Abstract
Tumours of cutaneous sweat glands are uncommon, with a wide histological spectrum, complex classification and many different terms often used to describe the same tumour. Furthermore, many eccrine/apocrine lesions coexist within hamartomas or within lesions with composite/mixed differentiation. In addition to the eccrine and apocrine glands, two other skin sweat glands have recently been described: the apoeccrine and the mammary‐like glands of the anogenital area. In this review (the second of two articles on skin adnexal neoplasms), common as well as important benign and malignant lesions of cutaneous sweat glands are described, and a summary for differentiating primary adnexal neoplasms from metastatic carcinoma is outlined, striving to maintain a common and acceptable terminology in this complex subject. Composite/mixed adnexal tumours are also discussed briefly.
The classification of cutaneous sweat gland lesions is very complex (table 1), as these lesions have a wide histological spectrum, and the pathogenesis and exact origin of many lesions is still under investigation and not clear.1,2 Many different terms are often used to describe the same tumour (table 2), making this subject more difficult for pathologists to reach a uniform terminology for the diagnosis of these lesions. Furthermore, the issue is complicated by the coexistence of eccrine and/or apocrine lesions within cutaneous hamartomas or within adnexal lesions with composite/mixed differentiation, which also includes those with follicular and sebaceous differentiation. A detailed practical approach to different skin adnexal lesions has been illustrated in the first review article of this series.3 Nonetheless, some important general features of sweat glands are reviewed here.
Table 1 Classification of cutaneous sweat gland adnexal lesions according to the current concept of the predominant “accepted” origin.
| Origin | Benign | Malignant |
|---|---|---|
| Eccrine and apocrine (mixed origin) | Hidrocystoma | Malignant mixed tumour of the skin (has eccrine/apocrine and mesenchymal components) |
| Apocrine/eccrine nevus | ||
| Tubulopapillary hidradenoma (including papillary eccrine adenoma and tubular apocrine adenoma) | ||
| Chondroid syringoma | ||
| Eccrine | Poroma | Porocarcinoma |
| Hidradenoma | Hidradenocarcinoma | |
| Spiradenoma | Spiradenocarcinoma | |
| Cylindroma | Malignant cylindroma | |
| Syringometaplasia | Syringoid carcinoma | |
| Syringoma | Microcystic adnexal carcinoma | |
| Syringofibroadenoma | Mucinous carcinoma | |
| Adenoid cystic carcinoma | ||
| Aggressive digital papillary adenocarcinoma | ||
| Apocrine | SCAP | Syringocystadenocarcinoma |
| Hidradenoma papilliferum | Apocrine carcinoma | |
| Extramammary Paget's disease | ||
| Composite/mixed cutaneous adnexal tumours |
SCAP, syringocystadenoma papilliferum.
Table 2 Cutaneous sweat gland lesions and their synonyms.
| Lesion | Synonyms |
|---|---|
| Hidrocystoma | Apocrine/eccrine hidrocystoma, cystadenoma |
| Syringofibroadenoma | Acrosyringeal adenomatosis, acrosyringeal nevus |
| Poroma | Hidroacanthoma simplex (intraepidermal poroma), eccrine poroma, and dermal duct tumour |
| Hidradenoma | Nodular hidradenoma, solid‐cystic hidradenoma, clear cell hidradenoma, clear cell acrospiroma, eccrine acrospiroma |
| Cylindroma (multiple) | Turban tumour |
| Tubulopapillary hidradenoma | Papillary eccrine adenoma, tubular apocrine adenoma, papillary apocrine fibroadenoma |
| SCAP | Papillary syringadenoma |
| Hidradenoma papilliferum | Papillary hidradenoma |
| Chondroid syringoma | Mixed tumour of the skin |
| Digital papillary adenocarcinoma | Aggressive digital papillary tumour, aggressive digital papillary adenoma/carcinoma, aggressive digital papillary adenoma |
| Sweat gland ductal carcinoma | Syringoid eccrine carcinoma, eccrine ductal carcinoma |
| Porocarcinoma | Malignant “eccrine” poroma |
| Microcystic adnexal carcinoma | Sclerosing sweat duct carcinoma |
| Hidradenocarcinoma | Malignant nodular/clear cell hidradenoma, malignant acrospiroma, malignant clear cell acrospiroma, clear cell eccrine carcinoma, primary mucoepidermoid cutaneous carcinoma |
| Apocrine carcinoma | Tubular apocrine carcinoma, malignant hidradenoma papilliferum |
SCAP, syringocystadenoma papilliferum.
Eccrine sweat glands are widely distributed almost everywhere in the skin. The cells in the excretory coil express positivity for low molecular weight keratin (LMWK), epithelial membrane antigen (EMA) and carcinoembryonic antigen (CEA), as well as S100 protein in the basal layer only. The myoepithelial cell layer may be highlighted by smooth muscle actin (SMA), p63 and calponin. Acrosyringeal cells (intraepidermal portion) stain for high molecular weight keratin (HMWK) and cytokeratin (CK) 14. A subpopulation of cells also expresses positivity to bcl‐2. Skin tumours with eccrine differentiation often also express positive immunohistochemical staining for oestrogen and progesterone receptors.4 The positivity of some eccrine carcinomas to oestrogen receptors has important clinical implications, as affected patients may be partially treated with hormonal therapy.5 Unfortunately, positivity for oestrogen receptors and progesterone receptors does not distinguish cutaneous eccrine tumours from cutaneous metastases of breast carcinoma,6 in which case clinical and radiological correlation is critical.
Apocrine lesions of the skin are rare and are found mainly in body folds including the axillary, groin and anogenital regions, where apocrine glands are most concentrated, as well as in the umbilicus, eyelid (Moll's glands), areola and in external auditory meatus. They also occur as heterotopic lesions elsewhere in the body, exemplified by tubular apocrine adenoma and within hamartomatous adnexal lesions such as in nevus sebaceous of Jadassohn (NSJ) in the scalp. In lesions of apocrine origin, the cells have abundant eosinophilic cytoplasm and eccentric, basally located nuclei. In addition, there is usually evidence of decapitation secretion in the luminal cells, and the tumour cells contain periodic acid Schiff (PAS)‐positive diastase‐resistant granules, and iron pigment (best illustrated with Prussian blue stain). The luminal cells are immunoreactive to CEA and EMA. The secretory cells are positive for LMWK, and the glandular epithelium often also expresses gross cystic disease fluid protein 15 (GCDFP‐15) and androgen receptors, which may be useful in the assessment of lesions suspicious of apocrine carcinoma. However, oestrogen receptors/progesterone/bcl‐2 negativity is more consistent, and is considered more helpful to confirm the diagnosis of apocrine carcinoma than GCDFP‐15/androgen receptor positivity.7
Apoeccrine glands are mostly found in the axillary region, and within lesions of NSJ.8 Their presence helps to explain the existence of some adnexal lesions that have both eccrine and apocrine components, including syringocystadenoma papilliferum (SCAP)1 and Fox–Fordyce disease.9 Some authors10 believe that these glands are synonymous with cutaneous mammary‐like glands (MLG), which are now recognised to be a normal component of the skin in the anogenital region, including the perianal skin.11 MLG are unique in having features of apocrine, eccrine and mammary glands. The previous assumption that these glands represent ectopic/accessory breast tissue lying along the milk line (mammary ridge) is now believed to be incorrect.12 Many adnexal lesions of the anogenital area are now recognised to be of MLG origin, including “apocrine” adenoma/fibroadenoma, hidrocystoma, hidradenoma papilliferum, extramammary Paget's disease and adenocarcinoma.11,12,13,14 These lesions often have papillary/cribriform growth pattern and a fibrous stroma reminiscent of fibroepithelial lesions of the breast. A helpful feature in attributing MLG origin to an adnexal tumour is the presence of normal MLG in the deep dermis and subcutaneous fatty tissue, in the vicinity of the lesion of interest,13 with a transition zone between benign and lesional areas.12
Tumours of pure apocrine origin appear to be much less common than those of eccrine origin, by virtue of the localisation of apocrine glands in the body. However, it is important to mention at the outset of this review that many of the sweat gland tumours traditionally assumed to have an eccrine origin are now recognised to have apocrine counterparts as well, as evidence of apocrine differentiation has been elucidated in many of them. These tumours include hidrocystoma, poroma, cylindroma, spiradenoma and chondroid syringoma. In addition, many sweat gland lesions have both eccrine and apocrine differentiation (as well as pilosebaceous occasionally) within the same tumour. Examples of this are the many composite/mixed adnexal tumours and hamartomas that continually appear in the literature. Therefore, it was felt necessary in this review to drop the prefix “eccrine” from many of these terms.
This review will concentrate on common and/or important benign as well as malignant tumours of cutaneous sweat glands (tables 3 and 4, respectively), with emphasis on a diagnostic approach. Many benign sweat gland tumours have malignant counterparts, which are discussed under the same subheading. Some entities are referred to only in the tables, but not considered in the text. After reading this review, the practising pathologist will be able to diagnose most of the entities considered herein. However, when a tumour is difficult to classify, it is more important to differentiate between benign and malignant lesions, and in that setting it may be sufficient to describe the tumour as a benign or malignant cutaneous sweat gland tumour, with predominant eccrine or apocrine differentiation. Furthermore, complete excision of all skin adnexal lesions is always advisable. The differentiation of sweat gland tumours from metastatic carcinomas is discussed where appropriate.
Table 3 Morphological approach to benign cutaneous sweat gland lesions.
| Predominant morphology | Lesion | General features |
|---|---|---|
| Solid tumours with prominent basaloid component | Poroma, hidroacanthoma simplex and dermal duct tumour | Large nodules of monomorphic small “poroid” cells connected to the epidermis |
| Nodular hidradenoma | Large dermal nodules | |
| Cylindroma | Jigsaw‐puzzle appearance | |
| Thickened basement membrane | ||
| Spiradenoma | Basaloid cells with two cell types | |
| Cystic lesions | Hidrocystoma | Simple cyst lined by two rows of cells |
| Lining may have eccrine or apocrine epithelial features | ||
| Papillary projections or solid buds of secretory cells may be seen | ||
| Solid‐cystic tumours | Nodular hidradenoma | Solid lobules with cystic changes |
| May have spindle or squamous differentiation | ||
| Eosinophilic or clear cell change may be prominent | ||
| Mixed tumour of the skin | Tubular or anastomosing ductal/glandular proliferation | |
| Chondromyxoid stroma | ||
| Eccrine more common than apocrine | ||
| May also have “hamartomatous” components | ||
| Glandular tumours with eccrine differentiation | Syringoma | “Tadpole” or “comma shaped” small ducts with two rows of cells |
| Tubulopapillary hidradenoma with eccrine differentiation (papillary eccrine adenoma) | Dermal/subcutaneous tubular structures | |
| Intraluminal papillary projections | ||
| Syringofibroadenoma (acrosyringeal adenomatosis) | Diffuse proliferation of anastomosing acrosyringeal epithelium in epidermis and dermis | |
| Glandular tumours with apocrine differentiation | Hidradenoma papilliferum | Vulvar/perianal |
| Mostly in females | ||
| Prominent papillary fronds | ||
| Fibrous stroma | ||
| Tubulopapillary hidradenoma with apocrine differentiation (tubular apocrine adenoma and fibroadenoma) | Dermal/subcutaneous tubular apocrine structures | |
| Lobular pattern of with epidermal connection | ||
| Intraluminal papillary projections | ||
| SCAP | Mostly over scalp in young | |
| Elaborate epithelial papillary infolding | ||
| Open to epidermis | ||
| Plasma cell‐rich stroma | ||
| Apocrine differentiation is not always apparent | ||
| Tumours demonstrating clear cell change | Nodular hidradenoma | This may occur in many sweat gland tumours |
| Syringoma | Should be considered in conjunction with other features | |
| Poroma | Follicular lesions with trichilemmal differentiation are also in the differential | |
| Spiradenoma | ||
| Miscellaneous/hamartomatous/hyperplastic lesions | Apocrine/eccrine nevus | Localised proliferation of apocrine/eccrine glandular/ductal structures within deep dermis |
| Syringometaplasia | Mostly reaction to damaged eccrine ductal epithelium induced by different stimuli; especially chemotherapeutic drugs. Metaplasia of eccrine duct epithelium into squamous cells with intercellular bridges similar to the stratum spinosum | |
| Keratinisation and focal necrosis110 | ||
| Apocrine hyperplasia | Axillae and groin | |
| Nipple adenomatosis | Nipple | |
| Papillated ducts open to surface | ||
| Apocrine differentiation | ||
| Composite/mixed skin adnexal tumours | Have two or more lines of differentiation towards eccrine, apocrine, sebaceous or pilar structures |
SCAP, syringocystadenoma papilliferum.
Table 4 Malignant cutaneous sweat gland tumours that have relatively constant identifiable features (discussed in text).
| Eccrine | Apocrine |
|---|---|
| Porocarcinoma | Apocrine carcinoma |
| Hidradenocarcinoma | Extramammary Paget's disease |
| Spiradenocarcinoma | |
| Malignant cylindroma | |
| Syringoid carcinoma | |
| Malignant chondroid syringoma | |
| Aggressive digital papillary adenocarcinoma | |
| Microcystic adnexal carcinoma | |
| Adenoid cystic carcinoma | |
| Mucinous carcinoma |
Poromas
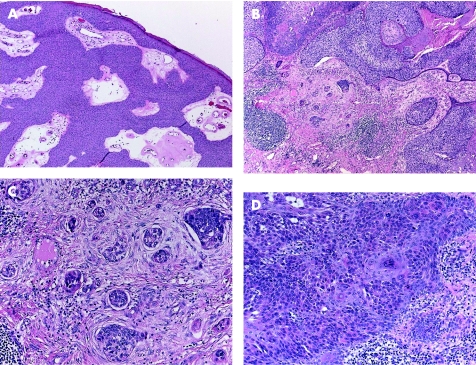
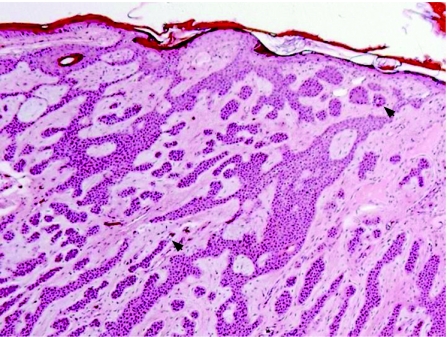
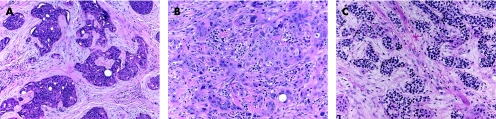
Most of these tumours originate from the outer cells of the intraepidermal (acrosyringeal) excretory ducts of eccrine sweat gland. They usually occur in adults, with predilection to palms and soles, and less frequently in the head and neck, and trunk regions. Clinically they can be solitary or multiple, and can present as superficial plaques or dermal nodules, with tendency to ulceration and bleeding. Histologically, three main benign tumours are recognised and distinguished according to their location in relation to the epidermis:15,16 (1) poroma (fig 1A), involving both epidermis and dermis; (2) hidroacanthoma simplex, also known as intraepidermal poroma, confined within the epidermis; and (3) dermal duct tumour, limited to the dermis, with no epidermal attachment. These tumours consist of solid sheets and nodules of basaloid poroid cells with pushing borders that emanate from the epidermis. The neoplastic cells are small, monomorphous and polyhedral cuboidal, with well‐defined cell membrane. They have small, centrally located bland nuclei, and a variable amount of cytoplasm that ranges from scant to ample, eosinophilic to clear glycogenated PAS positive and diastase sensitive. Variable numbers of mitotic figures can be identified. The tumour cells are sharply delineated from adjacent keratinocytes. Foci of squamous differentiation and clear cell change are commonly seen. The intervening stroma is characteristically very vascular. Inconspicuous to prominent CEA‐positive small ducts lined by cuboidal cells, and PAS‐positive eosinophilic cuticle material are present in most tumours. Poroma cells are also reactive for cytokeratin 17.17 More recently, there have been reports of poromas with sebaceous, follicular and apocrine differentiation, suggesting a possible apocrine origin. These cases have been labelled as “apocrine” poromas.18
Figure 1 (A) Poroma: multiple epidermal attachments with downgrowth into the dermis. (B) Porocarcinoma, with clear cell change. (C) Higher power of porocarcinoma demonstrating cellular atypia, tumour necrosis, infiltration and budding into adjacent desmoplastic stroma. (D) In situ component adjacent to the invasive porocarcinoma depicted in (B) and (C).
Porocarcinomas (malignant eccrine poromas; fig 1B) are usually found on the lower extremities. They usually arise in pre‐existing benign poroid tumours, and are histologically characterised by asymmetrical, solid, nodular growth pattern, with infiltrative or pushing borders. The neoplastic cells in porocarcinoma may have basaloid features, resembling those of poroma, but show varying degrees of cytonuclear atypia, hyperchromatic nuclei, and prominent nucleoli. Large polyhedral cells with abundant clear cytoplasm can be present. Foci of squamous and spindle cell differentiation are common. Evidence of eccrine differentiation in the form of CEA‐positive ductal formation is present in most of the cases. Brisk mitotic activity, necrosis and desmoplastic stromal reaction can be prominent (fig 1C). Continuity with a benign pre‐existing poroma and prominent intraepidermal component, occasionally showing severe cellular pleomorphism and resembling Paget's disease, can be present (fig 1D).19,20 A pure “porocarcinoma in situ” adjacent to a benign poroma may also occur. Porocarcinomas are aggressive tumours with potential for local recurrence, and nodal and distant metastases.21 It has been suggested that more than 14 mitoses per high power field, tumour depth >7 mm and presence of lymphovascular invasion are associated with more aggressive clinical course.20 The main differential diagnosis of porocarcinoma is squamous cell carcinoma, which lacks intracytoplasmic lumina and ductal formation. The differential diagnosis of porocarcinoma in situ includes clonal seborrhoeic keratosis, clonal squamous cell carcinoma in situ (Bowen's disease), melanoma in situ and Paget's disease.22 The presence of an adjacent benign poroid component is a helpful feature in establishing the diagnosis. In clonal Bowen's disease, the tumour cells are more atypical and there is more severe architectural abnormality. Clonal seborrhoeic keratosis and Bowen's disease are negative for CEA, cytokertain 7 and S100, which are often positive in porocarcinoma.16 Negativity for melan‐A and HMB45 helps rule out melanoma in situ. In Paget's disease, the cells are large, with clear PAS‐positive cytoplasm.
Hidradenoma and hidradenocarcinoma
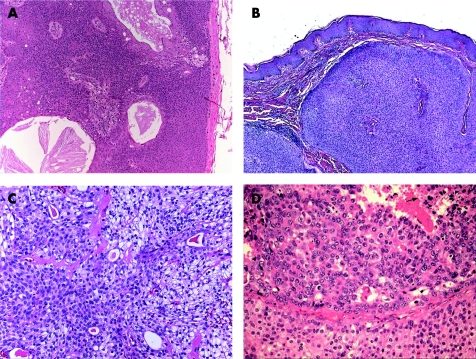
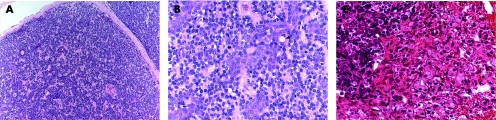
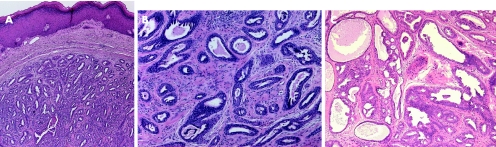
Hidradenoma is a benign tumour, which usually presents as a solitary, skin‐coloured lesion and occurs more commonly in females.16 Hidradenoma may have variable histomorphological patterns (fig 2A–C), reflected by the various terms used to describe this entity: nodular hidradenoma, eccrine acrospiroma, solid‐cystic hidradenoma clear cell hidradenoma, and clear cell acrospiroma. It shows variably sized nests and nodules of neoplastic epithelial cells, with small ductular lumens, confined within the upper dermis. The tumour cells are small, monomorphous and polyhedral, resembling those of poroma cells. In fact, some tumours have epidermal attachment, and occasionally may also have features overlapping with those of typical poromas.23 Clear cell change and/or squamous metaplasia may be prominent. However, squamoid change does not seem to denote a worse prognosis. Occasionally, focal apocrine components may also be present. The lesion is also characterised by its pushy, but well‐circumscribed, peripheral border. Nodular hidradenoma should be fully excised, as malignant transformation may be present in other areas of the lesion. Furthermore, hidradenoma has a recurrence rate of approximately 12% if not fully excised,24 especially in lesions with irregular peripheral margins.23
Figure 2 (A) Hidradenoma—solid‐cystic variant. (B) Hidradenoma—solid variant. (C) Hidradenoma—tubular/ductal differentiation and clear cell change (clear cell hidradenoma). (D) Hidradenocarcinoma—cellular atypia and tumour necrosis (arrow).
Although most cases of hidradenocarcinoma arise de novo, the tumour may also arise in pre‐existing hidradenoma.23 Hidradenocarcinoma is also often referred to as malignant nodular/clear cell hidradenoma, malignant clear cell acrospiroma, clear cell eccrine carcinoma or primary mucoepidermoid cutaneous carcinoma. Histologically (fig 2D), it is a multinodular solid malignant neoplasm, showing ductal structures and intracytoplasmic tubular vacuoles, with areas of tumour necrosis. The tumour cells have similar morphology as those of nodular hidradenoma, but may also show cytonuclear atypia and increased mitotic activity. Apocrine differentiation is frequently seen. Evidence of nodular hidradenoma remnants may be present. An infiltrative growth pattern is not universally seen, and the carcinoma is distinguished from benign hidradenoma by the presence of brisk mitotic activity and cellular pleomorphism. The tumour cells stain positively for LMWK, and the ductal structures/luminal surfaces are highlighted by EMA and CEA. Although these rare tumours do not always behave aggressively, they may have an aggressive course with metastasis and/or local recurrence. The primary treatment is wide local excision with or without lymph node dissection.25,26
Interestingly, clear cell hidradenoma and hidradenocarcinoma may occasionally mimic metastatic clear cell carcinomas including thyroid, lung or renal cell carcinomas. However, the first two are usually distinguished by their positivity to thyroid transcription factor‐1 (TTF‐1), and the latter by its prominent vascularity, and the presence of haemorrhage and focal granular necrosis within the lesion.22 Renal cell carcinoma also expresses both EMA and CD10.
Syringoma and syringoid eccrine carcinoma
Syringomas constitute a spectrum of benign sporadic tumours that arise from the straight segment of the intradermal eccrine sweat duct. They predominantly affect middle‐aged women, and most commonly involve the head and neck region, with a predilection for the eyelids. An uncommon variant is eruptive syringoma, a clinical condition characterised by multiple yellow–brown‐coloured, firm papules, with no specific site predilection in young adults.27 The pathogenesis of eruptive syringoma is controversial, and it has been suggested that it represents a reactive eccrine gland ductal proliferation following cutaneous inflammatory conditions.28 There is also an increased incidence of eruptive syringoma in patients with Down's syndrome, and, rarely, syringomas can occur in familial form.29
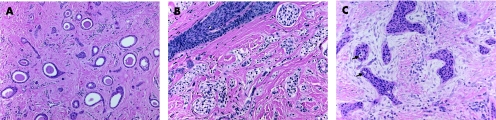
Histologically, syringomas are symmetrical, well circumscribed, confined to the upper dermis, and usually have no connection with the overlying epidermis. The tumours consist of proliferation of epithelial cells, and are arranged in nests, cords or tubules, in which some display a “comma” or “tadpole” shape (fig 3A). The tubules are lined by single or double layers of bland, monomorphous, cuboidal epithelial cells with small normochromic nuclei, and small to moderate amount of pale eosinophilic cytoplasm. In some tumours, the neoplastic cells predominantly exhibit abundant clear cytoplasm30 (clear cell syringoma; fig 3B). A PAS‐positive eosinophilic material is often present within the tubular lumina. Syringoma should be distinguished from the extremely rare malignant counterparts, syringoid eccrine carcinoma and microcystic adnexal carcinoma. In contrast with benign syringomas, syringoid eccrine carcinoma (fig 3C) exhibits cytonuclear atypia, mitotic activity, lymphovascular and perineural invasion, and infiltrative growth pattern, with tumour extension into the deep dermis and subcutaneous tissue, and potential for local recurrence and distant metastases.31,32 In superficial skin biopsies, microcystic adnexal carcinoma may be misdiagnosed as syringoma (see below). Therefore, re‐evaluation of these tumours and especially their base after complete excision is important.
Figure 3 (A) Syringoma: typical eccrine ducts with comma shape and intraductal eosinophilic material. (B) Clear cell syringoma. (C) Syringoid eccrine carcinoma: hyperchromatic nuclei, ductular/tubular structures (arrows), infiltrating into a desmoplastic stroma are illustrated.
Chondroid syringoma (mixed tumour of the skin) and malignant chondroid syringoma
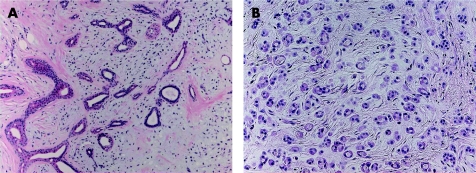
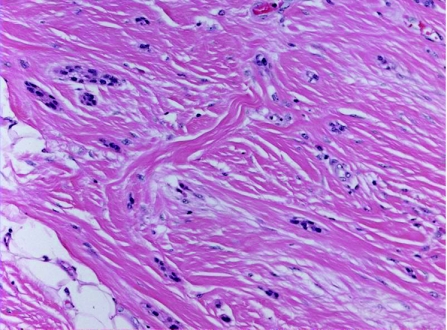
This uncommon tumour presents as a slowly growing, painless, firm nodule, generally occurring in the head and neck region, but most commonly affecting the nose, cheek and upper lip.1,33 Less frequently, the extremities, trunk or back may also be affected. Histologically, chondroid syringoma is similar to the benign mixed tumour of the salivary glands, having both epithelial and mesenchymal stromal components. The tumour shows a circumscribed nodule, consisting of bland epithelial cells, arranged in cords, ducts (fig 4A), or tubules (fig 4B). The stromal component is most often myxoid‐cartilaginous (chondromyxoid), but may also be hyalinised/fibrous, fatty, osteoid, or may even be minimal or absent.16 The stromal cells show myoepithelial differentiation, illustrated by positivity to S100, vimentin and neurone‐specific enolase, and less commonly by glial fibrillary acid protein, SMA, calponin or p63. Desmin is usually negative.1,33 Chondroid syringoma is classified into apocrine and eccrine types, based on the histopathological appearance of the sweat gland lumina within the tumour. In the apocrine type, lesions incorporating areas with pilar and sebaceous differentiation have been described repeatedly,34 suggesting that some tumours are best viewed as hamartomatous lesions of the pilosebaceous‐apocrine unit. Chondroid syringoma is best completely excised because of the possibility of local recurrence and malignant transformation.
Figure 4 (A) Chondroid syringoma: proliferating ductuloglandular eccrine epithelium in chondromyxoid stroma. (B) Chondroid syringoma with prominent tubular proliferation in chondroid stroma.
Malignant chondroid syringoma is very rare, and may arise de novo or from a pre‐existing chondroid syringoma which has undergone malignant transformation.35,36 Histologically, there is cellular atypia, increased mitotic activity, infiltrative margins, satellite tumour nodules and tumour necrosis.1 Haematogenous and lymphatic spread to lymph nodes, lungs and bone may occur. The term “atypical chondroid syringoma” is used to describe tumours with histological features of malignancy as well as recurrence, local invasion and satellite tumour nodules, but without proved metastases.37
Syringofibroadenoma (acrosyringeal adenomatosis)
This is a rare benign eccrine tumour of acrosyringeal origin, with variable clinical presentation. Histologically, there are reticulated and irregularly anastomosing epithelial cords and strands extending from the epidermis into the superficial dermis (fig 5), usually embedded in a fibrovascular stroma. The cells are basophilic and monomorphous, and are smaller than adjacent epidermal keratinocytes. They are positive for HMWK and cytokertain 14. Occasional ductal structures may be identified, and enhanced with CEA or EMA. Areas of clear cell change may also occur. This lesion has indeterminate pathogenesis, and is labelled variously as a neoplasm, hamartoma, nevus or reactive hyperplasia; therefore, it probably represents a group of heterogeneous disorders.38,39
Figure 5 Syringofibroadenoma reticulated and irregularly anastomosing epithelial cords with areas of ductal differentiation (arrows).
Spiradenoma and spiradenocarcinoma
Spiradenomas are commonly encountered benign tumours originating from the straight intradermal eccrine duct. They usually present as a solitary, painful, nodular lesion in young adults, with a predilection to the trunk and upper extremities. Spiradenomas can rarely be associated with Brooke–Spiegler syndrome, a rare autosomal dominant inherited disorder, characterised by the development of multiple skin adnexal tumours.40 Microscopically, they involve a well‐circumscribed nodular tumour in the dermis and subcutaneous tissue, encapsulated by a well‐defined fibrous capsule, and lacking a connection to the epidermis. The neoplastic nodules are solid, variable in size and characteristically formed of two epithelial cell types (fig 6A,B): (1) large cells with round vesicular nuclei prominent nucleoli, and abundant pale cytoplasm, located in the centre of the neoplastic nodules; (2) small cells with hyperchromatic nuclei and scant cytoplasm rimming the periphery of the neoplastic nodules. Mitotic figures can be present. The cells can be arranged in cords, or exhibit tubular and alveolar differentiation. Globules of dense eosinophilic PAS‐positive basement membrane material are present within the tumour lobules. Richly vascular stroma with occasional thrombi, foci of squamous differentiation, fibrosis and cystic degenerative changes are commonly seen. Apocrine differentiation has also been reported in spiradenomas, leading some authors to suggest that not all spiradenomas are of eccrine origin.41
Figure 6 (A) Spiradenoma: a lobulated mass composed of multiple nests of small basophilic cells, admixed with squamoid cells and clear cells, with hyalinised basement membrane. (B) Spiradenoma, high power: focal ductal differentiation is evident (arrow). (C) Spiradenocarcinoma: cellular atypia and increased mitotic activity. The lesion is seen adjacent to benign spiradenoma.
Spiradenocarcinoma (fig 6C) is a very rare malignant neoplasm arising from a pre‐existing spiradenoma and histologically characterised by loss of nodular growth pattern, infiltrative borders, cytonuclear pleomorphism, increased mitotic activity and tumour necrosis.42 The presence, at least in focal parts within the lesion, of dual population (central basaloid cells and larger peripheral cells) is required for the diagnosis of spiradenocarcinoma, which has a high recurrence rate, and can occasionally metastasise to lymph nodes and distant organs.
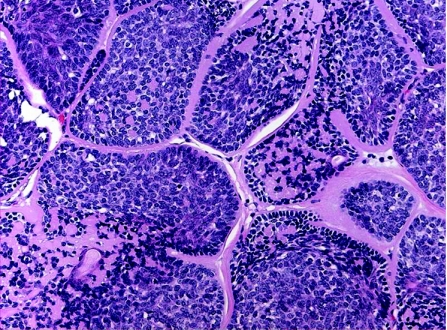
Cylindroma
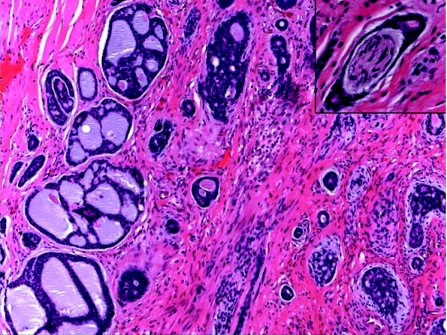
Cylindromas are benign neoplasms, usually affect adult females, with 90% occurring in the head and neck region, particularly the scalp and face. They can be sporadic and present as a solitary lesion, or less commonly as a variably sized, multiple coalescing tumour causing marked disfiguring of the scalp and face (turban tumour). Rarely, multiple cylindromas can be associated with familial cylindromatosis; an autosomal dominant disorder, and Brook–Spiegler syndrome, in which case there are multiple tumours that may also involve the trunk and extremities in children or young adults. Mutations in the cylindromatosis tumour suppressor gene (CYLD1) on chromosome 16q12–13 play an essential role in the pathogenesis of hereditary and sporadic cylindromas.43 This mutation is also associated with developing spiradenoma, trichoepithelioma, milia or a membranous variant of salivary gland basal cell adenoma. The histogenesis of cylindroma is controversial; where as accumulated evidences favour an eccrine origin from the coiled part of the intradermal duct,44 others suggest an apocrine differentiation.45 Histologically, these tumours are readily recognised and characterised by numerous well‐defined, non‐encapsulated variably sized islands and nodules of epithelial cells, lined by thick PAS‐positive basement membrane‐like, hyalinised material, and arranged in a characteristic “jigsaw puzzle” pattern (fig 7). Two populations of cells are usually identified within the tumour epithelial islands: (1) large cells with pale‐staining nuclei and moderate amount of amphophilic cytoplasm, located at the central zone, and (2) peripherally located, small, basaloid cells with dark‐staining nuclei and scant cytoplasm that exhibit a palisading pattern. Ductulotubular structures, lined by cuboidal or columnar cells and containing intraluminal eosinophilic material, are usually present. PAS‐positive hyaline globules are also found in the tumour islands. Some tumours, termed spiradenocylindromas, have features of both cylindromas and eccrine spiradenomas,46 the existence of which may suggest that both entities represent a continuous morphological spectrum of a single entity, or a transitional or maturing phase from one lesion to the other.32 In addition to the morphological similarity between cylindroma and spiradenoma, both entities express immunopositivity for S100 protein within the tubular and large, pale‐staining cells, human milk fat globulin and lysosome in tubular cells, SMA in small basaloid cells, and CK 7, 8, and 18.47
Figure 7 Cylindroma: jigsaw puzzle appearance and eosinophilic peripheral basement membrane.
Malignant cylindroma is a rare tumour arising from pre‐existing benign cylindroma, both in solitary sporadic and in autosomal dominant multiple forms. Malignant cylindroma is clinically characterised by rapid growth, long‐standing ulceration or bleeding, and aggressive clinical behaviour, with local recurrence and potential for distant metastases.48,49 Histologically, there is an infiltrative growth pattern with cytonuclear pleomorphism, increased mitotic activity, and loss of jigsaw appearance, hyaline sheath and peripheral palisading.
SCAP and syringocystadenocarcinoma papilliferum
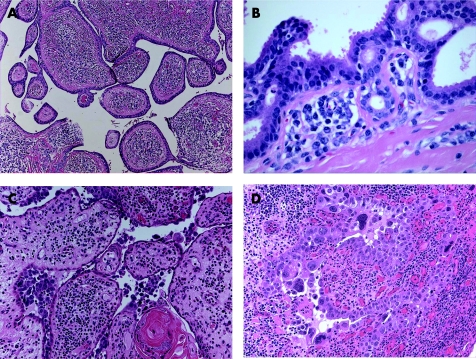
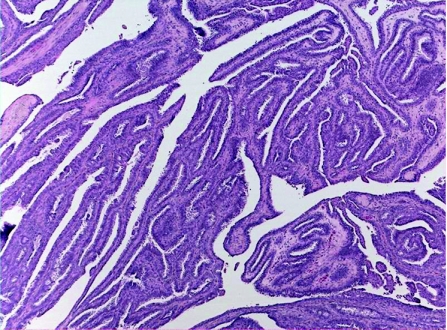
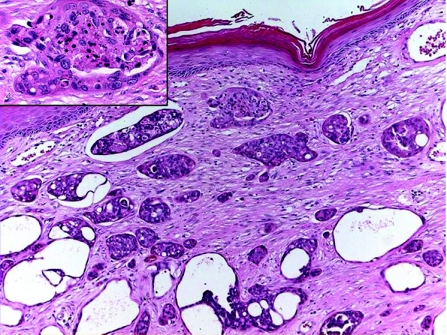
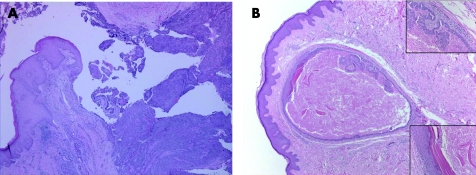
SCAP is a benign hamartomatous tumour usually affecting the young adults, and occurs as a solitary or less commonly multiple lesion, most commonly in the face and scalp. They can arise within a pre‐existing NSJ,50 and on rare occasions can be associated with papillary eccrine adenoma,51 tubular apocrine adenoma52 and hidradenoma papilliferum.13 Mutations in tumour suppressor gene p16 may contribute to the pathogenesis of SCAP.53 The tumour is variably denoted to be derived from eccrine, apocrine or apoeccrine origins.1,2 Histologically, SCAP is a verrucous tumour that displays multiple epithelial invaginations extending down from the epidermis, and contains numerous papillae (fig 8A,B). These papillary structures consist of a dermal fibrovascular core, lined by two layers of epithelial cells, luminal columnar cells with decapitation secretion and outer cuboidal cells. A characteristically marked plasmacytic inflammatory cell infiltrate is present within the fibrous cores.54 Apocrine sweat glands are often identified at the deep part of the tumour. Immunohistochemically, the luminal columnar cells express GCDFP‐15, CEA, and CK 7 and 19, whereas the basal cuboidal cells express CK 5, 7, 8 and 14.55 Syringocystadenocarcinoma papilliferum (fig 8C,D) is very rare, and as characterised histologically by disorderly arrangement of papillary projections, cytological atypia and loss of double‐layered epithelium.56
Figure 8 (A) Syringocystadenoma papilliferum (SCAP): papillary epithelial projections with opening into the surface. (B) Decapitation secretion and prominent plasma cell stromal component in SCAP. (C) Syringocystadenocarcinoma papilliferum. (D) Syringocystadenocarcinoma papilliferum: cellular atypia, tumour necrosis and mitoses.
Hidradenoma papilliferum
Hidradenoma papilliferum is a benign tumour, and occurs almost exclusively in females, with predilection to the vulva and perianal region. Rare cases of hydradenoma papilliferum have been reported in males,57 and in extra‐anogenital locations, particularly in the head and neck.58 The tumours are solitary, and are usually small and asymptomatic. Occasionally, the tumours can be large and elevated, forming a reddish‐brown mass with an ulcerated and bleeding surface.59 Human papilloma virus may have a potential role in the pathogenesis of hydradenoma papilliferum,60 an observation that needs to be confirmed by further study. Histologically (fig 9), hidradenoma papilliferum is a well‐circumscribed solid or cystic dermal nodular lesion, not connected to the epidermis. It is formed of frond‐like papillae or tubulopapillary structures that are lined by a two‐cell layer: luminal cuboidal or low columnar epithelial cells with apical secretions, resting on an outer myoepithelial cell layer. The epithelial cells typically have histochemical characteristics in keeping with an apocrine origin. hidradenoma papiulliferum lacks cytonuclear atypia, mitotic activity or tumour necrosis. Morphological features analogous to benign breast diseases can be frequently encountered, and include oxyphilic (apocrine) metaplasia, sclerosing adenosis‐like changes, atypical apocrine adenosis‐like changes, ductal epithelial hyperplasia, clear cell change of the myoepithelium, foamy histiocyte reaction and stromal fibrosis.60 Rare malignant transformation in hidradenoma papiulliferum (apocrine adenocarcinoma) is characterised by cytological pleomorphism, mitoses and necrosis with or without epidermal pagetoid spread of the malignant cells.61 Immunohistochemically, the tumour cells often express CEA,62 GCDFP‐15,63 lysosome and CD‐15 (Leu M1)64 Furthermore, oestrogen receptors, progesterone receptors, and androgen receptors are commonly expressed in hidradenoma papiulliferum.65 This and the presence of histomorphological features analogous to benign breast diseases lend further evidence to a possible origin of hidradenoma papiulliferum from MLG.
Figure 9 Hidradenoma papilliferum: a cystic and papillary dermal neoplasm demonstrating decapitation secretion characteristic of apocrine differentiation.
Tublopapillary hidradenoma with eccrine/apocrine differentiation (including papillary eccrine adenoma, tubular apocrine adenoma, and papillary apocrine fibroadenoma)
Papillary eccrine adenoma (PEA) and tubular apocrine adenoma (TAA) occur most commonly on the scalp, but rare variants of TAA have also been described in the vulva66 and in the perianal skin.67 In the anogenital skin, apocrine adenoma is believed to arise from MLG.11,68 As TAA may be histologically indistinguishable from PEA (fig 10), the term “tubulopapillary hidradenoma” was recently introduced to describe benign sweat gland tumours characterised by combining ductal as well as apocrine and eccrine glandular differentiation, incorporating features of TAA and/or PEA, with a predominance of eccrine or apocrine differentiation.45,69 This incorporates entities previously designated as PEA, TAA and other related lesions. Clinically, these lesions appear as well‐circumscribed superficially located solitary dermal/subcutaneous nodules. Microscopically, the tumour is composed of dilated tubular/ductular structures of varying size, lined by a double layer of epithelial cells and a peripheral myoepithelial cell layer. Delicate intraluminal papillary projections are commonly seen. Immunohistochemical evidence of both eccrine and apocrine differentiation may be found,45 raising the possibility that such lesions have dual origin or arise from apoeccrine glands70 or MLG (in lesions of the anogenital area).45,69 The lumina are usually filled by eosinophilic amorphous material. In lesions of tubulopapillary hidradenoma with apocrine differentiation, irregularly shaped tubular structures lined by two layers of epithelial cells with decapitation secretion in the lumina are evident in many areas.69 Immunohistochemically, the tumour is reactive to S100 protein, CEA and EMA.71 The coexistence of SCAP with TAA72 and with PEA51 has been reported, suggesting that TAA, PEA and SCAP may represent a spectrum of inter‐related tumours.
Figure 10 (A) Tubulopapillary hidradenoma. (B) Tubulopapillary hidradenoma with eccrine differentiation (papillary eccrine adenoma). (C) Tubulopapillary hidradenoma with apocrine differentiation (tubular apocrine adenoma).
Hidradenoma papilliferum is distinguished from these tumours by its more closely arranged tumour cells and glands,13 and by its almost consistent presence in the female anogenital area.
Microcystic adnexal carcinoma
Microcystic adnexal carcinomas (MACS; sclerosing sweat duct carcinomas) are uncommon malignant neoplasms. They are widely believed to be of eccrine origin. However, the issue is complicated by the presence of keratinous cysts and the description of apocrine and sebaceous differentiation in many cases.73 This may suggest that the tumour has a mixed adnexal lineage with eccrine, apocrine and pilosebaceous features. Microcystic adnexal carcinoma occurs as a solitary lesion in middle‐aged adults, commonly in the face, with predilection to the upper and lower lip.32 Risk factors include previous radiation therapy and immunosuppression.74 Histologically (fig 11), microcystic adnexal carcinoma consists of poorly circumscribed proliferation of deceptively bland epithelial neoplastic cells infiltrating the dermis and subcutaneous tissue, and exhibits prominent perineural and intraneural invasion. The neoplastic cells are small, uniform and basaloid, or less commonly clear, and have no or minimal cytological atypia and mitotic activity. Keratin‐filled microcysts are often present in the superficial dermis. Tubular and ductal structures lined by a single layer of neoplastic cells dominate the deeper parts of the lesion. Small, solid clusters and single tumour cells often infiltrate the subcutaneous and skeletal muscle tissues, as well as into the perichondrium and periosteum. The tumour stroma is characteristically sclerotic. The ductal elements are usually positive for EMA and CEA. Recently, CD5 was found to stain the epithelial component in most cases.75 MAC is locally aggressive, with propensity to invade the nerves and cause local tissue destruction, and has a high recurrence rate. Occasionally, nodal and distant metastases also occur.76 The differential diagnosis of microcystic adnexal carcinoma includes syringoma, desmoplastic trichoepithelioma, sclerosing/morpheic basal cell carcinoma and papillary eccrine adenoma (table 5).22,77 In all cases, the base of the lesion must be observed to rule out this tumour. If a syringoma‐like lesion infiltrates deeply into the underlying tissues with/without perineural involvement, it is highly suspicious for MAC.
Figure 11 Microcystic adnexal carcinoma: deceptively benign‐looking epithelial proliferation reaching the subcutis.
Table 5 Histological features of microcystic adnexal carcinoma and its differential diagnoses.
| Microcystic adnexal carcinoma | The epidermis is usually not involved |
| Poorly circumscribed | |
| Deep invasion into deep dermis and underlying tissues | |
| Depth greater than width | |
| Keratinous microcysts in superficial part | |
| Deeper areas with glandular and ductal differentiation | |
| Sclerotic stroma | |
| Cells are not pleomorphic | |
| Mitoses are rare to absent | |
| Perineural/intraneural involvement | |
| CEA and EMA highlight ductal structures | |
| Syringoma | Well‐circumscribed strands of basaloid cells |
| Confined to upper to mid dermis | |
| Well‐differentiated ductal/glandular structures | |
| “Tadpole” or “comma” shape | |
| Small horn cysts | |
| Width greater than depth | |
| Desmoplastic stroma | |
| No perineural involvement | |
| Desmoplastic trichoepithelioma | Superficial |
| Numerous small keratin cysts | |
| Foreign body reaction | |
| Calcification | |
| Lacks eccrine duct formation | |
| No perineural involvement | |
| Sclerosing/morpheic basal cell carcinoma | Infiltrative basaloid cells |
| Prominent sclerotic stroma | |
| Papillary eccrine adenoma | Fairly well circumscribed |
| Epithelial islands | |
| Glandular differentiation | |
| Luminal micropapillary projections | |
| Tumour stroma is fibrotic and frequently hyalinised |
CEA, carcinoembryonic antigen; EMA, epithelial membrane antigen.
Primary cutaneous mucinous carcinoma
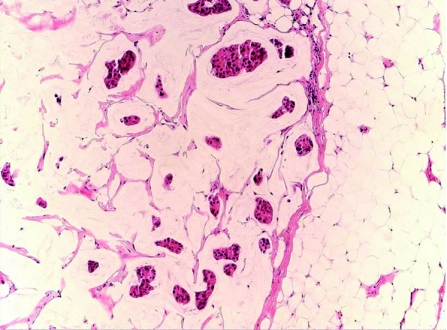
Primary cutaneous mucinous carcinoma (PCMC) is a rare low‐grade malignancy tumour that affects older adults, and occurs most frequently in the head and neck, and axillary regions, with a predilection to the eyelids. Histologically, the tumour has a morphological spectrum comparable to its mammary counterpart,22,78 and usually appears as a well‐demarcated, lobular dermal lesion, with frequent extension into the subcutaneous tissue and no connection with the overlying epidermis. It consists of PAS‐positive, diastase‐resistant multilocular pools of mucin, in which nests of bland, monotonous, polygonal epithelial cells are embedded (fig 12). The tumour cells possess round, hyperchromatic nuclei, inconspicuous nucleoli, moderate amount of pale eosinophilic to clear cytoplasm and well‐defined cell membrane. Secretory snouts may be present. Mitotic figures are rare. Solid sheets of tumour cells and single neoplastic cells arranged in an Indian‐file pattern can be seen.79 Immunohistochemically, the tumour cells are often immunoreactant to EMA, LMWK and CEA,19 as well as to GCDFP‐15, CK 7, oestrogen receptors and progesterone receptors.80 PCMC should be differentiated from metastasising mucinous carcinoma originating in extracutaneous organs; therefore, an extensive clinical examination to exclude metastasis from breast or visceral mucinous carcinoma is essential. Histologically, an in situ component in the form of epithelial islands bounded by a myoepithelial layer, and highlighted by CK 5/6, p53, calponin and SMA, is frequently present in PCMC in comparison to metastatic mucinous carcinoma.78 In comparison, breast mucinous carcinoma metastatic to the skin mostly presents with lesions on chest wall, breast and axillae, and these locations can serve as clue to the breast origin. In addition, these metastatic lesions lack an in situ component histologically. PCMC is typically an indolent tumour with tendency for local recurrence; however, distant metastasis is rare.22
Figure 12 Primary cutaneous mucinous carcinoma in pools of mucinous stroma.
Adenoid cystic carcinoma
Adenoid cystic carcinoma (ACC) is a very rare malignant tumour affecting older adults, with female predominance. It can occur in different anatomical sites, most commonly in the scalp.81 The tumour has a 50% local recurrence rate,81 but metastasis to regional lymph nodes and distant organs is exceedingly rare.82 Histologically, ACC is indistinguishable from its salivary gland counterpart, usually presenting as a dermal tumour that consists of bland, monomorphous basaloid cells with round or angulated hyperchromatic nuclei, indistinct nucleoli and inconspicuous cytoplasm, arranged in cribriform, solid or trabecular growth patterns (fig 13). A basement membrane‐like PAS‐positive, diastase‐resistant eosinophilic material is often present within the microcystic spaces. The tumour cells are immunoreactant to LMWK and pankeratin (AE1/AE3). EMA and CEA are frequently expressed in the luminal cells.83 Inconstant expression of actin was described previously, which is possibly an indication of the presence of a myoepithelial cell population.81 Before diagnosing primary cutaneous ACC, it is essential to clinically rule out the presence of salivary gland ACC and exclude other cutaneous basaloid tumours, especially adenoid BCC. The lack of epidermal connection and absence of peripheral palisading of neoplastic cells, and the presence of PAS‐positive material and perineural space invasion favour the diagnosis of adenoid cystic carcinoma over adenoid BCC.19 In addition, CEA and CD117 (c‐kit protein) are seen in adenoid cystic carcinoma but not in BCC.84 Adenoid cystic carcinoma may have squamous metaplasia, in which case it must also be distinguished from squamous cell carcinoma with adenoid (pseudoglandular) features. The positivity for CEA and S100 protein, and negativity for p63 favour the diagnosis of ACC.
Figure 13 Adenoid cystic carcinoma, with perineural invasion (inset).
Digital papillary adenocarcinoma (aggressive digital papillary tumour, aggressive digital papillary adenoma/carcinoma, aggressive digital papillary adenoma)
These are rare sweat gland neoplasms, largely accepted to be of eccrine origin, that occur in middle‐aged adults (with male predominance) and have a mostly digital location (most frequently on fingers, but also on toes, palms and soles).85 They typically have clinically aggressive growth behaviour. Histologically (fig 14), the tumour shows intradermal multinodular solid and cystic growth pattern, composed of cuboidal/low columnar epithelial cells, with back‐to‐back/cribriform glands. Multiple cystic spaces, containing eosinophilic material, with luminal papillary projections are characteristically present. Not uncommonly, there may be cytonuclear atypia, increased mitotic activity or tumour necrosis. Rarely, decapitation secretion (traditionally attributed to apocrine glands) may be seen in parts of the lesion. Areas of squamous, clear cell or spindle cell change are less frequently observed. There is no connection to the overlying epidermis. The tumour expresses CEA within the luminal cells and S100 protein within other tumour cells. The presence of poor glandular differentiation, necrosis, cytological atypia, increased mitotic rate, and invasion of soft tissue, bone and blood vessels was deemed important in labelling these tumours as adenocarcinomas. However, the distinction between adenoma and carcinoma on histological grounds alone does not seem to predict the clinical progression of these tumours,86 which often have a high rate of local recurrence and potential for metastasis.87 Therefore, all these tumours are best considered malignant, and they should be completely excised with or without amputation of the affected digit, as they may recur in 50% of cases, with 14% risk for metastasis. Sentinel lymph node biopsy has also been suggested in the staging of these tumours.88 All patients should be followed up for potential local recurrence/metastatic disease, even after apparent complete excision, in which case the risk of local recurrence is estimated at 5%.
Figure 14 Digital papillary adenocarcinoma. Tumour necrosis is more evident in the inset.
The differential diagnosis of this tumour includes spiradenocarcinoma, which should have at least the foci of a two‐cell population, and hidradenocarcinoma, which is usually less circumscribed and lacks the papillary pattern and back‐to‐back glandular differentiation.86 Papillary eccrine adenoma is well circumscribed and lacks the back‐to‐back glandular differentiation and the cystic component of digital papillary adenocarcinoma. This tumour may also resemble breast carcinoma; however, it is unusual for an invasive papillary breast carcinoma to metastasi
Apocrine carcinoma
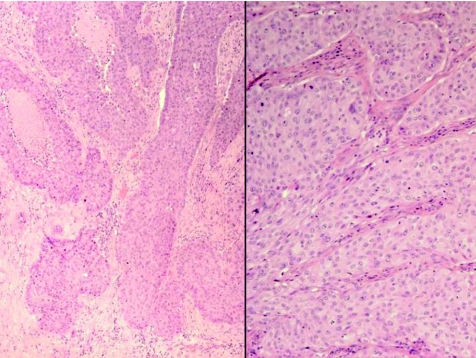
Apocrine carcinoma is a rare and highly aggressive cutaneous adenocarcinoma, more commonly seen in middle‐aged women, and mostly occurs in areas rich in apocrine glands, especially the axilla.89,90 Other possible sites include eyelid (in Moll's glands), ear and scalp.91 These tumours have also been described in NSJ.92 Histologically (fig 15), the tumour shows an asymmetric poorly demarcated malignant tumour, composed of conspicuous tubular and ductal structures. Papillary projections within the lumina may be present. The tumour has infiltrative margins, occupies the dermis and may extend to the subcutis. Cytonuclear pleomorphism, increased mitotic activity and areas of tumour necrosis are also noted. Decapitation secretion, as a sign of apocrine differentiation, is often present. PAS‐positive diastase‐resistant material is identified in the cells or lumen. The tumour cells are positive for LMWK, and the luminal cells are reactive for CEA, EMA and GCDFP‐15. S100 protein is also occasionally positive.91 Although positivity to androgen receptors and GCDFP‐15 is considered helpful, negativity of these tumours to ER and PR is more relevant from a practical point of view. The use of calponin and SMA as markers for the peripheral myoepithelium is helpful in delineating invasion.1
Figure 15 Apocrine carcinoma, with infiltrative growth and tumour necrosis (left side), and cellular atypia, prominent nucleoli and mitotic activity (right side).
Of interest is the possible association between apocrine carcinoma and benign apocrine lesions (apocrine hyperplasia and apocrine adenoma), which is reported in the axillary region in five elderly male Japanese patients,90 and a single case in the perianal region reported from the Department of Laboratory Medicine and Pathobiology (University of Toronto and University Health Network, Toronto, Ontario, Canada).93 In another case we encountered, in a perianal skin tag, the lesion was present within an apocrine tubulopapillary hidradenoma and showed features reminiscent of breast lesions, specifically ductal carcinoma in situ, with no break‐up of the peripheral myoepithelial layer, possibly arising in MLG.94 Helpful features in distinguishing tubular apocrine carcinoma from metastatic visceral carcinoma are the presence of decapitation secretion and attachment of the lesion to the neighbouring follicular epithelium, or the presence of benign apocrine component within the lesion.
Extramammary Paget's disease
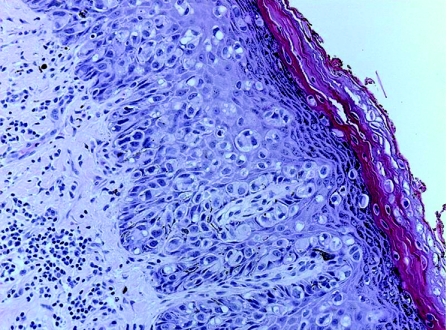
Extramammary Paget's disease (EMPD) is an uncommon cutaneous malignant neoplasm arising in areas rich in apocrine glands, most commonly in the vulvar and perianal region of middle‐aged females. It has also been described in the axillae, eyelids, external auditory canal, penis and scrotum. Apocrine gland origin or apocrine differentiation of cells in EMPD has been generally accepted. However, some authors believe that EMPD arises from the intraepidermal stem cell of sweat ducts.23 An alternative origin from Toker cells, with possible association to MLG, has also been suggested.14 Lesions usually present with erythematous eczematoid plaques and erosive ulceration. Histologically, there is proliferation in a Pagetoid pattern within the epidermis of single and small clusters of large malignant cells (fig 16), which have less defined intercellular bridges and exhibit glandular differentiation,22 occasionally showing intracytoplasmic luminal and ductal formation. The tumour cells contain alcian blue, mucicarmine and PAS‐positive mucin in most cases. AE1/AE3, LMWK and CK 7 are typically positive,95 with variable positivity seen for EMA and CEA. GCDFP‐15, androgen receptors and Her2/neu are also commonly expressed in EMPD, whereas oestrogen receptors and progesterone receptors are not.96,97
Figure 16 Extramammary Paget's disease: Single units and small clusters of atypical cells scattered throughout epidermal layers.
In contrast with mammary Paget's disease, EMPD is most commonly a primary intraepidermal lesion, with only 20% of cases demonstrating a dermal invasive element.12 When the invasive component is <1.0 mm in depth, this is considered minimally invasive disease, and complete excision is usually curative.98 EMPD may also be associated with an underlying cutaneous adnexal carcinoma, usually an apocrine adenocarcinoma.23 In up to 30% of cases, it represents metastatic spread from a regional visceral malignancy, such as cervical99 rectal,100 prostate101 or transitional cell carcinoma,102 in which case the tumour cells are positive to CK 7 and possibly GCDFP‐15,98 but they also demonstrate immunohistochemical profile similar to the original carcinoma, including positivity to CK 20 or CA19.9 in genitourinary or gastrointestinal carcinomas,23 or prostate‐specific antigen in prostate cancer.101 An underlying regional visceral malignancy or cutaneous adnexal carcinoma is more commonly seen in perianal EMPD compared with the vulvar variant. Primary intraepidermal EMPD typically has a CK 7+/GCDFP‐15+/CK 20–/prostate‐specific antigen−immunohistochemical profile.
Anaplastic cases of Paget's disease have also been described, in which there is full‐thickness epidermal atypia, loss of nuclear polarity, marked cytologic anaplasia and inconspicuous glandular differentiation.22,103 It is distinguished from Bowen's disease by sparing the basal cell layer and the presence of mucin‐containing cells. Furthermore, the lesion in Bowen's disease is positive for HMWK but not for CK 7. In contrast, EMPD expresses positivity to CEA, CK 7, GCDFP‐15, AR and CD5.22 In addition to Bowen's disease and metastatic visceral malignancy, the differential diagnosis of EMPD also includes other lesions that exhibit Pagetoid spread. In melanoma, the tumour cells usually have melanin granules in their cytoplasm, and are positive for melan‐A and HMB45 immunostains.
Other sweat gland carcinoma
Apart from the well‐defined entities aforementioned, there are many malignant adnexal tumours that have prominent eccrine component/differentiation. These tumours have no characteristic clinical picture, and it is usually very difficult to differentiate them from metastatic carcinomas, as they usually lack an epidermal connection and simulate carcinomas from other parts of the body, including thyroid, breast, salivary glands and renal cell carcinomas. This group includes eccrine ductal carcinoma, basaloid eccrine carcinoma, clear cell eccrine carcinoma and other non‐specified sweat gland carcinomas.
Eccrine ductal carcinoma (fig 17A) resembles breast ductal carcinomas, with different growth patterns possibly seen in isolation or in combination, including solid, papillary, tubular and cribriform. On most occasions, only a clinical history may help differentiate these tumours from their mammary counterparts. In eccrine carcinomas with basaloid morphology, the tumour cells are small, with scanty cytoplasm and prominent nuclei. Apoptotic cells, geographical necrosis and desmoplastic stroma are usually seen. The differential diagnosis of basaloid eccrine carcinoma is wide, and includes Merkel cell carcinoma, Ewing's sarcoma, metastatic carcinoma, as well as small cell melanoma and squamous cell carcinoma. Immunohistochemically, the eccrine carcinomas are positive for AE1/AE3 and CEA, and occasionally for S100 protein. Typically, chromogranin, melan‐A, HMB45 and CD45 are negative.16 Occasionally, eccrine carcinomas may exhibit squamous differentiation, and in some of them CK14 is positive, suggesting that such cases originate from the acrosyringium (acrosyringeal eccrine carcinoma; fig 17B).
Figure 17 (A) Eccrine ductal carcinoma. (B) Eccrine ductal carcinoma with cellular atypia, brisk mitotic activity and squamous differentiation, probably arising from the syringeum (acrosyringeal carcinoma). (C) Eccrine carcinoma with clear cell change.
Prominent clear cell change may be seen in benign as well as malignant sweat gland tumours, including poroma/porocarcinoma, hidradenoma/hidradenocarcinoma, syringoma, spiradenoma, microcystic adnexal carcinoma and other eccrine carcinomas (fig 17C). Furthermore, clear cell change may also be present in metastatic carcinomas, including thyroid, lung, urogenital and renal cell carcinomas. Differentiating these entities from each other is very important, but not always possible. Clinical history, other histomorphological features and immunohistochemical stains are very important in this regard. CK 5/6 is expressed by most skin adnexal carcinomas, but only in 33% of metastatic adenocarcinomas.104 CA125 may be helpful in identifying urogenital carcinomas, and positivity for thyroid transcription factor‐1 may be seen in thyroid and lung carcinomas.
Composite/mixed adnexal tumours
Cutaneous adnexal tumours may display a varied composition with a mixture of eccrine, apocrine, sebaceous and pilar differentiation.105,106,107,108 The diagnosis of these mixed lesions relies on histological evaluation, and they are usually classified according to the predominant morphological component. If no component is predominant, a different terminology is used to describe these lesions, including “combined adnexal tumours of the skin”,105 “benign adnexal tumour with multi‐directional differentiation”,108 “benign adnexal tumour of mixed lineage”2,16 and “composite adnexal tumours of the skin”.109 Clinically, these tumours are located in the head and neck and the extremities, and rarely elsewhere in the body, as in a case we diagnosed recently from the ventral aspect of the penis (fig 18A).109 They present as slowly enlarging solitary dermal/subcutaneous nodules.2,108 Histologically, they are characterised by well‐circumscribed, non‐encapsulated nodules composed of a lobular proliferation of epithelial cells displaying a spectrum of eccrine, apocrine, sebaceous and follicular differentiation. Figure 18B illustrates another example of these lesions.
Figure 18 (A) Composite adnexal tumour on penis, combining features of syringocystadenoma, trichilemmoma and benign eccrine ductal proliferation. (B) Composite adnexal tumour showing a mixed follicular cyst with infundibular and papillary apocrine components (insets).
The occurrence of these different elements together in one lesion may be explained by the common embryological origin of these structures in the primary epithelial germ of the superficial ectoderm.1 The existence of these cases highlights the difficulties encountered in the classification of some benign skin adnexal neoplasms, where more than one cell lineage is encountered. In our opinion, the term “composite adnexal tumours of the skin” better reflects the common embryological origin of these lesions, and the potential variable combination of eccrine, apocrine, sebaceous and pilar differentiation.
Take‐home messages
Many sweat gland tumours have both eccrine and apocrine origins.
A malignant counterpart of most benign lesions has been described. Features that suggest malignancy include asymmetry of the lesion, jagged/infiltrative borders, irregular arrangement of neoplastic cells, cytonuclear atypia and significantly increased mitotic activity.
Apocrine lesions are usually immunoreactant for androgen receptors and gross cystic disease fluid protein‐15, and negative for oestrogen and progesterone receptors, and bcl‐2.
Differentiating primary cutaneous sweat gland carcinomas from metastatic carcinoma is often difficult. Clinical history, other histomorphological features and immunohistochemical stains are all very important.
Composite/mixed adnexal tumours display a varied composition, with a mixture of eccrine, apocrine, sebaceous and pilar differentiation.
Abbreviations
ACC - adenoid cystic carcinoma
CEA - carcinoembryonic antigen
EMA - epithelial membrane antigen
EMPD - extramammary Paget's disease
GCDFP‐15 - gross cystic disease fluid protein 15
HMB45 - HMWK, high molecular weight keratin
LMWK - low molecular weight keratin
MAC - microcystic adnexal carcinoma
NSJ - nevus sebaceous of Jadassohn
PAS - periodic acid Schiff
PCMC - primary cutaneous mucinous carcinoma
PEA - papillary eccrine adenoma
SCAP - syringocystadenoma papilliferum
SMA - smooth muscle actin, TAA, tubular apocrine adenoma
References
- 1.Klein W, Chan E, Seykora J T. Tumors of the epidermal appendages. In: Elder DE (Editor‐in‐Chief). Lever's histopathology of the skin. 9th edn. Philadelphia, PA: Lippincott Williams & Wilikins, 2005867–926.
- 2.Weedon D. Tumors of cutaneous appendages. In: Weedon D. Skin pathology. 2nd adn. Edinburgh: Churchill Livingstone, 2002859–916.
- 3.Alsaad K O, Obaidat N A, Ghazarian D. Skin adnexal neoplasms ‐ part 1: an approach to tumours of the pilosebaceous unit. J Clin Pathol 200660129–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busam K J, Tan L K, Granter S R.et al Epidermal growth factor, estrogen, and progesterone receptor expression in primary sweat gland carcinomas and primary and metastatic mammary carcinomas. Mod Pathol 199912786–793. [PubMed] [Google Scholar]
- 5.Mirza I, Kloss R, Sieber S C. Malignant eccrine spiradenoma. Arch Pathol Lab Med 2002126591–594. [DOI] [PubMed] [Google Scholar]
- 6.Wallace M L, Longacre T A, Smoller B R. Estrogen and progesterone receptors and anti‐gross cystic disease fluid protein 15 (BRST‐2) fail to distinguish metastatic breast carcinoma from eccrine neoplasms. Mod Pathol 19958897–901. [PubMed] [Google Scholar]
- 7.Honma N, Takubo K, Akiyama F.et al Expression of GCDFP‐15 and AR decreases in larger or node‐positive apocrine carcinomas of the breast. Histopathology 200547195–201. [DOI] [PubMed] [Google Scholar]
- 8.van der Putte S C. Apoeccrine glands in nevus sebaceous. Am J Dermatopathol 19941623–30. [DOI] [PubMed] [Google Scholar]
- 9.Kamada A, Saga K, Jimbow K. Apoeccrine sweat duct obstruction as a cause for Fox‐Fordyce disease. J Am Acad Dermatol 200348453–455. [DOI] [PubMed] [Google Scholar]
- 10.Pfeifer J D, Barr R J, Wick M R. Ectopic breast tissue and breast‐like sweat gland metaplasias: an overlapping spectrum of lesions. J Cutan Pathol 199926190–196. [DOI] [PubMed] [Google Scholar]
- 11.van der Putte S C. Mammary‐like glands of the vulva and their disorders. Int J Gynecol Pathol 199413150–160. [DOI] [PubMed] [Google Scholar]
- 12.Abbott J J, Ahmed I. Adenocarcinoma of mammary‐like glands of the vulva: report of a case and review of the literature. Am J Dermatopathol 200628127–133. [DOI] [PubMed] [Google Scholar]
- 13.Nishie W, Sawamura D, Mayuzumi M.et al Hidradenoma papilliferum with mixed histopathologic features of syringocystadenoma papilliferum and anogenital mammary‐like glands. J Cutan Pathol 200431561–564. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y H, Wong T W, Lee J Y Y. Depigmented genital extramammary Paget's disease: a possible histogenetic link to Toker's clear cells and clear cell papulosis. J Cutan Pathol 200128105–108. [DOI] [PubMed] [Google Scholar]
- 15.Garcia‐Rio I, Perez‐Gala S, Fraga J.et al Eccrine squamous syringometaplasia in a patient with systemic lupus erythematosus. J Eur Acad Dermatol Venereol 200620608–610. [DOI] [PubMed] [Google Scholar]
- 16.Kakinuma H, Miyamoto R, Iwasawa U.et al Three subtypes of poroid neoplasia in a single lesion: eccrine poroma, hidroacanthoma simplex, and dermal duct tumor. Histologic, histochemical, and ultrastructural findings. Am J Dermatopathol 19941666–72. [DOI] [PubMed] [Google Scholar]
- 17.Wick M R, Swanson P E, Barnhill R L. Sweat gland tumors. In: Barnhill RL, ed. Textbook of dermatopathology. 2nd edn. New York: McGraw‐Hill, 2004745–784.
- 18.Yamamoto O, Hisaoka M, Yasuda H.et al Cytokeratin expression of apocrine and eccrine poromas with special reference to its expression in cuticular cells. J Cutan Pathol 200027367–373. [DOI] [PubMed] [Google Scholar]
- 19.Kamiya H, Oyama Z, Kitajima Y. “Apocrine” poroma: review of the literature and case report. J Cutan Pathol 200128101–104. [DOI] [PubMed] [Google Scholar]
- 20.Urso C, Bondi R, Paglierani M.et al Carcinomas of sweat glands: report of 60 cases. Arch Pathol Lab Med 2001125498–505. [DOI] [PubMed] [Google Scholar]
- 21.Robson A, Greene J, Ansari N.et al Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol 200125710–720. [DOI] [PubMed] [Google Scholar]
- 22.Maeda T, Mori H, Matsuo T.et al Malignant eccrine poroma with multiple visceral metastases: report of a case with autopsy findings. J Cutan Pathol 199623566–570. [DOI] [PubMed] [Google Scholar]
- 23.Crowson A N, Magro C M, Mihm M C. Malignant adnexal neoplasms. Mod Pathol 200619(Suppl 2)S93–126. [DOI] [PubMed] [Google Scholar]
- 24.Patterson J W, Wick M R. Neoplasms and pseudoneoplastic proliferations of the sweat glands. In: Silverbeg SG, Sobin LH, eds. AFIP Atlas of tumor pathology. Non‐melanocytic tumors of the skin. Washington, DC: Armed Forces Institute of Pathology, 2006137–199.
- 25.Helwig E B. Eccrine acrospiroma. J Cutan Pathol 198411415–420. [DOI] [PubMed] [Google Scholar]
- 26.Headington J T, Niederhuber J E, Beals T F. Malignant clear cell acrospiroma. Cancer 197841641–647. [DOI] [PubMed] [Google Scholar]
- 27.Gortler I, Koppl H, Stark G B.et al Metastatic malignant acrospiroma of the hand. Eur J Surg Oncol 200127431–435. [DOI] [PubMed] [Google Scholar]
- 28.Soler‐Carrillo J, Estrach T, Mascaro J M. Eruptive syringoma: 27 new cases and review of the literature. J Eur Acad Dermatol Venereol 200115242–246. [DOI] [PubMed] [Google Scholar]
- 29.Guitart J, Rosenbaum M M, Requena L. “Eruptive syringoma”: a misnomer for a reactive eccrine gland ductal proliferation? J Cutan Pathol 200330202–205. [DOI] [PubMed] [Google Scholar]
- 30.Patrizi A, Neri I, Marzaduri S.et al Syringoma: a review of twenty‐nine cases. Acta Dermatol Venereol 199878460–462. [DOI] [PubMed] [Google Scholar]
- 31.Ohnishi T, Watanabe S. Immunohistochemical analysis of keratin expression in clear cell syringoma. A comparative study with conventional syringoma. J Cutan Pathol 199724370–376. [DOI] [PubMed] [Google Scholar]
- 32.Ohnishi T, Kaneko S, Egi M.et al Syringoid eccrine carcinoma: report of a case with immunohistochemical analysis of cytokeratin expression. Am J Dermatopathol 200224409–413. [DOI] [PubMed] [Google Scholar]
- 33.Storm C A, Seykora J T. Cutaneous adnexal neoplasms. Am J Clin Pathol 2002118533–549. [DOI] [PubMed] [Google Scholar]
- 34.Satter E K, Graham B S. Chondroid syringoma. Cutis 20037149–52. [PubMed] [Google Scholar]
- 35.Yamamoto O, Yasuda H. An immunohistochemical study of the apocrine type of cutaneous mixed tumors with special reference to their follicular and sebaceous differentiation. J Cutan Pathol 199926232–241. [DOI] [PubMed] [Google Scholar]
- 36.Nather A, Sutherland I H. Malignant transformation of a benign cutaneous mixed tumour. J Hand Surg [Br] 198611139–143. [DOI] [PubMed] [Google Scholar]
- 37.Metzler G, Schaumburg‐Lever G, Hornstein O.et al Malignant chondroid syringoma: immunohistopathology. Am J Dermatopathol 19961883–89. [DOI] [PubMed] [Google Scholar]
- 38.Bates A W, Baithun S I. Atypical mixed tumor of the skin: histologic, immunohistochemical, and ultrastructural features in three cases and a review of the criteria for malignancy. Am J Dermatopathol 19982035–40. [DOI] [PubMed] [Google Scholar]
- 39.Takeda H, Mitsuhashi Y, Hayashi M.et al Eccrine syringofibroadenoma: case report and review of the literature. J Eur Acad Dermatol Venereol 200015147–149. [DOI] [PubMed] [Google Scholar]
- 40.Fouilloux B, Perrin C, Dutoit M.et al Clear cell syringofibroadenoma (of Mascaro) of the nail. Br J Dermatol 2001144625–627. [DOI] [PubMed] [Google Scholar]
- 41.Uede K, Yamamoto Y, Furukawa F. Brooke‐Spiegler syndrome associated with cylindroma, trichoepithelioma, spiradenoma and syringoma. J Dermatol 20043132–38. [DOI] [PubMed] [Google Scholar]
- 42.Michal M. Spiradenoma associated with apocrine adenoma component. Pathol Res Pract 19961921135–1139. [DOI] [PubMed] [Google Scholar]
- 43.Granter S R, Seeger K, Calonje E.et al Malignant eccrine spiradenoma (spiradenocarcinoma): a clinicopathologic study of 12 cases. Am J Dermatopathol 20002297–103. [DOI] [PubMed] [Google Scholar]
- 44.Leonard N, Chaggar R, Jones C.et al Loss of heterozygosity at cylindromatosis gene locus, CYLD, in sporadic skin adnexal tumours. J Clin Pathol 200154689–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Penneys N S, Kaiser M. Cylindroma expresses immunohistochemical markers linking to eccrine coil. J Cutan Pathol 19932040–43. [DOI] [PubMed] [Google Scholar]
- 46.Tellechea O, Reis J P, Marques C.et al Tubular apocrine adenoma with eccrine and apocrine immunophenotypes or papillary tubular adenoma? Am J Dermatopathol 199517499–505. [DOI] [PubMed] [Google Scholar]
- 47.Michal M, Lamovec J, Mukensnabl P.et al Spiradenocylindromas of the skin: tumors with morphological features of spiradenoma and cylindroma in the same lesion: report of 12 cases. Pathol Int 199949419–425. [DOI] [PubMed] [Google Scholar]
- 48.Meybehm M, Fischer H P. Spiradenoma and dermal cylindroma: comparative immunohistochemical analysis and histogenetic considerations. Am J Dermatopathol 199820315–317. [DOI] [PubMed] [Google Scholar]
- 49.Gerretsen A L, van der Putte S C, Deenstra W.et al Cutaneous cylindroma with malignant transformation. Cancer 1993721618–1623. [DOI] [PubMed] [Google Scholar]
- 50.Durani B K, Kurzen H, Jaeckel A.et al Malignant transformation of multiple dermal cylindromas. Br J Dermatol 2001145653–656. [DOI] [PubMed] [Google Scholar]
- 51.Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceous: a study of 596 cases. J Am Acad Dermatol 200042263–268. [DOI] [PubMed] [Google Scholar]
- 52.Coyne J D, Fitzgibbon J F. Mixed syringocystadenoma papilliferum and papillary eccrine adenoma occurring in a scrotal condyloma. J Cutan Pathol 200027199–201. [DOI] [PubMed] [Google Scholar]
- 53.Ansai S, Watanabe S, Aso K. A case of tubular apocrine adenoma with syringocystadenoma papilliferum. J Cutan Pathol 198916230–236. [DOI] [PubMed] [Google Scholar]
- 54.Boni R, Xin H, Hohl D.et al Syringocystadenoma papilliferum: a study of potential tumor suppressor genes. Am J Dermatopathol 20012387–89. [DOI] [PubMed] [Google Scholar]
- 55.Vanatta P R, Bangert J L, Freeman R G. Syringocystadenoma papilliferum. A plasmacytoid tumor. Am J Surg Pathol 19859678–683. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto O, Doi Y, Hamada T.et al An immunohistochemical and ultrastructural study of syringocystadenoma papilliferum. Br J Dermatol 2002147936–945. [DOI] [PubMed] [Google Scholar]
- 57.Ishida‐Yamamoto A, Sato K, Wada T.et al Syringocystadenocarcinoma papilliferum: case report and immunochemical comparison with its benign counterpart. J Am Acad Dermatol 200145755–759. [DOI] [PubMed] [Google Scholar]
- 58.Loane J, Kealy W F, Mulcahy G. Perianal hidradenoma papilliferum occurring in a male: a case report. Irish J Med Sci 199816726–27. [DOI] [PubMed] [Google Scholar]
- 59.Vang R, Cohen P R. Ectopic hidradenoma papilliferum: a case report and review of the literature. J Am Acad Dermatol 199941115–118. [DOI] [PubMed] [Google Scholar]
- 60.Handa Y, Yamanaka N, Inagaki H.et al Large ulcerated perianal hidradenoma papilliferum in a young female. Dermatol Surg 200329790–792. [DOI] [PubMed] [Google Scholar]
- 61.Kazakov D V, Mikyskova I, Kutzner H.et al Hidradenoma papilliferum with oxyphilic metaplasia: a clinicopathological study of 18 cases, including detection of human papillomavirus. Am J Dermatopathol 200527102–110. [DOI] [PubMed] [Google Scholar]
- 62.Pelosi G, Martignoni G, Bonetti F. Intraductal carcinoma of mammary‐type apocrine epithelium arising within a papillary hydradenoma of the vulva. Report of a case and review of the literature. Arch Pathol Lab Med 19911151249–1254. [PubMed] [Google Scholar]
- 63.Tamaki K, Furue M, Matsukawa A.et al Presence and distribution of carcinoembryonic antigen and lectin‐binding sites in benign apocrine sweat gland tumours. Br J Dermatol 1985113565–571. [DOI] [PubMed] [Google Scholar]
- 64.Mazoujian G, Margolis R. Immunohistochemistry of gross cystic disease fluid protein (GCDFP‐15) in 65 benign sweat gland tumors of the skin. Am J Dermatopathol 19881028–35. [DOI] [PubMed] [Google Scholar]
- 65.Ansai S, Koseki S, Hozumi Y.et al An immunohistochemical study of lysosome, Cd‐15 (Leu M1), and gross cystic disease fluid protein‐15 in various skin tumors. Assessment of the specifity and sensitivity of markers of apocrine differentiation. Am J Dermatopathol 199517249–255. [DOI] [PubMed] [Google Scholar]
- 66.Offidani A, Campanati A. Papillary hidradenoma: immunohistochemical analysis of steroid receptor profile with a focus on apocrine differentiation. J Clin Pathol 199952829–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Higgins C M, Strutton G M. Papillary apocrine fibroadenoma of the vulva. J Cutan Pathol 199724256–260. [DOI] [PubMed] [Google Scholar]
- 68.Assor D, Davis J B. Multiple apocrine fibroadenomas of the anal skin. Am J Clin Pathol 197768397–399. [DOI] [PubMed] [Google Scholar]
- 69.Donati P, Amantea A. Adenoma of anogenital mammary‐like glands. Am J Dermatopathol 19961873–76. [DOI] [PubMed] [Google Scholar]
- 70.Falck V G, Jordaan H F. Papillary eccrine adenoma. A tubulopapillary hidradenoma with eccrine differentiation. Am J Dermatopathol 1986864–72. [DOI] [PubMed] [Google Scholar]
- 71.Fox S B, Cotton D W. Tubular apocrine adenoma and papillary eccrine adenoma. Entities or unity? Am J Dermatopathol 199214149–154. [DOI] [PubMed] [Google Scholar]
- 72.Megahed M, Holzle E. Papillary eccrine adenoma. A case report with immunohistochemical examination. Am J Dermatopathol 199315150–155. [DOI] [PubMed] [Google Scholar]
- 73.Ishiko A, Shimizu H, Inamoto N, Nakmura K. Is tubular apocrine adenoma a distinct clinical entity? Am J Dermatopathol 199315482–487. [DOI] [PubMed] [Google Scholar]
- 74.Pujol R M, LeBoit P E, Su W P. Microcystic adnexal carcinoma with extensive sebaceous differentiation. Am J Dermatopathol 199719358–362. [DOI] [PubMed] [Google Scholar]
- 75.Lei J Y, Wang Y, Jaffe E S.et al Microcystic adnexal carcinoma associated with primary immunodeficiency, recurrent diffuse herpes simplex virus infection, and cutaneous T‐cell lymphoma. Am J Dermatopathol 200022524–529. [DOI] [PubMed] [Google Scholar]
- 76.Bogner P N, Su L D, Fullen D R. Cluster designation 5 staining of normal and non‐lymphoid neoplastic skin. J Cutan Pathol 20053250–54. [DOI] [PubMed] [Google Scholar]
- 77.Ban M, Sugie S, Kamiya H.et al Microcystic adnexal carcinoma with lymph node metastasis. Dermatology 2003207395–397. [DOI] [PubMed] [Google Scholar]
- 78.Fischer S, Breuninger H, Metzler G.et al Microcystic adnexal carcinoma: an often misdiagnosed, locally aggressive growing skin tumor. J Craniofac Surg 20051653–58. [DOI] [PubMed] [Google Scholar]
- 79.Kazakov D V, Suster S, LeBoit P E.et al Mucinous carcinoma of the skin, primary, and secondary: a clinicopathologic study of 63 cases with emphasis on the morphologic spectrum of primary cutaneous forms: homologies with mucinous lesions in the breast. Am J Surg Pathol 200529764–782. [DOI] [PubMed] [Google Scholar]
- 80.Breier F, Clabian M, Pokieser W.et al Primary mucinous carcinoma of the scalp. Dermatology 2000200250–253. [DOI] [PubMed] [Google Scholar]
- 81.Hanby A M, McKee P, Jeffery M.et al Primary mucinous carcinomas of the skin express TFF1, TFF3, estrogen receptors and progesterone receptors. Am J Surg Pathol 1998221125–1131. [DOI] [PubMed] [Google Scholar]
- 82.van der Kwast T H, Vuzevski V D, Ramaekers F.et al Primary cutaneous adenoid cystic carcinoma: case report, immunohistochemistry, and review of the literature. Br J Dermatol 1988118567–577. [DOI] [PubMed] [Google Scholar]
- 83.Chu S S, Chang Y L, Lou P J. Primary cutaneous adenoid cystic carcinoma with regional lymph node metastasis. J Laryngol Otol 2001115673–675. [DOI] [PubMed] [Google Scholar]
- 84.Bergman R, Lichtig C, Moscona R A.et al A comparative immunohistochemical study of adenoid cystic carcinoma of the skin and salivary glands. Am J Dermatopathol 199113162–168. [DOI] [PubMed] [Google Scholar]
- 85.Mino M, Pilch B Z, Faquin W C. Expression of KIT (CD117) in neoplasms of the head and neck: an ancillary marker for adenoid cystic carcinoma. Mod Pathol 2003161224–1231. [DOI] [PubMed] [Google Scholar]
- 86.Kao G F, Helwig E B, Graham J H. Aggressive digital papillary adenoma and adenocarcinoma. A clinicopathological study of 57 patients, with histochemical, immunopathological, and ultrastructural observations. J Cutan Pathol 198714129–146. [DOI] [PubMed] [Google Scholar]
- 87.Duke W H, Sherrod T T, Lupton G P. Aggressive digital papillary adenocarcinoma (aggressive digital papillary adenoma and adenocarcinoma revisited). Am J Surg Pathol 200024775–784. [DOI] [PubMed] [Google Scholar]
- 88.Jih D M, Elenitsas R, Vittorio C C.et al Aggressive digital papillary adenocarcinoma: a case report and review of the literature. Am J Dermatopathol 200123154–157. [DOI] [PubMed] [Google Scholar]
- 89.Malafa M P, McKesey P, Stone S.et al Sentinel node biopsy for staging of aggressive digital papillary adenocarcinoma. Dermatol Surg 200026580–583. [DOI] [PubMed] [Google Scholar]
- 90.Chamberlain R S, Huber K, White J C.et al Apocrine gland carcinoma of the axilla. Review of the literature and recommendations for treatment. Am J Clin Oncol 199922131–135. [DOI] [PubMed] [Google Scholar]
- 91.Miyamoto T, Hagari Y, Inoue S.et al Axillary apocrine carcinoma with benign apocrine tumours: a case report involving a pathological and immunohistochemical study and review of the literature. J Clin Pathol 200558757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paties C, Taccagni G L, Papotti M.et al Apocrine carcinoma of the skin. A clinicopathologic, immunocytochemical, and ultrastructural study. Cancer. 1993;15 71375–381. [DOI] [PubMed] [Google Scholar]
- 93.Dalles S, Skowron F, Balme B.et al Apocrine carcinoma developed in nevus sebaceus of Jadassohn. Eur J Dermatol 200313487–489. [PubMed] [Google Scholar]
- 94.MacNeill K N, Riddell R H, Ghazarian D. Perianal apocrine adenocarcinoma arising in a benign apocrine adenoma; first case report and review of the literature. J Clin Pathol 200558217–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Obaidat N A, Awamleh A A, Ghazarian D M. Adenocarcinoma in situ arising in a tubulopapillary apocrine hidradenoma of the peri‐anal region: a report of the first case. Eur J Dermatol 200616576–578. [PubMed] [Google Scholar]
- 96.Willman J H, Golitz L E, Fitzpatrick J E. Vulvar clear cells of Toker: precursors of extramammary Paget's disease. Am J Dermatopathol 200527185–188. [DOI] [PubMed] [Google Scholar]
- 97.Diaz de Leon E, Carcangiu M L, Prieto V G.et al Extramammary Paget disease is characterized by the consistent lack of estrogen and progesterone receptors but frequently expresses androgen receptor. Am J Clin Pathol 2000113572–575. [DOI] [PubMed] [Google Scholar]
- 98.Liegl B, Horn L C, Moinfar F. Androgen receptors are frequently expressed in mammary and extramammary Paget's disease. Mod Pathol 2005181283–1288. [DOI] [PubMed] [Google Scholar]
- 99.Goldblum J R, Hart W R. Vulvar Paget's disease: a clinicopathologic and immunohistochemical study of 19 cases. Am J Surg Pathol 1997211178–1187. [DOI] [PubMed] [Google Scholar]
- 100.McKee P H, Hertogs K T. Endocervical adenocarcinoma and vulval Paget's disease: a significant association. Br J Dermatol 1980103443–448. [DOI] [PubMed] [Google Scholar]
- 101.Battles O E, Page D L, Johnson J E. Cytokeratins, CEA, and mucin histochemistry in the diagnosis and characterization of extramammary Paget's disease. Am J Clin Pathol 19971086–12. [PubMed] [Google Scholar]
- 102.Allan S J, McLaren K, Aldridge R D. Paget's disease of the scrotum: a case exhibiting positive prostate‐specific antigen staining and associated prostatic adenocarcinoma. Br J Dermatol 1998138689–691. [DOI] [PubMed] [Google Scholar]
- 103.Wilkinson E J, Brown H M. Vulvar Paget disease of urothelial origin: a report of three cases and a proposed classification of vulvar Paget disease. Hum Pathol 200233549–554. [DOI] [PubMed] [Google Scholar]
- 104.Rayne S C, Santa Cruz D J. Anaplastic Paget's disease. Am J Surg Pathol 1992161085–1091. [DOI] [PubMed] [Google Scholar]
- 105.Plumb S J, Argenyi Z B, Stone M S.et al Cytokeratin 5/6 immunostaining in cutaneous adnexal neoplasms and metastatic adenocarcinoma. Am J Dermatopathol 200426447–451. [DOI] [PubMed] [Google Scholar]
- 106.Apisarnthanarax P, Bovenmyer D A, Mehregan A H. Combined adnexal tumor of the skin. Arch Dermatol 1984120231–233. [PubMed] [Google Scholar]
- 107.Poomeechaiwong S, Bonelli J E, Golitz L E. Mixed tumor of the pilosebaceous type: mixed tumor of the skin with apocrine, follicular and sebaceous differentiation. J Cutan Pathol 19881533–38. [Google Scholar]
- 108.Sanchez Yus E, Requena L, Simon P.et al Complex adnexal tumor of the primary epithelial germ with distinct patterns of superficial epithelioma with sebaceous differentiation, immature trichoepithelioma, and apocrine adenocarcinoma. Am J Dermatopathol 199214245–252. [DOI] [PubMed] [Google Scholar]
- 109.Wong T Y, Suster S, Cheek R F.et al Benign cutaneous adnexal tumors with combined folliculosebaceous, apocrine, and eccrine differentiation: clinicopathologic and immunohistochemical study of eight cases. Am J Dermatopathol 199618124–136. [DOI] [PubMed] [Google Scholar]
- 110.Obaidat N A, Ghazarian D M. A ‘proliferating' composite adnexal tumour of the penis: report of the first case. J Clin Pathol. 2006. In press [DOI] [PMC free article] [PubMed]