Abstract
Haemophagocytic lymphohistiocytosis (HLH) comprises primary and secondary forms; the secondary form is most commonly triggered by the Epstein–Barr virus (EBV; EBV‐HLH). Patients with EBV‐HLH usually exhibit oligoclonal or monoclonal T cell proliferation, which may mimic T cell lymphoproliferative disorder (T‐LPD). This article reports on EBV‐HLH in a 17‐month‐old girl with an extreme surge of reactive T lymphocytosis (absolute count 167×109/l) with CD5 down regulation. Bone marrow aspirate and trephine contained florid haemophagocytosis and massive infiltration of CD3+ Epstein–Barr virus‐encoded RNA+ lymphocytes, as seen by double labelling. These lymphocytes were monoclonal for EBV and T cell receptor γ chain gene rearrangement. The patient responded dramatically to intravenous immunoglobulin, interferon α2b, ganciclovir and prednisolone, suggesting restoration of her immune system and eradication of the clonal T cells through these immunoregulatory agents. Thus, careful clinicopathological correlation is warranted in the interpretation of immunophenotyping and clonality data in T cell proliferation in association with EBV‐HLH to avoid erroneous diagnosis of T‐LPD.
Haemophagocytic lymphohistiocytosis (HLH) is a clinical syndrome in which T cells, natural killer cells and macrophages are aberrantly activated, resulting in hypercytokinaemia that causes cellular damage and dysfunction of various organs.1 HLH is characterised by fever, splenomegaly, cytopenia, hypertriglyceridaemia, coagulopathy and reactive histiocytic proliferation with haemophagocytosis in the bone marrow, spleen and lymph nodes.1,2 HLH comprises primary (familial) and secondary forms, and both conditions are associated with a high mortality. Secondary HLH is usually triggered by an infectious agent, most commonly Epstein–Barr virus (EBV; EBV‐HLH). Primary EBV infection occurring in early childhood is often asymptomatic. Delayed primary infection in adolescents and young adults may cause infectious mononucleosis, in which EBV infects B cells, with polyclonal B cell expansion accompanied by antigen‐driven oligoclonal or monoclonal proliferation of CD8+ cytotoxic T –cells .3 On the other hand, in EBV‐HLH, the targets of EBV infection are T cells, leading to polyclonal or monoclonal proliferation of EBV‐containing T cells.4,5 In rare occasions, T cell lymphoproliferative disorder (T‐LPD) may develop in association with acute or chronic EBV infection and EBV‐HLH.6,7 Differentiating reactive T cell lymphocytosis from T‐LPD is a diagnostic challenge. In this report, we describe a unique case of EBV‐HLH with an extreme surge of monoclonal, cytotoxic T cells with CD5 down regulation, mimicking T‐LPD.
Case report
A 17‐month‐old girl, without a family history of HLH, presented with fever, prostration, hepatosplenomegaly and massive ascites. Laboratory examination showed anaemia, thrombocytopenia, leukocytosis (white cells 5.8×109/l with 67% lymphocytes), coagulopathy, impaired liver function tests and hypertriglyceridaemia. Her serum was positive for EBV viral capsid antigen immunoglobulin (Ig)G and negative for viral capsid antigen IgM, early antigen IgA, Epstein–Barr nuclear antigen 1 IgA and monospot test. Her white cell counts drastically increased to a maximum of 199×109/l with 84% lymphocytes (absolute count 167×109/l) on day 4 (fig 1A) in the hospital. She responded dramatically to intravenous immunoglobulin, interferon α2b, ganciclovir and prednisolone, and her white cell counts decreased steadily to become normal from hospital day 9. She is alive and well 3 years later.
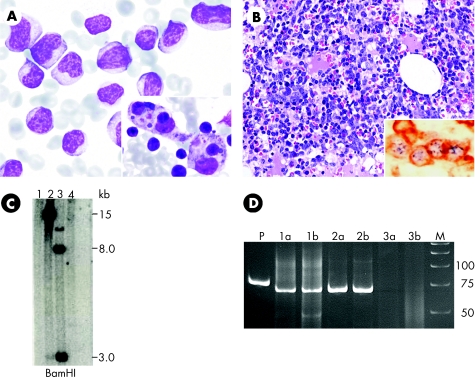
Figure 1 (A) Peripheral blood smear at onset contains numerous reactive lymphocytes. Inset shows reactive haemophagocytosis in the bone marrow aspirate. (B) Haematoxylin and eosin staining of the bone marrow biopsy specimen shows massive lymphocytic infiltration. CD3/Epstein–Barr virus (EBV)‐encoded RNA (EBER) double labelling (immunohistochemistry and in situ hybridisation) in the inset shows punctuated, blue EBER‐positive signals in the nuclei and brown cytoplasmic staining with CD3 in the same T –cells . In double labelling with CD79A/EBER, the cells with membrane staining of CD79A are negative for EBER, whereas EBER‐positive cells are negative for CD79A (data not shown). (C) Southern blot analysis using a hybridisation probe against the BamNJ fragment for the EBV terminal repeats shows clonal proliferation of EBV‐infected cells. Lane 1, normal control; lane 2, bone marrow at onset; lane 3, positive control; lane 4, second normal control. (D) Polyacrylamide gel electrophoresis of polymerase chain reaction products from T cell receptor γ gene rearrangement study. Lane P, positive control; lanes 1a and 1b, duplicates of bone marrow biopsy specimen at onset, showing a distinct monoclonal band of the same size as those of lanes 2a and 2b from peripheral blood mononuclear cells (PBMCs) 1 month after onset; lanes 3a and 3b, polyclonal pattern of PBMCs at 6 months; lane M, molecular weight marker.
The bone marrow aspirate at onset contained massive infiltration of reactive lymphocytes and many reactive histiocytes with haemophagocytosis (fig 1A inset). Two‐colour flow cytometry (FACSCalibur, Becton Dickinson, San Jose, California, USA, with CellQuest software) showed a CD3+ CD8+ HLA‐DR+ phenotype with down regulation of CD5 (table 1). These lymphocytes were shown to be CD3+ T cells harbouring Epstein–Barr virus (EBV)‐encoded RNA signals by double labelling (CD3 and EBER1/2, Dako, Carpinteria, California, USA) with immunohistochemistry and in situ hybridisation (fig 1B).
Table 1 Sequential flow cytometric immunophenotyping of peripheral blood lymphocytes.
| Time | WBC (×109/l) | Lym (×109/l) | CD2 | CD3 | CD4 | CD5 | CD7 | CD8 | CD10 | CD19 | CD16+56 | HLA‐DR | CD4/CD8 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Onset | 5.8 | 1.7 (33%) | ND | 93 | 40 | ND | ND | 47 | ND | 3 | 3 | 37 | 0.85 |
| Day 4 | 199.0 | 189.1 (95%) | 95 | 97 | 1 | 8 | 93 | 99 | 1 | 0 | ND | 98 | 0.01 |
| Day 11 | 3.5 | 3.2 (92%) | 82 | 80 | 14 | 27 | 76 | 65 | 1 | 6 | ND | 71 | 0.22 |
| 2 months | 8.0 | 5.5 (69%) | ND | 69 | 31 | ND | ND | 20 | ND | 17 | 10 | 8 | 1.50 |
HLA‐DR, human leucocyte antigen DR; Lym, lymphocyte; ND, not done; WBC, white cell.
CD antigens are expressed as a percentage of positive cells among gated lymphocytes except CD4/CD8 which is expressed as a ratio.
The EBV DNA viral load in serum was determined by a real‐time quantitative polymerase chain reaction system directed towards the EBV BamHI‐W fragment region and analysed with an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, California, USA) . The viral load was 287 copies/ml at onset and was undetectable 1 week later. Southern blotting using a hybridisation probe for the EBV terminal repeats showed monoclonal results (fig 1C). We followed the protocol of McCarthy et al8 for T cell receptor γ (TCRγ) chain gene rearrangement. The DNA extracted from the marrow trephine at onset and from the peripheral blood mononuclear cells (PBMCs) at 1 month yielded monoclonal results for TCRγ with the same‐sized single band (fig 1D), although it became polyclonal using PBMCs at 6 months. The DNA sample from PBMCs drawn at 1 month after disease onset was negative for perforin gene mutation, thus excluding type 2 familial HLH.9
Discussion
In acute infectious mononucleosis, oligoclonal or monoclonal proliferation of cytotoxic T cells is the principal method for controlling primary EBV infection. In contrast with the preferential targeting of B cells in patients with infectious mononucleosis, the targets of EBV in EBV‐HLH are T cells and natural killer cells. In these patients, the EBV‐infected cells are most commonly oligoclonally or monoclonally proliferating, and hypercytokinaemia has a major role in causing haemophagocytosis, cellular damage and dysfunction of various organs.1 The clinical and laboratory features of our patient fulfilled the criteria for EBV‐HLH; the unusual features were the tremendous increase in CD8+ T cells with down regulation of CD5 and monoclonality for EBV and TCRγ.
HLH may develop in patients with EBV‐associated T‐LPD as the initial presentation, at the time of lymphoma relapse and during clinical remission.7 The clinical course of EBV‐associated T‐LPD is fulminant, with a short survival despite aggressive treatment. Therefore, it is important to identify possible T‐LPD in patients with EBV‐HLH. In our patient, the extremely high count of reactive lymphocytes with CD5 down regulation was suggestive of T‐LPD, one of the most challenging diagnoses in haematopathology using flow cytometric immunophenotyping. Unlike the more common B cell lymphoproliferative disorders, in which clonality is often readily established by monotypic immunoglobulin light chain expression, no specific immunophenotypic features are diagnostic of a clonal T cell population. Gorczyca et al10 analysed a large series of patients with T‐LPD and controls, and concluded that the most suspicious flow cytometric features for malignancy were the loss or markedly dim expression of CD45 and complete loss of one or more pan‐T antigens. In a similar study of T‐LPD, Jamal et al11 found that the most common abnormally expressed antigen (overexpression, underexpression, or absent expression) in patients with T‐LPD was CD3, followed by CD7, CD5 and CD2. However, these aberrant antigenic expressions might be present in various reactive conditions. For example, using PBMCs, Weisberger et al12 found that all 25 patients with infectious mononucleosis exhibited an activated CD8+ cytotoxic T population with down‐regulated CD7 and, in addition, 2 (8%) patients had down‐regulated CD5.
Take‐home messages
Hemophagocytic lymphohistiocytosis (HLH) comprises primary and secondary forms, the latter been most commonly triggered by EBV (EBV‐HLH)
We report a young girl with EBV‐HLH and extreme surge of reactive T‐lymphocytosis characterised by CD5 down regulation and monoclonality for EBV and T‐cell receptor γ chain gene rearrangement, who responded dramatically to immunoregulatory agents.
Reactive T‐lymphocytosis in EBV‐HLH may mimic T‐cell malignancy in both clinical and laboratory aspects. Careful clinicopathological correlations are warranted to avoid erroneous diagnosis.
Monoclonal T cell proliferation usually indicates T‐LPD; however, this may rarely be present in non‐neoplastic T cells.13 The regression of monoclonal T cell proliferation with CD5 down regulation in our patient after immunoregulatory treatment suggested that this florid clonal T cell proliferation was reactive, probably driven by a strong immune reaction against EBV infection instead of a neoplastic process. The reduction in EBV load paralleled her recovery, consistent with the observation by Teramura et al14 that the EBV genome content in serum or plasma reflects clinical features and outcome in EBV‐HLH, and the quantitative analysis of cell‐free EBV genome copy number is useful for evaluating disease activity and for predicting the response to treatment.
EBV‐HLH is a systemic disease and is usually manifested as a persistent fever unresponsive to antibiotics. The EBV‐infected cytotoxic T cells or natural killer cells proliferate and cause hypercytokinaemia. Death due to EBV‐HLH is largely the result of the cytokine storm, resulting in multiple organ failure, severe haemorrhage and neutropenia, which promotes opportunistic pulmonary infections. The best way to reduce these risks is early interruption of the inflammatory cascade. Once the cytokine storm is under control, the proliferating EBV+ T cells or natural killer cells can be eradicated by etoposide and other chemotherapeutic agents, depending on the severity of the disease.1 In our patient, her cytokine storm was effaced by intravenous immunoglobulin, interferon α2b, ganciclovir and prednisolone. Surprisingly, the proliferating EBV+ monoclonal T cells disappeared simultaneously without cytotoxic chemotherapy. It seems that immunoregulatory agents alone might be effective in some patients with EBV‐HLH. Those who have persistent EBV+ T cell or natural killer cell proliferation should receive cytotoxic chemotherapy or bone marrow transplantation.
In summary, we observed a transient monoclonal proliferation of EBV‐infected cytotoxic T cells with CD5 down regulation in a girl with EBV‐HLH, who survived without cytotoxic chemotherapy. Careful clinicopathological correlation is warranted in the interpretation of immunophenotypic and clonality data in T‐LPD, especially in patients with EBV‐associated infectious mononucleosis and HLH, to avoid an erroneous diagnosis of T‐LPD and unnecessary cytotoxic chemotherapy.
Acknowledgements
We thank Dr Ikuyo Ueda and Professor Shinsaku Imashuku for performing the perforin gene mutation assay.
Abbreviations
EBV - Epstein–Barr virus
HLH - haemophagocytic lymphohistiocytosis
PBMC - peripheral blood mononuclear cell
TCR - T cell receptor
T‐LPD - T cell lymphoproliferative disorder
Footnotes
Competing interests: None declared.
References
- 1.Imashuku S. Clinical features and treatment strategies of Epstein‐Barr virus‐associated hemophagocytic lymphohistiocytosis. Crit Rev Oncol Hematol 200244259–272. [DOI] [PubMed] [Google Scholar]
- 2.Henter J I, Elinder G, Ost A. Diagnostic guidelines for hematophagocytic lymphohistiocytosis. The FEL Group of the Histiocytic Society. Semin Oncol 19911829–33. [PubMed] [Google Scholar]
- 3.Callan M F, Steven N, Krausa P.et al Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat Med 19962906–911. [DOI] [PubMed] [Google Scholar]
- 4.Kawaguchi H, Miyashita T, Herbst H.et al Epstein‐Barr virus‐infected T lymphocytes in Epstein‐Barr virus‐associated hemophagocytic syndrome. J Clin Invest 1993921444–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imashuku S, Hibi S, Ohara T.et al Effective control of Epstein‐Barr virus‐related hemophagocytic lymphohistiocytosis with immunochemotherapy. Blood 1999931869–1874. [PubMed] [Google Scholar]
- 6.Quintanilla‐Martinez L, Kumar S, Fend F.et al Fulminant EBV(+) T‐cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood 200096443–451. [PubMed] [Google Scholar]
- 7.Yao M, Cheng A L, Su I J.et al Clinicopathological spectrum of haemophagocytic syndrome in Epstein‐Barr virus‐associated peripheral T‐cell lymphoma. Br J Haematol 199487535–543. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy K P, Sloane J P, Kabarowski J H.et al A simplified method of detection of clonal rearrangements of the T‐cell receptor‐gamma chain gene. Diagn Mol Pathol 19921173–179. [PubMed] [Google Scholar]
- 9.Ishii E, Ueda I, Shirakawa R.et al Genetic subtypes of familial hemophagocytic lymphohistiocytosis: correlations with clinical features and cytotoxic T lymphocyte/natural killer cell functions. Blood 20051053442–3448. [DOI] [PubMed] [Google Scholar]
- 10.Gorczyca W, Weisberger J, Liu Z.et al An approach to diagnosis of T‐cell lymphoproliferative disorders by flow cytometry. Cytometry 200250177–190. [DOI] [PubMed] [Google Scholar]
- 11.Jamal S, Picker L J, Aquino D B.et al Immunophenotypic analysis of peripheral T‐cell neoplasms. A multiparametric flow cytometric approach. Am J Clin Pathol 2001116512–526. [DOI] [PubMed] [Google Scholar]
- 12.Weisberger J, Cornfield D, Gorczyca W.et al Down‐regulation of pan‐T‐cell antigens, particularly CD7, in acute infectious mononucleosis. Am J Clin Pathol 200312049–55. [DOI] [PubMed] [Google Scholar]
- 13.Cairns S M, Taylor J M, Gould P R.et al Comparative evaluation of PCR‐based methods for the assessment of T cell clonality in the diagnosis of T cell lymphoma. Pathology 200234320–325. [DOI] [PubMed] [Google Scholar]
- 14.Teramura T, Tabata Y, Yagi T.et al Quantitative analysis of cell‐free Epstein‐Barr virus genome copy number in patients with EBV‐associated hemophagocytic lymphohistiocytosis. Leuk Lymphoma 200043173–179. [DOI] [PubMed] [Google Scholar]