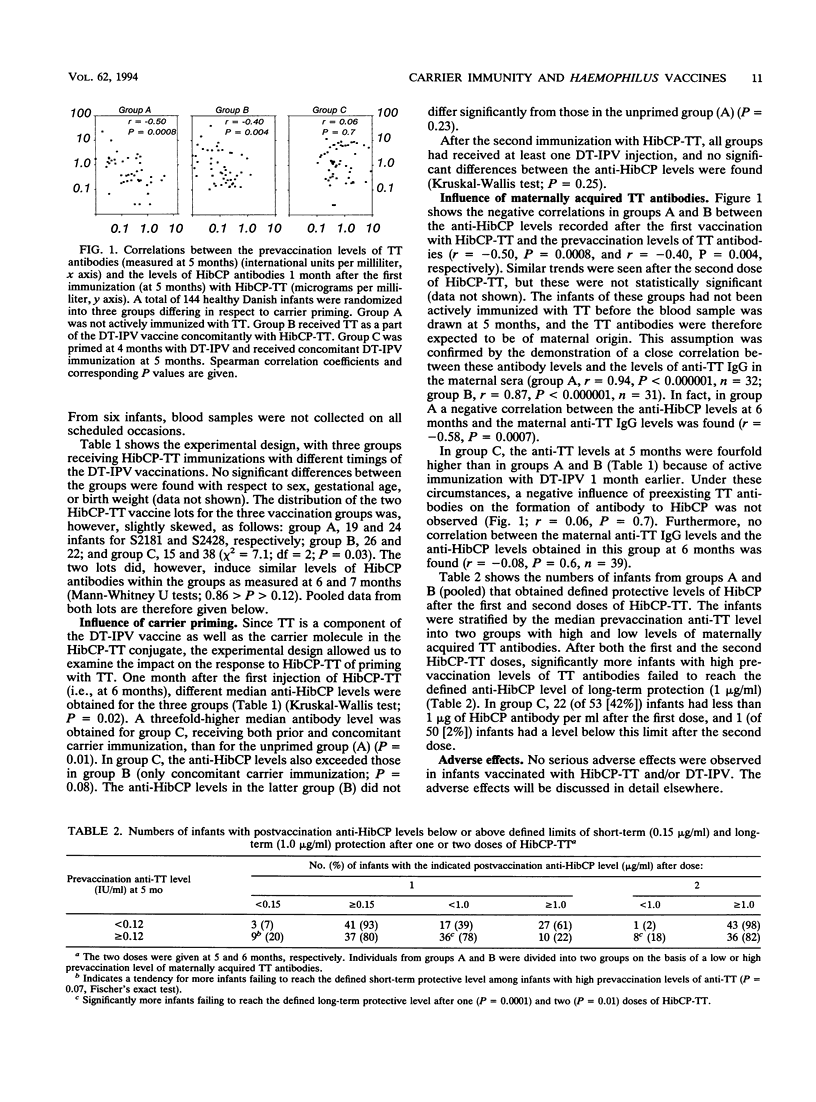
Vaccination of infants with Haemophilus influenzae type b (Hib) capsular polysaccharide (HibCP) coupled to carrier proteins has proven protective against invasive Hib diseases in several trials. However, insufficient immunogenicity has been noted in certain populations. Therefore, studies analyzing factors influencing the antibody response to conjugate vaccines are needed. In this study, the response to HibCP coupled to tetanus toxoid (TT) was examined in relation to (i) priming with or coadministration of the carrier protein and (ii) the levels of passively acquired maternal TT antibodies. One hundred forty-four infants were vaccinated with HibCP-TT at 5 and 6 months. They were randomized into three groups that received TT as part of a diphtheria-tetanus-polio vaccine at either 6 and 7 months (group A), 5 and 6 months (group B), or 4 and 5 months (group C). Maternally acquired TT antibodies inhibited the anti-HibCP response to the first HibCP-TT dose in groups A and B (r = -0.5 and -0.4, respectively; P < 0.005). In these groups, infants with prevaccination anti-TT levels above the median failed to reach the defined long-term protective level of HibCP antibodies (1 microgram/ml) more often than infants with low prevaccination levels after the first (P = 0.0001) and the second (P = 0.01) doses of HibCP-TT. In contrast, active priming with TT at 4 months resulted in a threefold-higher median level of anti-HibCP (group C; 1.34 micrograms/ml) than in the unprimed group (group A; 0.40 microgram/ml) after the first dose of HibCP-TT (P = 0.01). Coadministration of TT had no enhancing effect (group B; 0.58 microgram/ml). No significant differences between the median anti-HibCP levels were seen after the second HibCP-TT dose (6.72, 9.63, and 11.44 micrograms/ml in groups A, B, and C, respectively; P = 0.25).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. Antibody responses to Haemophilus influenzae type b and diphtheria toxin induced by conjugates of oligosaccharides of the type b capsule with the nontoxic protein CRM197. Infect Immun. 1983 Jan;39(1):233–238. doi: 10.1128/iai.39.1.233-238.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Pichichero M., Edwards K., Porch C. R., Insel R. Priming and induction of Haemophilus influenzae type b capsular antibodies in early infancy by Dpo20, an oligosaccharide-protein conjugate vaccine. J Pediatr. 1987 Nov;111(5):644–650. doi: 10.1016/s0022-3476(87)80237-8. [DOI] [PubMed] [Google Scholar]
- Avery O. T., Goebel W. F. CHEMO-IMMUNOLOGICAL STUDIES ON CONJUGATED CARBOHYDRATE-PROTEINS : II. IMMUNOLOGICAL SPECIFICITY OF SYNTHETIC SUGAR-PROTEIN ANTIGENS. J Exp Med. 1929 Sep 30;50(4):533–550. doi: 10.1084/jem.50.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barington T., Skettrup M., Juul L., Heilmann C. Non-epitope-specific suppression of the antibody response to Haemophilus influenzae type b conjugate vaccines by preimmunization with vaccine components. Infect Immun. 1993 Feb;61(2):432–438. doi: 10.1128/iai.61.2.432-438.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlmer H. A., van Alphen L. A prospective, population-based study of Haemophilus influenzae type b meningitis in The Gambia and the possible consequences. J Infect Dis. 1992 Jun;165 (Suppl 1):S29–S32. doi: 10.1093/infdis/165-supplement_1-s29. [DOI] [PubMed] [Google Scholar]
- Black S. B., Shinefield H. R., Fireman B., Hiatt R. Safety, immunogenicity, and efficacy in infancy of oligosaccharide conjugate Haemophilus influenzae type b vaccine in a United States population: possible implications for optimal use. J Infect Dis. 1992 Jun;165 (Suppl 1):S139–S143. doi: 10.1093/infdis/165-supplement_1-s139. [DOI] [PubMed] [Google Scholar]
- Claesson B. A., Schneerson R., Robbins J. B., Johansson J., Lagergard T., Taranger J., Bryla D., Levi L., Cramton T., Trollfors B. Protective levels of serum antibodies stimulated in infants by two injections of Haemophilus influenzae type b capsular polysaccharide-tetanus toxoid conjugate. J Pediatr. 1989 Jan;114(1):97–100. doi: 10.1016/s0022-3476(89)80611-0. [DOI] [PubMed] [Google Scholar]
- Coulehan J. L., Michaels R. H., Hallowell C., Schults R., Welty T. K., Kuo J. S. Epidemiology of Haemophilus influenzae type B disease among Navajo Indians. Public Health Rep. 1984 Jul-Aug;99(4):404–409. [PMC free article] [PubMed] [Google Scholar]
- Decker M. D., Edwards K. M., Bradley R., Palmer P. Comparative trial in infants of four conjugate Haemophilus influenzae type b vaccines. J Pediatr. 1992 Feb;120(2 Pt 1):184–189. doi: 10.1016/s0022-3476(05)80424-x. [DOI] [PubMed] [Google Scholar]
- Einhorn M. S., Weinberg G. A., Anderson E. L., Granoff P. D., Granoff D. M. Immunogenicity in infants of Haemophilus influenzae type B polysaccharide in a conjugate vaccine with Neisseria meningitidis outer-membrane protein. Lancet. 1986 Aug 9;2(8502):299–302. doi: 10.1016/s0140-6736(86)90001-2. [DOI] [PubMed] [Google Scholar]
- Eskola J., Käyhty H., Peltola H., Karanko V., Mäkelä P. H., Samuelson J., Gordon L. K. Antibody levels achieved in infants by course of Haemophilus influenzae type B polysaccharide/diphtheria toxoid conjugate vaccine. Lancet. 1985 May 25;1(8439):1184–1186. doi: 10.1016/s0140-6736(85)92863-6. [DOI] [PubMed] [Google Scholar]
- Eskola J., Käyhty H., Takala A. K., Peltola H., Rönnberg P. R., Kela E., Pekkanen E., McVerry P. H., Mäkelä P. H. A randomized, prospective field trial of a conjugate vaccine in the protection of infants and young children against invasive Haemophilus influenzae type b disease. N Engl J Med. 1990 Nov 15;323(20):1381–1387. doi: 10.1056/NEJM199011153232004. [DOI] [PubMed] [Google Scholar]
- Etlinger H. M., Gillessen D., Lahm H. W., Matile H., Schönfeld H. J., Trzeciak A. Use of prior vaccinations for the development of new vaccines. Science. 1990 Jul 27;249(4967):423–425. doi: 10.1126/science.1696030. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Anderson E. L., Osterholm M. T., Holmes S. J., McHugh J. E., Belshe R. B., Medley F., Murphy T. V. Differences in the immunogenicity of three Haemophilus influenzae type b conjugate vaccines in infants. J Pediatr. 1992 Aug;121(2):187–194. doi: 10.1016/s0022-3476(05)81186-2. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Boies E. G., Munson R. S., Jr Immunogenicity of Haemophilus influenzae type b polysaccharide--diphtheria toxoid conjugate vaccine in adults. J Pediatr. 1984 Jul;105(1):22–27. doi: 10.1016/s0022-3476(84)80350-9. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Rathore M. H., Holmes S. J., Granoff P. D., Lucas A. H. Effect of immunity to the carrier protein on antibody responses to Haemophilus influenzae type b conjugate vaccines. Vaccine. 1993;11 (Suppl 1):S46–S51. doi: 10.1016/0264-410x(93)90160-y. [DOI] [PubMed] [Google Scholar]
- Hansman D., Hanna J., Morey F. High prevalence of invasive Haemophilus influenzae disease in central Australia, 1986. Lancet. 1986 Oct 18;2(8512):927–927. doi: 10.1016/s0140-6736(86)90451-4. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Paul W. E., Goidl E. A., Benacerraf B. Carrier function in anti-hapten immune responses. I. Enhancement of primary and secondary anti-hapten antibody responses by carrier preimmunization. J Exp Med. 1970 Aug 1;132(2):261–282. doi: 10.1084/jem.132.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käyhty H., Eskola J., Peltola H., Rönnberg P. R., Kela E., Karanko V., Saarinen L. Antibody responses to four Haemophilus influenzae type b conjugate vaccines. Am J Dis Child. 1991 Feb;145(2):223–227. doi: 10.1001/archpedi.1991.02160020117030. [DOI] [PubMed] [Google Scholar]
- Noelle R. J., Snow E. C. T helper cell-dependent B cell activation. FASEB J. 1991 Oct;5(13):2770–2776. doi: 10.1096/fasebj.5.13.1833257. [DOI] [PubMed] [Google Scholar]
- Peeters C. C., Tenbergen-Meekes A. M., Poolman J. T., Beurret M., Zegers B. J., Rijkers G. T. Effect of carrier priming on immunogenicity of saccharide-protein conjugate vaccines. Infect Immun. 1991 Oct;59(10):3504–3510. doi: 10.1128/iai.59.10.3504-3510.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola H., Käyhty H., Sivonen A., Mäkelä H. Haemophilus influenzae type b capsular polysaccharide vaccine in children: a double-blind field study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics. 1977 Nov;60(5):730–737. [PubMed] [Google Scholar]
- Rajewsky K., Schirrmacher V., Nase S., Jerne N. K. The requirement of more than one antigenic determinant for immunogenicity. J Exp Med. 1969 Jun 1;129(6):1131–1143. doi: 10.1084/jem.129.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santosham M., Rivin B., Wolff M., Reid R., Newcomer W., Letson G. W., Almeido-Hill J., Thompson C., Siber G. R. Prevention of Haemophilus influenzae type b infections in Apache and Navajo children. J Infect Dis. 1992 Jun;165 (Suppl 1):S144–S151. doi: 10.1093/infdis/165-supplement_1-s144. [DOI] [PubMed] [Google Scholar]
- Sarvas H., Mäkelä O., Toivanen P., Toivanen A. Effect of carrier preimmunization on the anti-hapten response in the chicken. Scand J Immunol. 1974;3(4):455–460. doi: 10.1111/j.1365-3083.1974.tb01278.x. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R., Robbins J. B., Chu C., Sutton A., Vann W., Vickers J. C., London W. T., Curfman B., Hardegree M. C., Shiloach J. Serum antibody responses of juvenile and infant rhesus monkeys injected with Haemophilus influenzae type b and pneumococcus type 6A capsular polysaccharide-protein conjugates. Infect Immun. 1984 Sep;45(3):582–591. doi: 10.1128/iai.45.3.582-591.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella P. P., Ellis R. W. Immunogenicity of Haemophilus influenzae type b conjugate vaccines in infant rhesus monkeys. Pediatr Res. 1991 Jan;29(1):10–13. doi: 10.1203/00006450-199101000-00003. [DOI] [PubMed] [Google Scholar]
- Ward J. I., Margolis H. S., Lum M. K., Fraser D. W., Bender T. R., Anderson P. Haemophilus influenzae disease in Alaskan Eskimos: characteristics of a population with an unusual incidence of invasive disease. Lancet. 1981 Jun 13;1(8233):1281–1285. doi: 10.1016/s0140-6736(81)92458-2. [DOI] [PubMed] [Google Scholar]
- Ward J., Brenneman G., Letson G. W., Heyward W. L. Limited efficacy of a Haemophilus influenzae type b conjugate vaccine in Alaska Native infants. The Alaska H. influenzae Vaccine Study Group. N Engl J Med. 1990 Nov 15;323(20):1393–1401. doi: 10.1056/NEJM199011153232006. [DOI] [PubMed] [Google Scholar]


