Abstract
In order study patterns of local antibody responses following mucosal immunization of mice via different routes, a method for collection of secretions directly from mucosal surfaces was developed. Mice were immunized on days 0, 10, 17, and 24 by administration of cholera toxin into the oral cavity, stomach, colon-rectum, or vagina. At sacrifice on day 32, absorbent wicks were placed in the oral cavity and, via an applicator tube, into the vagina and distal colon-rectum and along the entire small intestine after flushing of luminal contents. Protein was quantitatively extracted from wicks, and specific anti-cholera toxin immunoglobulin A (IgA) and IgG were measured by enzyme-linked immunosorbent assay. Concentrations of specific IgA in secretions at various mucosal sites were dramatically influenced by the route of immunization. Oral immunization effectively induced IgA in saliva, and the intragastric route was optimal for induction of IgA in the small intestine. High levels of specific IgA appeared on the colonic-rectal mucosal surface only after rectal delivery of antigen. Oral, gastric, and rectal immunizations also produced distant responses in the vagina. Following vaginal immunization, however, neither local nor distant IgA responses were detected. These results suggest that vaccines intended for protection of colonic-rectal and vaginal mucosal surfaces might best be administered by the rectal route.
Full text
PDF

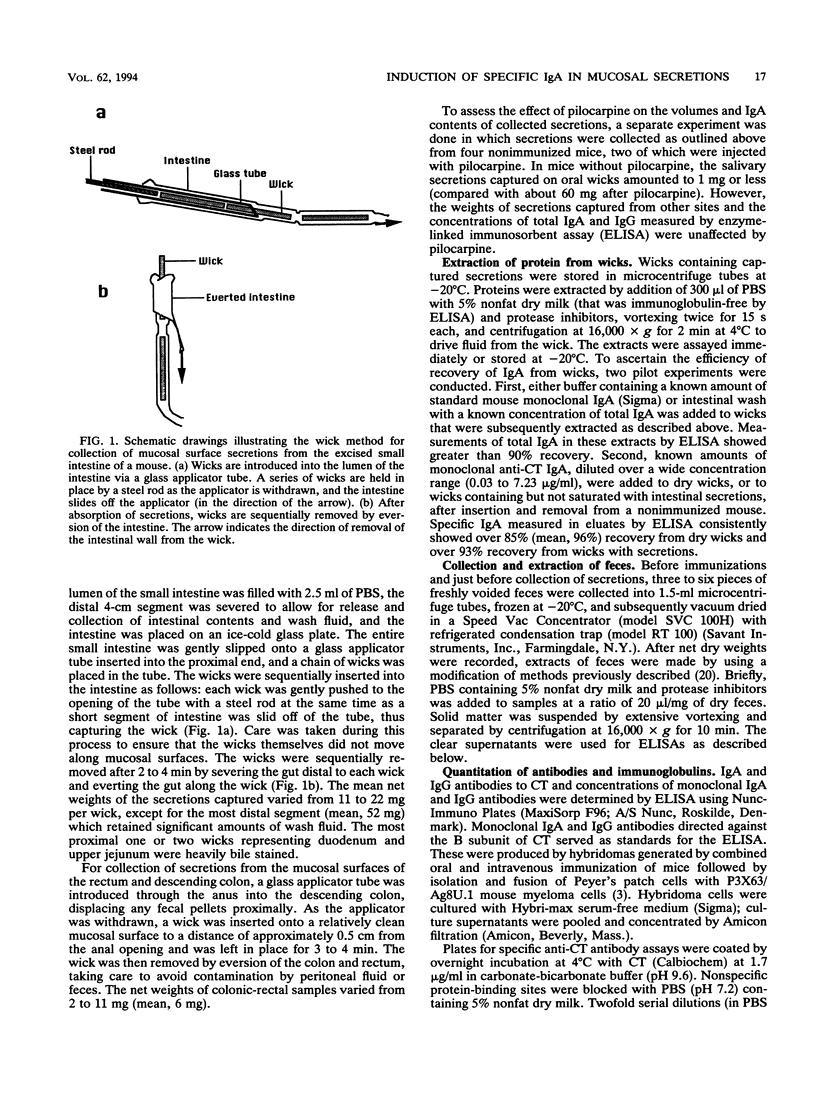

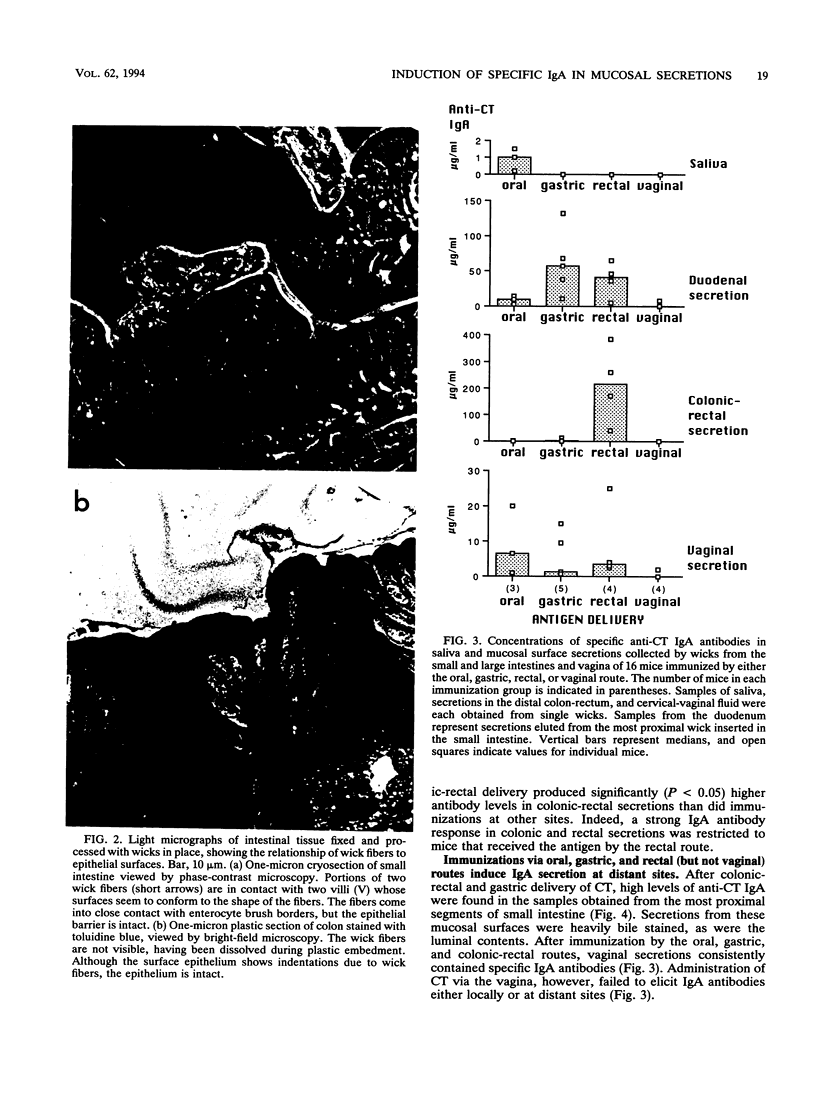
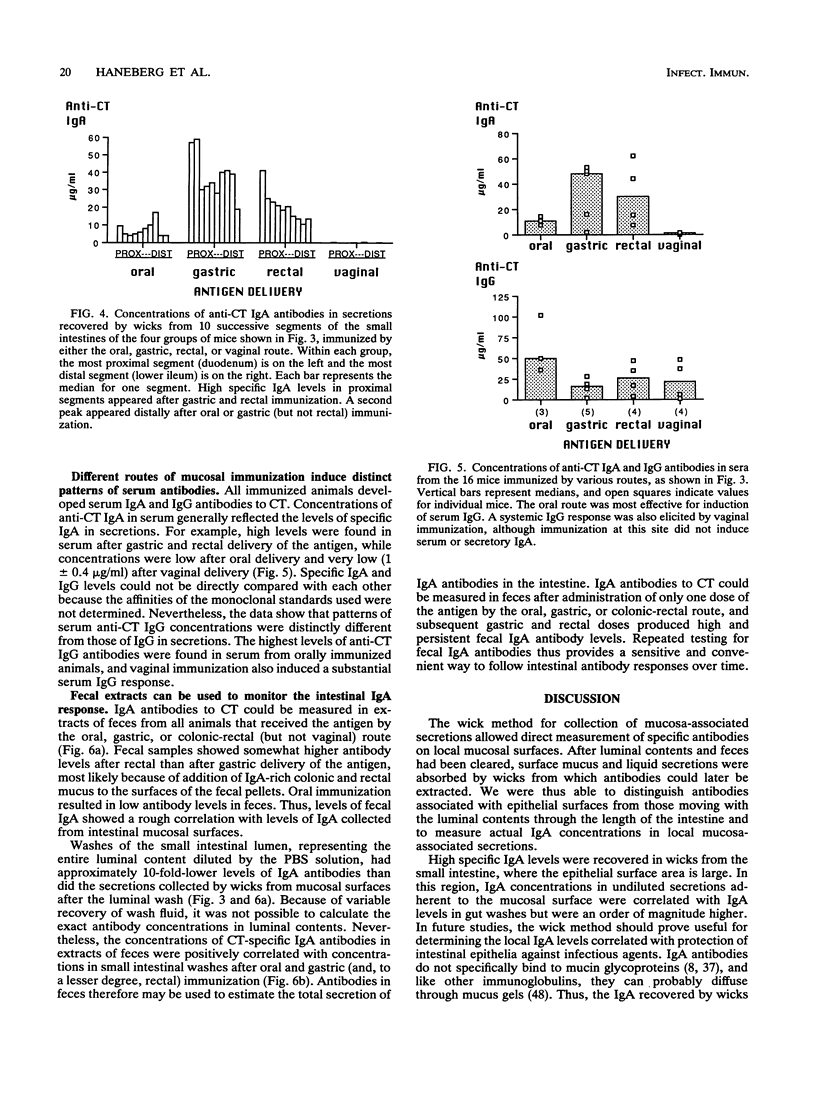
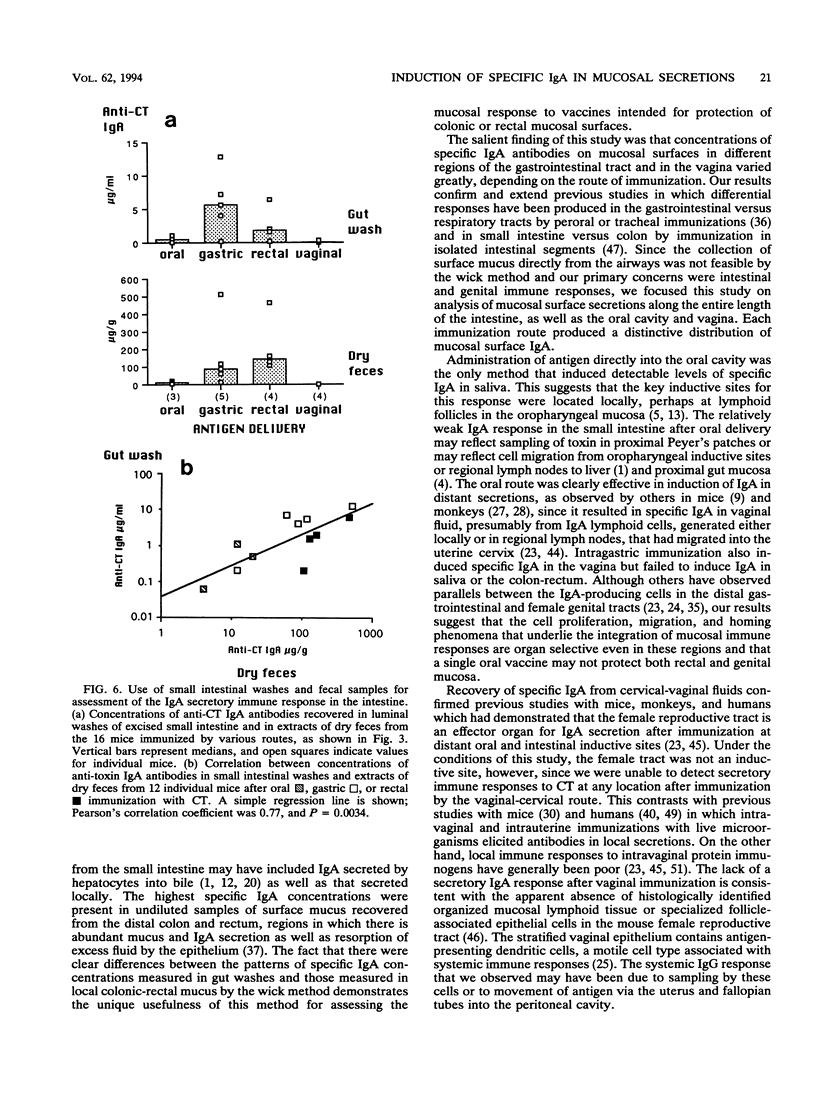


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altorfer J., Hardesty S. J., Scott J. H., Jones A. L. Specific antibody synthesis and biliary secretion by the rat liver after intestinal immunization with cholera toxin. Gastroenterology. 1987 Sep;93(3):539–549. doi: 10.1016/0016-5085(87)90917-6. [DOI] [PubMed] [Google Scholar]
- Amerongen H. M., Weltzin R., Farnet C. M., Michetti P., Haseltine W. A., Neutra M. R. Transepithelial transport of HIV-1 by intestinal M cells: a mechanism for transmission of AIDS. J Acquir Immune Defic Syndr. 1991;4(8):760–765. [PubMed] [Google Scholar]
- Apter F. M., Lencer W. I., Finkelstein R. A., Mekalanos J. J., Neutra M. R. Monoclonal immunoglobulin A antibodies directed against cholera toxin prevent the toxin-induced chloride secretory response and block toxin binding to intestinal epithelial cells in vitro. Infect Immun. 1993 Dec;61(12):5271–5278. doi: 10.1128/iai.61.12.5271-5278.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg E. L., Goldstein L. A., Jutila M. A., Nakache M., Picker L. J., Streeter P. R., Wu N. W., Zhou D., Butcher E. C. Homing receptors and vascular addressins: cell adhesion molecules that direct lymphocyte traffic. Immunol Rev. 1989 Apr;108:5–18. doi: 10.1111/j.1600-065x.1989.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Overview of the mucosal immune system. Curr Top Microbiol Immunol. 1989;146:13–25. doi: 10.1007/978-3-642-74529-4_2. [DOI] [PubMed] [Google Scholar]
- Cebra J. J., Gearhart P. J., Kamat R., Robertson S. M., Tseng J. Origin and differentiation of lymphocytes involved in the secretory IgA responses. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):201–215. doi: 10.1101/sqb.1977.041.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z. D., Tristram D., LaScolea L. J., Kwiatkowski T., Jr, Kopti S., Ogra P. L. Induction of antibody response to Chlamydia trachomatis in the genital tract by oral immunization. Infect Immun. 1991 Apr;59(4):1465–1469. doi: 10.1128/iai.59.4.1465-1469.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerkinsky C., Prince S. J., Michalek S. M., Jackson S., Russell M. W., Moldoveanu Z., McGhee J. R., Mestecky J. IgA antibody-producing cells in peripheral blood after antigen ingestion: evidence for a common mucosal immune system in humans. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2449–2453. doi: 10.1073/pnas.84.8.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerkinsky C., Svennerholm A. M., Quiding M., Jonsson R., Holmgren J. Antibody-producing cells in peripheral blood and salivary glands after oral cholera vaccination of humans. Infect Immun. 1991 Mar;59(3):996–1001. doi: 10.1128/iai.59.3.996-1001.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacroix D. L., Malburny G. N., Vaerman J. P. Hepatobiliary transport of plasma IgA in the mouse: contribution to clearance of intravascular IgA. Eur J Immunol. 1985 Sep;15(9):893–899. doi: 10.1002/eji.1830150906. [DOI] [PubMed] [Google Scholar]
- Dolen W. K., Spofford B., Selner J. C. The hidden tonsils of Waldeyer's ring. Ann Allergy. 1990 Oct;65(4):244–248. [PubMed] [Google Scholar]
- Elson C. O., Ealding W., Lefkowitz J. A lavage technique allowing repeated measurement of IgA antibody in mouse intestinal secretions. J Immunol Methods. 1984 Feb 24;67(1):101–108. doi: 10.1016/0022-1759(84)90089-9. [DOI] [PubMed] [Google Scholar]
- Forrest B. D. Indirect measurement of intestinal immune responses to an orally administered attenuated bacterial vaccine. Infect Immun. 1992 May;60(5):2023–2029. doi: 10.1128/iai.60.5.2023-2029.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest B. D., Shearman D. J., LaBrooy J. T. Specific immune response in humans following rectal delivery of live typhoid vaccine. Vaccine. 1990 Jun;8(3):209–212. doi: 10.1016/0264-410x(90)90047-p. [DOI] [PubMed] [Google Scholar]
- Haneberg B., Aarskog D. Human faecal immunoglobulins in healthy infants and children, and in some with diseases affecting the intestinal tract or the immune system. Clin Exp Immunol. 1975 Nov;22(2):210–222. [PMC free article] [PubMed] [Google Scholar]
- Jiang Z. D., Nelson A. C., Mathewson J. J., Ericsson C. D., DuPont H. L. Intestinal secretory immune response to infection with Aeromonas species and Plesiomonas shigelloides among students from the United States in Mexico. J Infect Dis. 1991 Nov;164(5):979–982. doi: 10.1093/infdis/164.5.979. [DOI] [PubMed] [Google Scholar]
- Koertge T. E., Butler J. E. Dimeric mouse IgA is transported into rat bile five times more rapidly than into mouse bile. Scand J Immunol. 1986 Nov;24(5):567–574. doi: 10.1111/j.1365-3083.1986.tb02172.x. [DOI] [PubMed] [Google Scholar]
- Kraehenbuhl J. P., Neutra M. R. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol Rev. 1992 Oct;72(4):853–879. doi: 10.1152/physrev.1992.72.4.853. [DOI] [PubMed] [Google Scholar]
- Kristiansen B. E. Collection of mucosal secretion by synthetic discs for quantitation of secretory IgA and bacteria. J Immunol Methods. 1984 Oct 26;73(2):251–257. doi: 10.1016/0022-1759(84)90399-5. [DOI] [PubMed] [Google Scholar]
- Kutteh W. H., Hatch K. D., Blackwell R. E., Mestecky J. Secretory immune system of the female reproductive tract: I. Immunoglobulin and secretory component-containing cells. Obstet Gynecol. 1988 Jan;71(1):56–60. [PubMed] [Google Scholar]
- Langhoff E., Terwilliger E. F., Bos H. J., Kalland K. H., Poznansky M. C., Bacon O. M., Haseltine W. A. Replication of human immunodeficiency virus type 1 in primary dendritic cell cultures. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7998–8002. doi: 10.1073/pnas.88.18.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langman J. M., Rowland R. The number and distribution of lymphoid follicles in the human large intestine. J Anat. 1986 Dec;149:189–194. [PMC free article] [PubMed] [Google Scholar]
- Lehner T., Bergmeier L. A., Panagiotidi C., Tao L., Brookes R., Klavinskis L. S., Walker P., Walker J., Ward R. G., Hussain L. Induction of mucosal and systemic immunity to a recombinant simian immunodeficiency viral protein. Science. 1992 Nov 20;258(5086):1365–1369. doi: 10.1126/science.1360702. [DOI] [PubMed] [Google Scholar]
- McDermott M. R., Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979 May;122(5):1892–1898. [PubMed] [Google Scholar]
- McDermott M. R., Brais L. J., Evelegh M. J. Mucosal and systemic antiviral antibodies in mice inoculated intravaginally with herpes simplex virus type 2. J Gen Virol. 1990 Jul;71(Pt 7):1497–1504. doi: 10.1099/0022-1317-71-7-1497. [DOI] [PubMed] [Google Scholar]
- McGhee J. R., Mestecky J. In defense of mucosal surfaces. Development of novel vaccines for IgA responses protective at the portals of entry of microbial pathogens. Infect Dis Clin North Am. 1990 Jun;4(2):315–341. [PubMed] [Google Scholar]
- Merchant A. A., Groene W. S., Cheng E. H., Shaw R. D. Murine intestinal antibody response to heterologous rotavirus infection. J Clin Microbiol. 1991 Aug;29(8):1693–1701. doi: 10.1128/jcm.29.8.1693-1701.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987 Jul;7(4):265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- Nedrud J. G., Liang X. P., Hague N., Lamm M. E. Combined oral/nasal immunization protects mice from Sendai virus infection. J Immunol. 1987 Nov 15;139(10):3484–3492. [PubMed] [Google Scholar]
- Neutra M. R., Kraehenbuhl J. P. Transepithelial transport and mucosal defence I: the role of M cells. Trends Cell Biol. 1992 May;2(5):134–138. doi: 10.1016/0962-8924(92)90099-9. [DOI] [PubMed] [Google Scholar]
- O'Leary A. D., Sweeney E. C. Lymphoglandular complexes of the colon: structure and distribution. Histopathology. 1986 Mar;10(3):267–283. doi: 10.1111/j.1365-2559.1986.tb02481.x. [DOI] [PubMed] [Google Scholar]
- Ogra P. L., Karzon D. T. Distribution of poliovirus antibody in serum, nasopharynx and alimentary tract following segmental immunization of lower alimentary tract with poliovaccine. J Immunol. 1969 Jun;102(6):1423–1430. [PubMed] [Google Scholar]
- Ogra P. L., Ogra S. S. Local antibody response to poliovaccine in the human female genital tract. J Immunol. 1973 May;110(5):1307–1311. [PubMed] [Google Scholar]
- Owen R. L., Ermak T. H. Structural specializations for antigen uptake and processing in the digestive tract. Springer Semin Immunopathol. 1990;12(2-3):139–152. doi: 10.1007/BF00197502. [DOI] [PubMed] [Google Scholar]
- Owen R. L., Piazza A. J., Ermak T. H. Ultrastructural and cytoarchitectural features of lymphoreticular organs in the colon and rectum of adult BALB/c mice. Am J Anat. 1991 Jan;190(1):10–18. doi: 10.1002/aja.1001900103. [DOI] [PubMed] [Google Scholar]
- Parr E. L., Parr M. B. A comparison of antibody titres in mouse uterine fluid after immunization by several routes, and the effect of the uterus on antibody titres in vaginal fluid. J Reprod Fertil. 1990 Jul;89(2):619–625. doi: 10.1530/jrf.0.0890619. [DOI] [PubMed] [Google Scholar]
- Parr M. B., Parr E. L. Immunohistochemical localization of immunoglobulins A, G and M in the mouse female genital tract. J Reprod Fertil. 1985 Jul;74(2):361–370. doi: 10.1530/jrf.0.0740361. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr Determinants of the localization, magnitude, and duration of a specific mucosal IgA plasma cell response in enterically immunized rats. J Immunol. 1982 Mar;128(3):1311–1315. [PubMed] [Google Scholar]
- Radomsky M. L., Whaley K. J., Cone R. A., Saltzman W. M. Macromolecules released from polymers: diffusion into unstirred fluids. Biomaterials. 1990 Nov;11(9):619–624. doi: 10.1016/0142-9612(90)90018-l. [DOI] [PubMed] [Google Scholar]
- Waldman R. H., Cruz J. M., Rowe D. S. Immunoglobulin levels and antibody to Candida albicans in human cervicovaginal secretions. Clin Exp Immunol. 1972 Mar;10(3):427–434. [PMC free article] [PubMed] [Google Scholar]
- Weisz-Carrington P., Roux M. E., McWilliams M., PHILLIPS-Quagliata J. M., Lamm M. E. Organ and isotype distribution of plasma cells producing specific antibody after oral immunization: evidence for a generalized secretory immune system. J Immunol. 1979 Oct;123(4):1705–1708. [PubMed] [Google Scholar]
- Yang S. L., Schumacher G. F. Immune response after vaginal application of antigens in the rhesus monkey. Fertil Steril. 1979 Nov;32(5):588–598. doi: 10.1016/s0015-0282(16)44365-7. [DOI] [PubMed] [Google Scholar]



