Abstract
Tumours metastatic to the heart (cardiac metastases) are among the least known and highly debated issues in oncology, and few systematic studies are devoted to this topic. Although primary cardiac tumours are extremely uncommon (various postmortem studies report rates between 0.001% and 0.28%), secondary tumours are not, and at least in theory, the heart can be metastasised by any malignant neoplasm able to spread to distant sites. In general, cardiac metastases are considered to be rare; however, when sought for, the incidence seems to be not as low as expected, ranging from 2.3% and 18.3%. Although no malignant tumours are known that diffuse preferentially to the heart, some do involve the heart more often than others—for example, melanoma and mediastinal primary tumours. This paper attempts to review the pathophysiology of cardiac metastatic disease, epidemiology and clinical presentation of cardiac metastases, and pathological characterisation of the lesions.
Tumours metastatic to the heart (cardiac metastases) are among the least known and highly debated issues in oncology, and few systematic studies are devoted to this topic. Although primary cardiac tumours are extremely uncommon (various post mortem studies report rates between 0.001% and 0.28%), secondary tumours are not, and at least in theory, the heart can be metastasised by any malignant neoplasm able to spread to distant sites.1,2 In general, cardiac metastases are considered to be rare; however, when sought for, the incidence seems to be not as low as expected.3 The incidence of cardiac metastases reported in literature is highly variable, ranging from 2.3% and 18.3% (table 1).3,4,5,6,7,8,9,10,11,12,13,14 Although no malignant tumours are known which diffuse preferentially to the heart, some do involve the heart more often than others—for example, melanoma and mediastinal primary tumours. Therefore, as for every other site, the tumour's ability to spread to the heart depends on several factors, not only the characteristics of the primary tumour but also the specific histological and functional characteristics of the heart.15 This paper attempts to review the pathophysiology of cardiac metastatic disease, epidemiology and clinical presentation of cardiac metastases, and pathological characterisation of the lesions.
Table 1 Overview of the literature.
| Author, year of publication | Neoplasms (n) | Cardiac metastases,n (%) |
|---|---|---|
| Walther et al, 19484 | 2027 | 46 (2.3) |
| Willis et al, 19525 | 342 | 17 (4.9) |
| Hanfling et al, 19606 | 694 | 127 (18.3) |
| Berge et al, 19687 | 2595 | 122 (4.7) |
| Kline et al, 19728 | 716 | 61 (8.5) |
| Karwinski et al, 19899 | 2564 | 130 (5.1) |
| Mukai et al, 198810 | 6240 | 953 (15.0) |
| Manojlovic et al, 199011 | 477 | 39 (8.2) |
| MacGee et al, 199112 | 1311 | 57 (4.3) |
| Silvestri et al, 199713 | 1928 | 162 (8.4) |
| Butany et al, 200514 | NA* | 266 (2.3)* |
| Bussani et al (unpublished data) | 7289 | 662 (9.1) |
*Butany et al38 describe 266 cardiac neoplasms found among 11 432 consecutive autopsies.
Pathophysiology of cardiac metastases
By the term cardiac metastases, we define distant spread of a tumour to any of the structures composing the heart (pericardium, epicardium, myocardium, endocardium, great vessels and coronary arteries), and also tumours affecting the heart cavities or producing actual intracavitary neoplastic thrombi (figs 1–4).
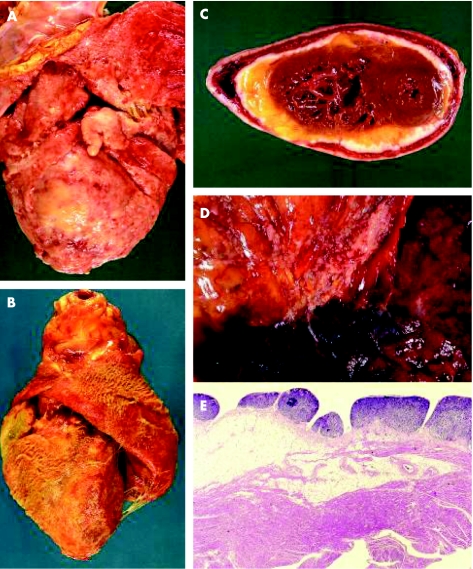
Figure 1 Pericardial metastases usually present clinically as pericardial effusion or pericarditis. (A) A massive infiltration of the pericardium by a non‐small cell lung carcinoma. (B) Fibrino‐haemorrhagic pericarditis in a case of squamous cell carcinoma of the oesophagus. (C) Another case of pericardial infiltrate by non‐small cell lung cancer; the lack of involvement of the myocardium is evident. (D) Neoplastic infiltration of the pericardium by direct invasion by a non‐small cell lung carcinoma. (E) Histology of metastatic pericardial nodules in a patient with poorly differentiated colon carcinoma.
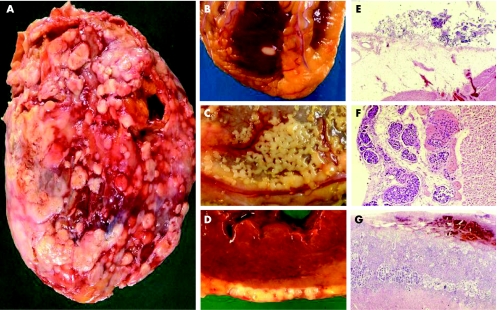
Figure 2 Epicardial metastases. (A,B) Epicardial invasions are shown by mesothelioma. (C,D) Poorly differentiated colon carcinoma. (E–G) Histology of epicardial lesion by cervical adenocarcinoma, prostate adenocarcinoma and non‐small cell lung carcinoma (adenocarcinoma).
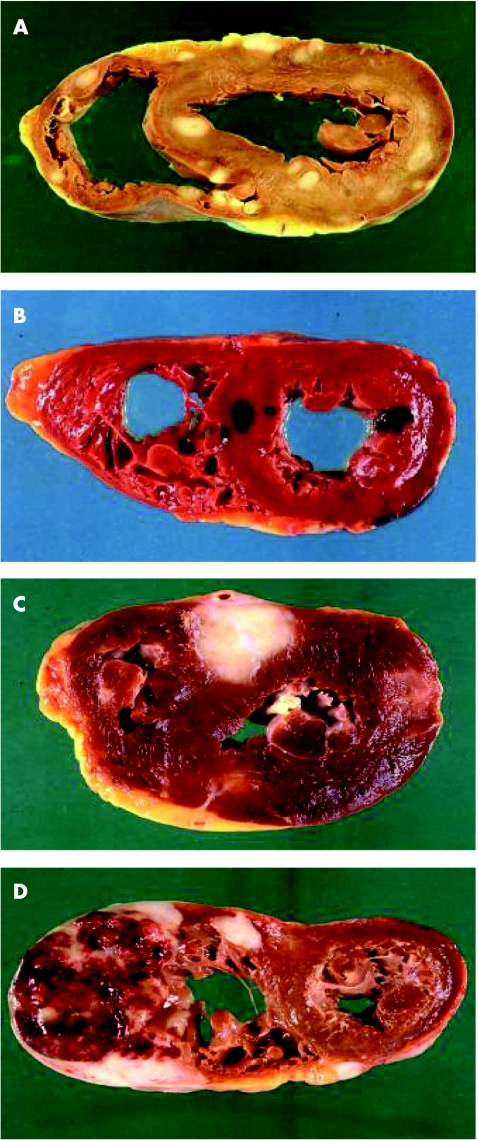
Figure 3 Myocardial metastases. Discrete myocardial lesions by metastases are shown. (A) Sarcoma of the skin; (B) melanoma; (C) urothelial carcinoma; (D) non‐Hodgkin's B cell lymphoma.
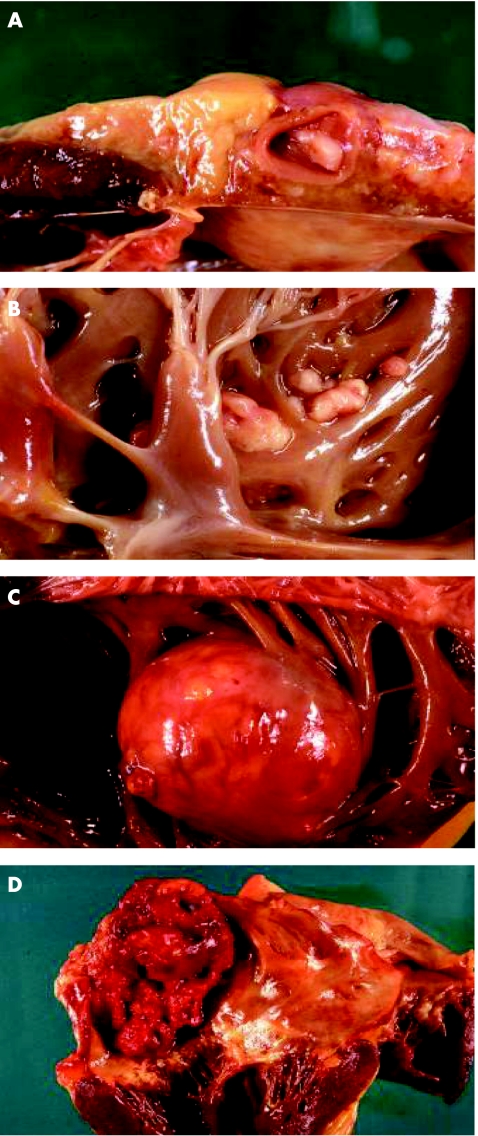
Figure 4 Endocardial metastases. (A) An endocardial metastasis by small cell lung cancer involving the coronary sinus, determining occlusion of the ostium of the right coronary. (B) Multiple small lesions in the endocardium of the right ventricle caused by squamous cell carcinoma of the pharynx. (C) A large endocardial metastasis by clear cell carcinoma of the kidney. (D) Massive neoplastic thrombosis of the left atrium in a patient with squamous cell lung carcinoma.
Tumours can spread to the heart through four alternative paths
by direct extension
through the bloodstream
through the lymphatic system and
by intracavitary diffusion through either the inferior vena cava or the pulmonary veins.
Clinical distinction should be made on the basis of which structure in the heart is primarly affected. For metastases involving the pericardium, pericardial involvement is either the result of direct invasion by an intrathoracic or mediastinal tumour, retrograde lymphatic spread through tracheal or bronchomediastinal lymphatic channels, or secondary involvement of the pericardium through spread from myocardial or epicardial metastases (fig 1).
Metastases to the myocardium or epicardium is almost exclusively the result of retrograde lymphatic spread through tracheal or bronchomediastinal channels and is usually secondary to prior diffusion of the tumour to the pericardium (figs 2 and 3).
Endocardial metastases are usually the result of the invasion from the bloodstream through the heart's chambers with intracavitary lodging. Endocardial metastasis secondary to diffusion from myocardial metastases is also possible (fig 4).
As described, the lymphatic system plays a major part in cardiac metastatic disease. The structure of the lymphatic system in the heart may explain the relatively low incidence of secondary tumours in the heart compared with other organs.
The heart lymphatic network is a three‐tiered structure: the subepicardial, the myocardial and the subendocardial nets. Drainage of the lymphatics forms a major trunk that drains the whole heart and rests against the anterior side of the pulmonary artery. Multiple lymph channels, however, drain from the heart to the mediastinum, and interestingly there are efferent lymph ducts common to the heart and lungs draining directly into the mediastinum.16
The direction of the flow of lymphatics in the heart is from the endocardium to the epicardium. Two major intracardiac ducts are formed by the conversion of multiple minor channels: the right and left lymphatic ducts. These ducts may be found in the vascular bundle of the coronary arteries and drain in the inverse direction compared with arterial flow. Once they reach the aorta, they converge into a single duct that drains into a major lymph node in the pretracheal region. After draining into the pretracheal node, another intracardiac lymph node is encountered: the cardiac lymphatic node. This node is found in the space between the superior vena cava and the anonymous artery. After the cardiac node, the duct drains into the right lymphatic duct.17
An alternative drainage route in the heart is a lymphatic duct that is found to be parallel to the right bundle branch, and that drains into the right cardiac duct at the level of the atrioventricular junction.
The subepicardial lymphatic plexus is composed of a net of capillaries and drainage ducts.17 The web of capillaries is distributed over the entire surface of the heart. Perpendicular to this web, lymphatic capillaries are found to transverse the myocardium parallel to the vascular supply to the myocardium. The lymphatics in the myocardium are characterised by capillary structures, and collecting ducts are not found. The small capillary structures in the myocardium drain directly into the collecting ducts found in the epicardium. The endocardial lymphatic plexus is represented by another web of capillaries that is disposed parallel to the internal surface of the heart.
As a particularity of lymphatics in the heart, the flow in the capillaries and ducts is high, determined by the effects of pressure deriving from the cardiac diastole and systole. During diastole, the filling of the ventricles drives drainage from the endocardial plexus into the myocardial web, and from the myocardial web to the epicardial plexus. The higher filling pressure in the ventricles at end diastole drives the drainage from the pericardial ducts into the mediastinal ducts. Cardiac contractions in systole apply pressure to the myocardial lymphatic capillaries, driving the drainage to the epicardial plexus.
The lymphatic flow in the heart is variable. Increased lymphatic flow is observed in hypoxic conditions and also in hypoproteinaemia associated with reduced oncotic pressure in the haematic capillaries. An increase in haematic flow results in an increase in lymphatic flow, and an impairment in haematic flow causes decrease in lymphatic flow.18 Experiments have also shown that, in the absence of coronary perfusion, no contrast medium is absorbed by the lymphatics, whereas as soon as the coronary flow is restored, absorption and lymph channels are re‐established and become visible once again.
Obstruction to the lymphatic flow results in impaired myocardial flow and dysfunction. Acute obstruction determines oedema in the myocardium, and systolic and diastolic dysfunction. A more chronic obstruction determines changes such as haemorrhages and fibrosis initially in the subendocardial plexus and ultimately in the myocardium, resulting in myocardial dysfunction. Changes in the subendocardial and myocardial structures may be focal, and are typically multifocal in nature. Focal fibrosis results in worsening impairment in venous and lymphatic drainage, creating therefore a vicious cycle leading to worsening oedema.
In the setting of cardiac metastases, if intramural lymphatics are obstructed by neoplastic emboli, lymph will stagnate in the myocardial regions upstream of the obstruction, and endocardial to epicardial drainage by the lymphatics will be partially or totally inhibited. This in turn leads to tissue damage due to lymph stasis and oedema, and favours increased proliferation of neoplastic cells in the undrained regions and retrograde lymph flow, which might disseminate metastases to the more internal areas. As a result of increased pressure, the lymphatic wall may also break, leading to interstitial tumour spread.
Once the lymphatics are involved by the neoplastic cells, myocardial contractions may have a dual effect, both hindering and promoting the formation of cardiac metastases: contractility on the one hand hinders the spreading of intramural tumour metastasis by facilitating lymph and blood drainage and therefore displacing any cardiac tumour‐produced emboli, but on the other it helps neoplastic cells diffuse along the epicardial surface.
Considering that lymphatics represent the major route to cardiac metastases, blockade of the common lymphatic node by neoplastic cells coming from metastasised mediastinum lymph nodes is a key event leading to the formation of metastases. Mediastinal duct blockages result in slow lymph flow in the myocardium, favouring proliferation and retrograde migration neoplastic cells towards the epicardium.
Once the neoplastic cells have colonised the epicardium, neoplastic emboli can penetrate the intramural lymphatics. The penetration into the myocardium is favoured by the stasis of the lymph flow caused by the epicardial involvement, and most importantly by the damage to the epicardial plexus caused by the stasis in part, but also by the direct effect of the neoplastic cells, and on many occasions also by the toxic effects of chemotherapy.
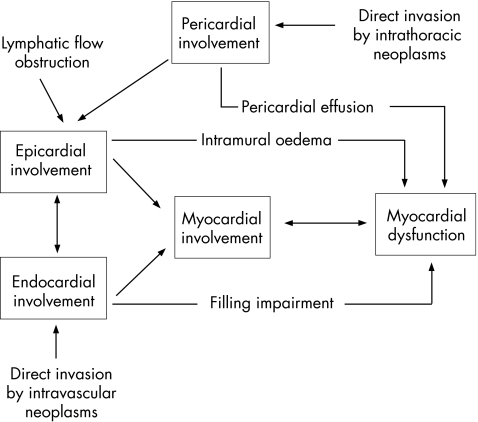
Figure 5 outlines the potential pathophysiological mechanisms of cardiac metastasis and cardiac dysfunction.
Figure 5 Pathophysiology. The figure outlines potential pathophysiological mechanisms of cardiac metastasis and cardiac dysfunction.
Epidemiology
The exact incidence of cardiac metastatic disease is unknown. Considering that most of the studies on the incidence of cardiac metastases derives from postmortem studies, it is not surprising that few papers have been published, in concordance with the fact that fewer and fewer postmortem examinations are performed.13 Few exceptions exist, with Trieste, Italy, being one of the them.
In our experience in the Department of Pathologic Anatomy, University of Trieste, Trieste, Italy, we have consistently carried out >80% of postmortem examinations of our in‐hospital deceased. In the time frame from 1994 to 2003, 18 751 postmortem studies were performed (9245 men and 9506 women). In 7289 cases, one or more malignant neoplasms were found (38.8% were evident at autopsy only) and 622 cases of heart metastases were ascertained, representing an incidence of 9.1% of all malignant tumours (table 2).
Table 2 Incidence of cardiac metastases and their location by type of primary neoplasm.
| Neoplasm type | n | Cardiac metastasis, n (%) | Prevalence/all neoplasms (%) | Prevalence/metastatic neoplosms (%) | % of all cardiac metastasis | Epicardial metastasis (%) | Myocardial metastasis (%) | Endocardial metastasis (%) |
|---|---|---|---|---|---|---|---|---|
| Adenocarcinoma of pancreas | 359 | 23 (6.4) | 7.9 | 3.5 | 47.6 | 17.6 | 41.2 | 5.8 |
| Adenocarcinoma of the lung | 460 | 97 (21.0) | 26.1 | 14.6 | 82.2 | 34.3 | 24.6 | 0 |
| Breast carcinoma | 427 | 66 (15.5) | 20.6 | 10.0 | 79.6 | 30.6 | 16.3 | 6.2 |
| Bronchoalveolar carcinoma | 61 | 6 (9.8) | 17.4 | 0.9 | 75.0 | 50.0 | 25.0 | 0 |
| Colon carcinoma | 1066 | 13 (1.2) | 1.9 | 2.0 | 60.0 | 40.0 | 40.0 | 0 |
| Hepatocellular carcinoma | 574 | 7 (1.2) | 2.4 | 1.0 | 40.0 | 40.0 | 20.0 | 20.0 |
| Leukaemia/lymphoma | 711 | 67 (9.4) | 17.3 | 10.1 | 38.0 | 44.0 | 64.0 | 8.0 |
| Melanoma | 79 | 22 (27.8) | 34.1 | 3.3 | 62.5 | 43.7 | 68.7 | 12.5 |
| Mesothelioma | 128 | 62 (48.4) | 54.2 | 9.4 | 89.2 | 28.3 | 28.3 | 4.3 |
| Oral cavity carcinoma | 75 | 4 (5.3) | 8.6 | 0.6 | 33.3 | 0 | 33.3 | 33.3 |
| Ovary carcinoma | 106 | 10 (10.3) | 11.6 | 1.5 | 87.5 | 12.5 | 0 | 0 |
| Poorly differentiated lung carcinoma | 420 | 82 (19.5) | 21.2 | 12.4 | 67.2 | 37.7 | 31.1 | 1.6 |
| Prostate carcinoma | 779 | 8 (1.0) | 2.6 | 1.2 | 83.3 | 50.0 | 0 | 0 |
| Renal cell carcinoma | 287 | 21 (7.3) | 16.3 | 3.2 | 53.3 | 20.0 | 33.3 | 26.7 |
| Squamous cell lung carcinoma | 428 | 78 (18.2) | 23.4 | 11.8 | 72.4 | 41.4 | 29.3 | 1.7 |
| Stomach carcinoma | 360 | 29 (8.0) | 9.8 | 4.4 | 91.5 | 19.5 | 19.0 | 4.7 |
| Urothelial carcinoma | 307 | 12 (3.9) | 6.4 | 1.8 | 44.4 | 44.4 | 44.4 | 0 |
| Others | 662 | 55 | 8.3 | |||||
| Total | 7289 | 662 (9.1) | 14.2 | 100 | 69.4 | 34.2 | 31.8 | 5.0 |
Although the frequency of primary tumours was found to be different for men and women (46% in men and 31.7% in women), no differences were detected in the incidence of cardiac metastases.
The incidence of primitive tumours tended to decrease with age (51.2% in patients aged ⩽64 years v 26.7% in patients >85 years), as well as with the incidence of cardiac metastases (16.8% in patients aged ⩽64 years v 4.9% in patients >85 years).
The tumours showing the highest rate of heart metastasis were the following: pleural mesothelioma (48.4%), melanoma (27.8%), lung adenocarcinoma (21%), undifferentiated carcinomas (19.5%), lung squamous cell carcinoma (18.2%) and breast carcinoma (15.5%). High rates of heart metastatisation have also been observed in patients affected by ovarian carcinoma (10.3%), lymphomyeloproliferative neoplasms (9.4%), bronchioalveolar carcinomas (9.8%), gastric carcinomas (8%), renal carcinomas (7.3%) and pancreatic carcinomas (6.4%).
When considering only tumours with multiple distant metastases, cardiac metastases were present in 14.2% of cases, with some tumours showing preferential cardiac involvement such as melanoma, bronchioalveolar carcinoma and renal carcinoma.
With the exception of mesothelioma, which was more often metastatic in the heart in men than in women (57.3% v 30%), cardiac metastases were present equally in both sexes.
In our case series, the heart was found to be the only target of metastasis, in as few as 10 of 662 cases, and precisely 4 cases of lung squamous cell carcinoma, 2 cases of breast carcinoma, 1 lymphoma, 1 oesophagus carcinoma, 1 pleural mesothelioma and 1 carcinoma of the kidney.
About two thirds of all cardiac metastases involved the pericardium (69.4%), one third the epicardium (34.2%) or the myocardium (31.8%) and only 5% the endocardium. The tumours most often involving the pericardium were mesothelioma and carcinoma of the lung, ovary, stomach and prostate. The epicardium was involved most often by melanoma, lung squamous cell carcinoma and bronchoalveolar carcinoma. The myocardium was most often involved by melanoma and lymphomyeloproliferative processes, whereas the endocardium was especially involved by melanoma, kidney carcinoma and liver carcinoma.
Although there are no marked age differences between the patients with heart metastases due to mesothelioma, or lung or breast carcinoma and those with metastases in other sites, this is not the case for other tumours, such as leukaemic or lymphomatous processes, prostatic or ovarian neoplasms, or stomach or pancreas cancers, where the age of the patients deceased with heart metastasis was considerably lower than in the group of patients with tumours spreading to other sites.
In lung carcinomas, rates of metastasis were shown to differ on the basis of the histotype. Adenocarcinoma spread to the heart in 26% of cases, squamous cell carcinoma in 23.4%, undifferentiated carcinoma in 21.2% and bronchoalveolar carcinoma in 17.4%. For all the lung cancer histotypes, however, the most involved area of spreading was the pericardium (for 82.2% of adenocarcinomas, 72.4% of squamous cell carcinomas and 67.2% of undifferentiated carcinomas). The remaining heart sites show similar rates for the various histotypes, although squamous cell carcinomas seem to prefer the epicardium as the site of metastasis (41.4%), whereas no cases of lung adenocarcinoma spreading to the endocardium were found.
Clinical manifestations
Clinical presentations of cardiac metastases are extremely variable. Although a cardiac metastasis may be the first or even the only manifestation of an undiagnosed malignant neoplasm, they often go unrecognised in vivo and are diagnosed only after death.
In general, although a focal lesion secondary to the myocardium may result in unclear symptoms that may go undetected, tumours spreading more extensively to the pericardium or to other cardiac sites may produce dramatic clinical patterns, causing medical emergencies. The most typical scenario would be a patient with lung or breast cancer presenting with increasing shortness of breath, hypotension and tachycardia and clinical findings suggestive of tamponade. Neoplastic pericardial effusions are indeed among the most feared complications. Although they may be mild and result in no symptoms, they are commonly symptomatic and often the ultimate cause of death.
Obviously, the symptoms differ greatly according to the most heavily involved site. A pericardial effusion, often haemorrhagic, is often the first manifestation of a cardiac metastasis.19 The effusion may be large in size, and can raise the central venous pressure, cause paradoxical pulse, a fall in QRS voltage and right atrioventricular diastolic collapse. When tamponade is diagnosed, immediate recourse to pericardiocentesis is imperative. In extreme forms characterised by massive effusion accompanied by severe periepicardial colonisation by the tumour, the heart might still be persistently constricted even after a pericardiocentesis has been performed. Tumour‐induced pericardial effusion and cardiac masses can be best viewed by means of transthoracic and transoesophageal ultrasound imaging, computed tomography scan, high resolution CT and magnetic resonance. Moreover, the pericardiocentesis specimen can be submitted to cytological analysis to typify the culprit cells and help identify the primary tumour site.
In the case of secondary tumours located in the myocardium, the clinical pattern will be proportional to the degree of myocardial infiltration, or in some way related to the wall infiltration site. Typical presentation includes arrhythmias, such as atrial flutter or fibrillation, premature beats or ventricular arrhythmias, conduction disturbances and complete atrioventricular blocks, especially if the conduction system has been infiltrated.20
If the ventricular involvement is diffuse, the clinical pattern may include congestive heart failure, with diastolic or systodiastolic dysfunction. Electrocardiographic changes are extremely common in cardiac metastasis.21 On many occasions, damage from direct infiltration of the tumour may not be differentiated from further damage to the myocardium produced by antineoplastic agents.
Rarely, myocardial infarction can also be induced. It can be due to a variety of causes: it can arise after a neoplasm‐induced embolus in the coronary circulation, or be due to perivascular compression–infiltration of the coronary arteries or even to external compression by a massive pericardial tumour‐induced effusion.
Some tumours diffuse along the inferior vena cava reaching the right atrium and producing an intracavitary lesion, leading occasionally to obstruction. Typically these include carcinoma of the kidney and hepatocellular carcinoma. This type of lesion can become so obstructive that it fills the chamber completely and blocks tricuspid movement, resulting in a clinical pattern similar to pericardial constriction or myocardial restrictive disease. Further, the metastatic mass can become eroded and shed small neoplastic emboli in the arterial blood circulation of the lungs.
Another risk is that neoplastic thrombi might be produced in the pectinate muscles of the right atrium or in the trabeculae of the right ventricle—in the second case, without clinical signs.
Neoplastic thrombi can also be produced in the left atrium, after tumour embolism through the venous circulation of the lungs, mostly in patients with lung carcinoma. Large thrombi can even block the mitral valve, and more commonly facilitate distant metastases.
Morphological aspects
Heart metastases can present a great variety of morphological aspects depending on tumour type, site, spreading capacity and mode of permeating the heart.
Pericardial metastases may present with focal, diffuse or massive infiltration with or without metaneoplastic fibrin–blood effusion (fig 1). The neoplasm may spread to the epicardium by micronodular, macronodular or diffuse invasion. Spread to the epicardium may occur not only through the lymphatic (multifocal or diffuse) route but also through the bloodstream. In the second case, the pattern tends to be microfocal, and often due to neoplastic spread from colonised intramural areas in the myocardium. In the case of diffuse carcinomatous spread, a tight yellow‐whitish mesh of lymphatics permeated with cancer cells is visible on the epicardium. A front wave extension phenomenon may be observed when the epicardial colonisation is due to lymphatic involvement and the centrifuge propulsion deriving from heart systole is lost (ie left ventricular dysfunction), leading to a marked decrease in lymph drainage and promotion of cancer spread.
The pericardial effusion seen in neoplastic pericarditis is often characterised by fully fledged haemorrhage, most likely due to thin capillary wall damage caused by the release of inflammation‐produced noxious substances and by hyperdilatation of the vascular lumen due to congestion.
Myocardial metastases can involve any one of the heart chambers. A preferential involvement of the right or left ventricle has been suggested by some authors but not by others.10,15,22,23,24,25,26,27 In our experience, we have observed no preferential localisation of the metastases.
The metastatic tumour cells targeting the myocardium may follow two routes: either they proliferate and extend along the lymphatics that run along the vessels from the epicardium to the myocardium, or they are transported in the bloodstream. Blood embolisation may result in lesions of remarkable size, sometimes compressing the surrounding myocardium and causing secondary hypoperfusion. If neoplastic emboli are produced that occlude the lumen of the coronary vessels, overt intramural metaneoplastic infarctions may be the result. As they increase in size, intramural myocardial metastases can involve both the epicardial and endocardial components.
Infiltration of the coronary sinus is an extremely uncommon finding: in this condition, the carcinomatous cells infiltrating the fatty tissue of the basal heart region invade the atrium and involve the coronary sinus, which has been occluded by a neoplastic thrombosis.
Neoplastic invasion secondary to lymphoma typically tends to replace the myocardial tissue, and broad heart areas are globally infiltrated by homogenised white–greyish tissue, with the typical “fish meat” appearance.28 Despite the massive loss of efficient contractile material, cardiac symptoms may be absent or aspecific.29 In the few existing reports, the heart seems to be more often involved in the case of non‐Hodgkin's lymphomas (78.3% v 66.7% of Hodgkin's lymphomas), whereas the pericardium is more often infiltrated in patients with Hodgkin's lymphoma (66.7%).30 Echocardiographic imaging may show a thickened myocardium, an abnormal myocardial structure and abnormal contractility.
In the endocardium, the metastatic lesions are mainly located in the right ventricle or atrium. Left ventricular lesions are uncommon. It is thought that anchorage of cancer cells to the right heart chambers is favoured by the low intracavitary pressure, by the slower blood flow and by the lighter contractile strength. Further, it is worth mentioning that the neoplastic cells usually come from the area of the inferior vena cava (tumours of the kidney, liver and uterus) or superior vena cava (thyroid tumours)), thus entering the right atrium first.
From a morphological viewpoint, small, multiple cancer thrombotic intratrabecular formations are often found to invade the right ventricle. At other times, larger thrombi can be observed in the right atrium or ventricle, where they often cause haemodynamic occlusion and sometimes superficial erosions resulting in pulmonary microembolism.
Valves are an unusual target for metastases, which might be because of two factors: the absence of vessels in the physiological valvular stroma and the constant cusp motion.31,32 A special form of valve involvement may be seen, however, when a “neoplastic” thrombotic endocarditis involving the valve is seen.33,34 In our experience of over a thousand postmortem examinations, however, we found only one case of true tricuspid valve neoplastic thrombotic endocarditis, in a patient with a poorly differentiated follicular carcinoma of the thyroid gland.35
Diagnosing neoplastic pericardial effusion
As a metastasis‐induced pericardial effusion is quite often the first clinical sign of a malignant tumour, cytodiagnostic examinations can be the first step in patients with no history of neoplasms. The histopathological assessment of pericardial biopsy specimens harvested after thoracotomy is another option.1 Patients with a metastatic carcinoma of unknown primary location pose a relatively common clinical problem, and many management and therapeutic aspects should be considered when endeavouring to solve it.36 A common example is the pericardial metastasis originating from an adenocarcinoma. This tumour is quite often devoid of any histopathological characteristic pointing to the anatomical location of the primary tumour: even though the most likely chance is a metastasis from a lung carcinoma, immunohistochemical markers should nevertheless be used to get the best possible indications on the origin of the tumour.37,38
The first goal is to understand whether the tumour is a lung adenocarcinoma, a reactive mesothelial proliferation or an epithelial mesothelioma. As to histochemistry, mucicarmine and periodic acid‐Schiff stain with diastase digestion are useful to characterise the cytoplasm mucin vacuoles, variable proportions of which are usually present in adenocarcinomas, whereas they are completely absent in mesothelioma cells. In mesotheliomas, cytoplasmic jaluronic acid is found by means of colloidal gold or Alcian Blue. The most likely immunohistochemical algorithm for lung adenocarcima is the following: peripheral or membrane keratin positivity and thyroid transcription factor (TTF) 1, BerEP4, leu‐M1 and CK7 positivity. Mesotheliomas on the contrary are always positive to keratins, but only to the cytoplasmic component, and to vimentin and calretinin. Unfortunately, there is no mesothelioma‐positive and adenocarcinoma‐negative immunohistochemical marker as yet; therefore, the immunohistochemical diagnosis of mesothelioma can only be reached by exclusion.
The next step is tracing back other primary locations of adenocarcinomatous spread to the heart. In the opinion of some authors, a special panel of markers (BCA225, CEA, CA125, and CA19‐9) may be fairly predictive for a number of neoplasms such as colonic, breast, lung and ovarian carcinomas.39 However, the sensitivity of these immunophenotypes is indisputably low, as they are expressed in varying rates by 32–39% of metastatic cells.
Other markers can also be useful in this type of diagnosis: for instance, lung adenocarcinomas are CK7 posive and CK20 negative, whereas colonic adenocarcinomas are also CK7 negative. Conversely, it is easy to confirm the thyroid or prostatic origin of a pericardial metastasis, as TTF1 and thyoglobulin can be used for thyroid carcinomas and for prostatic‐specific antigen for prostatic carcinomas (fig 1).
Cell clusters originating from a metastasised squamous cell carcinoma of the lung are generally CK5 positive and CK7, CK20 and TTF1 negative, whereas the undifferentiated lung carcinomas are CK5 negative but neuron‐specific enolase, cromogranine and calcitonin positive.
No specific markers exist for breast carcinomas either.40 Besides morphological data, CK7 positivity and CK20 negativity, oestrogen and progestogen receptors can be used, and in the past few years also the so called “gross cystic disease fluid protein‐15”, which seems to correlate significantly with this neoplasm.
A number of specific immunohistochemical panels now allow exact classification in the case of pericardial metastases secondary to various neoplasms, such as lymphomas (CD3, CD20, CD10, CD15, CD30, etc), melanomas (S100, HMB45), myelomas (light‐chain immunoglobulins), tumours, testicular neoplasms (CD30, human chorionic gonadotropin, carcinoembryonic antigen, α fetoprotein) and those of the urothelial area (CK5‐6, CK7, CK20).
Take-home messages
Although primary cardiac are extremely uncommon secondary tumours are not.
Cardiac metastases are found in 9% of autopsy where a primary tumour is found and in 14% of metastatic cancer.
Pericardial metastases are the most common type of cardiac metastasis, followed by epicardial and myocardial metastasis.
Endocardial metastases, usually localised to the right heart, are rare and usually associated with tumours with endovascular growth such as renal, liver and uterine cancers.
Clinical presentations of cardiac metastasis are highly variable and dependent on most involved site. While they are commonly asymptomatic, pericardial effusion may be the sole presentation of an unrecognized metastatic cancer.
Further, immunohistochemical markers are a crucial tool in the diagnosis of spread from sarcomas (keratin negative and keratin positive to specific mesenchimal line markers).
Acknowledgements
We thank Dr Vera Di Trocchio (Virginia Commonwealth University) for her editorial support.
Abbreviations
TTF - thyroid transcription factor
Footnotes
Competing interests: None declared.
References
- 1.Virmani R. Tumours metastatic to the heart and pericardium. In: Burke A, Virmani R, eds. Atlas of tumour pathology. Tumours of the heart and great vessels. 3rd Series Fascicle 16. Washington, DC: AFIP, 1995, 195–209,
- 2.McAllister H A., Jr Tumours of the heart and pericardium. In: Silver MD, ed. Cardiovascular pathology. Vol 2, 2nd edn. New York, NY: Churchill Livingstone, 19911297–1333.
- 3.Wenger N K. Pericardial disease in the elderly. Cardiovasc Clin 19922297–103. [PubMed] [Google Scholar]
- 4.Walther H E.Krebsmetastasen. Basel: Benno Schwabe, 194837–42.
- 5.Willis R A.The spread of tumours in the human body Vol 3, 2nd edn. London: Butterworths, 195242
- 6.Hanfling S M. Metastatic cancer to the heart. Review of the literature and report of 127 cases. Circulation 196022474–483. [DOI] [PubMed] [Google Scholar]
- 7.Berge T, Sievers J. Myocardial metastases. A pathological and electrocardiographic study. Br Heart J 196830383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kline I K. Cardiac lymphatic involvement by metastatic tumour. Cancer 197229799–808. [DOI] [PubMed] [Google Scholar]
- 9.Karwinski B, Svendsen E. Trends in cardiac metastasis. APMIS 1989971018–1024. [DOI] [PubMed] [Google Scholar]
- 10.Mukai K, Shinkai T, Tominaga K.et al The incidence of secondary tumours of the heart and pericardium: a 10‐year study. Jpn J Clin Oncol 198818195–201. [PubMed] [Google Scholar]
- 11.Manojlovic S. Metastatic carcinomas involving the heart. Review of post‐mortem examination. Zentralbl Allg Pathol Pathol Anat 1990136657–661. [PubMed] [Google Scholar]
- 12.MacGee W. Metastatic and invasive tumours involving the heart in a geriatric population: a necropsy study. Virchows Arch A Pathol Anat Histopathol 1991419183–189. [DOI] [PubMed] [Google Scholar]
- 13.Silvestri F, Bussani R, Pavletic N.et al Metastases of the heart and pericardium. G Ital Cardiol 1997271252–1255. [PubMed] [Google Scholar]
- 14.Butany J, Leong S W, Carmicheal K.et al A 30‐year analysis of cardiac neoplasms at autopsy. Can J Cardiol 200521675–680. [PubMed] [Google Scholar]
- 15.Prichard R W. Tumours of the heart: review of the subject and report of one hundred thirty‐seven cases. Arch Pathol 19515198–128. [PubMed] [Google Scholar]
- 16.Cui Y, Urschel J D, Petrelli N J. The effect of cardiopulmonary lymphatic obstruction in heart and lung function. Thorac Cardiovasc Surg 20014935–40. [DOI] [PubMed] [Google Scholar]
- 17.Patek P R. The morphology of the lymphatics of the mammalian heart. Am Heart J Anat 193964203–249. [Google Scholar]
- 18.Jellinek H, Gabor G, Solti F.et al The problem of the coronary changes due to disturbance of vascular wall permeability. Angiology 196718179–187. [DOI] [PubMed] [Google Scholar]
- 19.Vaitkus P T, Hermann H C, LeWinter M M. Treatment of malignant pericardial effusion. JAMA 199427259–64. [PubMed] [Google Scholar]
- 20.Wolver S, Franklin R W, Abbate A. ST‐segment elevation and new right‐bundle branch block: broadening the differential diagnosis. Int J Cardiol. 2006. Published Online First [DOI] [PubMed]
- 21.Cates C U, Virmani R, Vaughn W K.et al Electrocardiographic markers of cardiac metastasis. Am Heart J 19861121297–1303. [DOI] [PubMed] [Google Scholar]
- 22.Yater W M. Tumours of the heart and pericardium: pathology, symptomatology and report of 9 cases. Arch Int Med 193148627–666. [Google Scholar]
- 23.Scott R W, Garvin C F. Tumours of the heart and pericardium. Am Heart J 193917431–436. [Google Scholar]
- 24.Burnett R C, Shimkin M B. Secondary neoplasms of the heart. Arch Int Med 195433205–218. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser K, Mattfeldt T, Mobius H J. Herzmetastasierung beim primaren Bronchuskarzinom. Eine retrospektive Autopsiestudie. Pathologe 19867143–148. [PubMed] [Google Scholar]
- 26.Willis R A.The spread of tumours in the human body. London: J&A Churchill, 1933259–268.
- 27.Pratt C B, Dugger D L, Johnson W W.et al Metastatic involvement of the heart in childhood rhabdomyosarcoma. Cancer 1973311492–1497. [DOI] [PubMed] [Google Scholar]
- 28.Roberts W C, Glancy D L, De Vita V T., Jr Hodgkin's disease, lymphosarcoma, reticulum cell sarcoma and mycosis fungoides. A study of 196 autopsy cases. Am J Cardiol 19682285–107. [DOI] [PubMed] [Google Scholar]
- 29.Meng Q, Lai H, Lima J.et al Echocardiographic and pathological characteristics of cardiac metastasis in patients with lymphoma. Oncol Rep 2002985–88. [PubMed] [Google Scholar]
- 30.Moore J A, De Ran B P, Minor R.et al Transesophageal echocardiographic evaluation of intracardiac lymphoma. Am Heart J 1992124514–516. [DOI] [PubMed] [Google Scholar]
- 31.Hallahan D E, Vogelzang N J, Borow K M.et al Cardiac metastases from soft‐tissue sarcomas. J Clin Oncol 198641662–1669. [DOI] [PubMed] [Google Scholar]
- 32.Deck A J, True L D, Higano C S. Tricuspid valve metastasis from testicular carcinoma: a case report and review of the literature. Urology 200056330. [DOI] [PubMed] [Google Scholar]
- 33.Nand S, Messmore H. Hemostasis in malignancy. Am J Hematol 19903545–55. [DOI] [PubMed] [Google Scholar]
- 34.Patterson W P, Ringenberg Q S. The pathophysiology of thrombosis in cancer. Semin Oncol 199017140–146. [PubMed] [Google Scholar]
- 35.Bussani R, Silvestri F. Neoplastic thrombotic endocarditis of the tricuspid valve in a patient with carcinoma of the thyroid. Report of a case. Pathol Res Pract 1999195121–124. [DOI] [PubMed] [Google Scholar]
- 36.Dennis J L, Hvidsten T R, Wit E C.et al Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clin Cancer Res 2005113766–3772. [DOI] [PubMed] [Google Scholar]
- 37.Tamura A, Matsubara O, Yoshimura N H.et al Cardiac metastasis of lung cancer. A study of metastatic pathways and clinical manifestations. Cancer 199270437–442. [DOI] [PubMed] [Google Scholar]
- 38.Loire R, Hellal H. Neoplastic pericarditis. Study by thoracotomy and biopsy in 80 cases. Presse Med 199322244–248. [PubMed] [Google Scholar]
- 39.Brown R W, Campagna L B, Dunn K.et al Immunohistochemical identification of tumour markers in metastatic adenocarcinoma. A diagnostic adjunct in the determination of primary site. Am J Clin Pathol 199710712–19. [DOI] [PubMed] [Google Scholar]
- 40.Wick M R, Lillemoe T J, Copland G T.et al Gross cystic disease fluid protein‐15 as a marker for breast cancer: immunohistochemical analysis of 690 human neoplasms and comparison with alpha‐lactalbumin. Hum Pathol 19899418–26. [DOI] [PubMed] [Google Scholar]