Abstract
Chromosomal abnormalities and genomic instability are common features of, and possible driving forces in, tumorigenesis. Recently, several mitotic proteins that are critical to proper chromosome segregation have been identified. Members of the Aurora kinase family have been identified as having important roles in mitosis; overexpression induces multicellularity and fosters polyploidy. As aneuploidy is a common feature of malignant gliomas, particularly glioblastomas (GBMs), we examined 25 prospectively collected GBMs to assess the role that overexpression of one member of this family, Aurora B, might have in the clinical behaviour of GBMs. Aurora B expression levels were markedly correlated with a shortened survival. Aurora B expression was not directly related to age, tumour proliferation status or to several common molecular changes found in GBMs. These results suggest that Aurora B may be a prognostic feature of impaired survival and a novel therapeutic target in some patients.
Aneuploidy is a common feature of cancer cells, including malignant gliomas.1,2 Aurora kinases, which play critical parts in chromosome condensation and segregation, spindle assembly and mitotic checkpoint status, and cytokinesis and cell division, have recently been implicated in oncogenesis.3,4,5 Dysregulation of Aurora kinases is thought to cause mis‐segregation of individual chromosomes or polyploidisation, accompanied by centrosome amplification, leading to aneuploidy.3,4,5,6
In mammals, three related serine/threonine kinases have been characterised: Auroras A, B and C.3,4,5,6 Although all three possess similar structures, with variable amino terminal regulatory domains but a conserved carboxyl terminal catalytic domain, their expression patterns, subcellular localisations and activities differ markedly. Whereas Aurora C is expressed principally in the testis and localises to the spindle poles late in mitosis, Aurora A is essentially ubiquitous and localises to centrosomes during interphase and to spindle poles during early mitosis.3,4,5,6
Aurora B (ARUKB; OMIM entry 604970), located on chromosome 17p13.1, reaches maximum levels later in mitosis, regulates chromosome condensation and cohesion, bipolar chromosome attachment and coordinates chromosome segregation (http://www.ncbi.nlm.nih.gov).4 Aurora B forms a tight complex with inner centrosome protein and survivin; inactivation of any of these proteins causes similar defects in chromosome segregation, which, in tumour cells, leads to aneuploidy.1,4,6
Although recent studies have shown that Aurora A is both a cancer susceptibility gene and an oncogene, with raised levels in several solid epithelial cancers, including glioblastoma (GBM), there is still little evidence to link Aurora B directly to cancer.3,4,5,7,8,9 As changes along chromosome 17p are common in GBMs, we tested the hypothesis that overexpression of Aurora B may contribute to the aggressive behaviour of some GBMs.2
Materials and methods
Clinical and research samples
Tumours and clinical data were collected as part of an institutional review board‐approved study (table 1). Tissues from patients with newly diagnosed, previously untreated GBM who were short‐term survivors (STS) or long‐term survivors (LTS) were collected for immunohistochemistry (IHC) and fluorescence in situ hybridisation (FISH). Assays of p53 and Ki‐67 expression levels by IHC and EGFR amplification and overexpression by FISH and IHC were performed in the Pathology Department, Cleveland Clinic Foundation, Cleveland, Ohio, USA, and results were obtained from the pathology report. Malignant gliomas, HeLa cells (a known positive control for high‐level Aurora B expression3,4), and normal human astrocytes (Clonetics) were cultured on chamber slides (Nalge Nunc, Rochester, New York, USA) for FISH or in 75 cm2 flasks for western blotting, until 60–80% confluent.
Table 1 Clinical characteristics, molecular markers and Aurora B staining.
| Patient | Age (years), sex | Survival (months) | Treatment | p53 IHC (%) | EGFR FISH | Ki‐67 (%) | Aurora B IHC |
|---|---|---|---|---|---|---|---|
| STS1 | 64, F | 9.0 | OR, XRT, C | ND | NA | 18 | 1+ |
| STS2 | 56, M | 8.7 | OR, XRT, C×2 | ND | NA | N/D | 3+ |
| STS3 | 46, F | 8.4 | OR, XRT, C×2 | <10 | NA | 12 | 1+ |
| STS4 | 22, M | 8.7 | OR, XRT, C×3 | 75–100 | NA | 14 | – |
| STS5 | 63, M | 3.8 | OR, XRT, C | 0 | A | 16 | 1+ |
| STS6 | 64, M | 4.1 | OR, XRT, C | <10 | NA | 4 | – |
| STS7 | 59, F | 3.6 | OR, XRT | <10 | NA | 13 | 2+ |
| STS8 | 54, F | 7.7 | OR, XRT, C×2 | 75–100 | NA | 30 | 3+ |
| STS9 | 46, M | 6.1 | OR, XRT, C×2 | <10 | NA | 4 | – |
| STS10 | 48, F | 8.5 | OR, XRT, C, Bx, C | 1–2 | NA | 17.5 | 2+ |
| STS11 | 49, M | 7.1 | OR, XRT×2, C×2 | 100 | NA | 14 | 3+ |
| STS12 | 58, M | 5.1 | OR, XRT, C | 75–100 | NA | 6.5 | 3+ |
| Mean (SD) | 52.4 (11.7)† | 6.8 (2.1)* | 12.6 (8.1)‡ | ||||
| LTS1 | 58, M | 26 | OR, XRT, C×2 | 75–100 | A | 14.5 | – |
| LTS2 | 54, F | >24 | OR, XRT, C | <10 | A | 11.5 | Trace |
| LTS3 | 29, M | 29 | OR, XRT, C×2 | 75–100 | NA | 50 | – |
| LTS4 | 39, M | >24 | OR, XRT, C×2 | 25–50 | NA | 30 | Trace |
| LTS5 | 59, M | 27 | OR, XRT, C | <10 | A | 5 | – |
| LTS6 | 40, M | 37 | OR, XRT, C×2 | <25 | A | 12.5 | – |
| LTS7 | 50, M | >24 | OR, XRT, C | <10 | NA | 1.5 | – |
| LTS8 | 62, M | >26 | OR, XRT, C×2 | 75–100 | NA | 20 | – |
| LTS9 | 49, M | >24 | OR, XRT, C×2 | 75–100 | A | 15.5 | 3+ |
| LTS10 | 48, M | >32 | OR, XRT, C | 75–100 | A | 12 | – |
| LTS11 | 56, F | >26 | OR, XRT, C | <10 | NA | 9.5 | – |
| LTS12 | 51, M | >34 | OR, XRT, C×3 | <10 | A | 5 | – |
| LTS13 | 64, F | >27 | OR, XRT, C | <10 | NA | 15 | – |
| #Mean (SD) | 50.7 (10)† | >27.7 (4)* | 15.5 (12.6)‡ |
A, amplified and overexpressed; Bx, biopsy; C, chemotherapy regimen; EGFR, epidermal growth factor receptor; F, female; FISH, fluorescence in situ hybridisation; IHC, immunohistochemistry; Ki‐67%: Ki‐67 is a marker of cell proliferation in paraffin‐embedded tissues, with % of tumor cells that are stained by antibody to Ki‐67; LTS, long‐term survivor; M, male; NA, not amplified or overexpressed; ND, not determined; OR, gross total (100%) or near total (>95%) resection; p53 IHC %, the percentage of tumour cells that are stained by antibody to Tp53; STS, short‐term survivor; survival >, patient still alive; XRT, therapeutic radiotherapy; × followed by a number represents the number of radiotherapy or separate chemotherapy regimens administered or intended for treatment; −, no cells; 1+, <25% scattered cells; 2+, 25–50% of tumour cells; 3+, >50% cells, with heavy, diffuse staining.
STS v LTS (one‐sided Student's t test): *p<0.001 for survival.
†, age comparison, p = 0.34.
‡, Ki‐67 staining comparison, p = 0.76.
Immunohistochemistry, immunofluorescence11
Paraffin‐wax‐embedded tissue slides were stained with anti‐Aurora B rabbit anti‐human antibody (1:50 dilution; Zymed, South San Francisco, California, USA) at 4°C overnight. Detection was performed using an LSAB Kit (Dako, Carpinteria, California, USA) for tissue sections and Alexa fluor 594 goat‐anti‐rabbit IgG (Molecular Probes, Eugene, Oregon, USA) for cell lines. Chamber slides were mounted with 4′,6‐diamidino‐2‐phenylindole antifade medium (Vectashield; Vector Laboratories, California, USA). IHC staining was considered to be positive if signals were seen in either the nucleus or cytoplasm, and the grading was characterised as 0, absent or trace (rare cells, <5%); 1+, <25% scattered cells; 2+, 25–50% of tumour cells; 3+, >50%, with heavy, diffuse staining.
Western blotting11
A total of 5 μg of protein was separated on NuPAGE 10% bis‐TRIS gel (Invitrogen, San Diego, California, USA). Nitrocellulose membranes were incubated with mouse‐anti‐GAPDH (1:2000; Ambion, Austin, Texas, USA) or anti‐Aurora B antibodies (1:250), incubated with secondary, goat‐anti‐mouse–horseradish peroxidase antibodies (1:2000; Pierce, Rockford, Illinois, USA) and incubated in SuperSignal West Pico (Pierce). Signal was detected on BioMax MS film (Kodak).
Fluorescence in situ hybridisation12
Dual‐labelling hybridisation with hybridisation mix of Aurora B probe and directly labelled Spectrum Green‐labelled centromeric Chromosome 17 probe (Vysis, Des Plaines, Illinois, USA) was performed. Aurora B probe (BAC clone CTD‐2604F21; Invitrogen) was labelled with digoxygenin‐11‐dUTP by nick translation (nick translation kit, Roche). Target slides were denatured in 2× standard saline citrate/70% formamide, pH 7, at 75°C for 5 min and dehydrated in graded ethanol. Probes were denatured at 75°C for 5 min and applied to the target slides. Hybridisation was performed overnight at 37°C. After posthybridisation washes, Aurora B probe was detected by anti‐digoxigenin–rhodamine Fab fragments (Roche). Slides were counterstained with 4′,6‐diamidino‐2‐phenylindole antifade medium. The number of FISH signals was counted with an Olympus microscope equipped with a triple band pass filter. A minimum of 100 nuclei were evaluated. The criteria for Aurora B gene amplification was more Aurora B signals than chromosome 17 centromere (CEP 17) signals in >30% of cells and an Aurora B:CEP 17 ratio ⩾1.2.12
Results
Firstly, we performed IHC on unselected GBMs. About 32% (9 of 28) of the tumours on a retrospectively collected GBM tissue microarray stained 1+ to 3+ for Aurora B. Although detailed clinical information was incomplete, higher expression was seen in patients who tended to have a poorer outcome. To examine this issue directly, we prospectively collected 25 cases of GBM, for which detailed information was available: 12 were patients with survival of ⩽9 months (STS) and 13 were LTS (⩾24 months). All patients had undergone gross total or near‐total (>95%) resection, followed by radiation; all but one was treated with at least one full cycle of chemotherapy. Table 1 summarises the clinicopathological characteristics of patients.
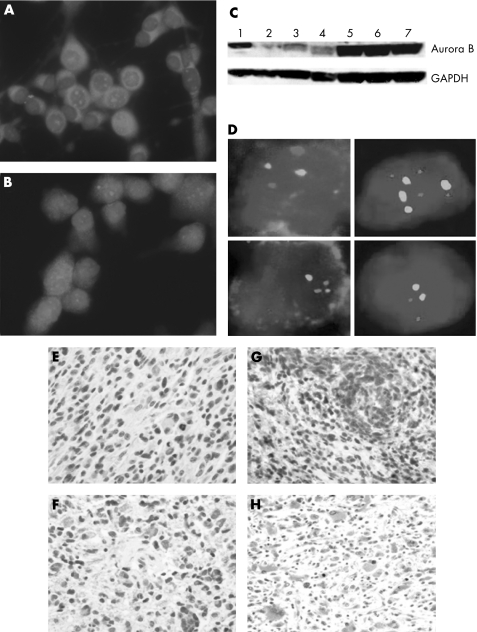
In all, 9 of 12 (75%) tumours expressed Aurora B (1+ to 3+) in STS, whereas only 3 of 13 (23.1%) LTS tumours expressed Aurora B (p<0.01, Student's one‐sided t test). Of the LTS tumours, only 1 (7.7%) had more than trace immunoreactivity (p<0.001). Overexpression of Aurora B, as documented by immunofluorescence, western blotting and FISH, was unrelated to gene amplification (fig 1A–D). Although U251 and U373 cells expressed high levels of Aurora B by immunofluorescence and western blotting compared with normal human astrocytes (expected to have low or no expression as these cells rarely divide) and HeLa (a positive control), only U373 showed more than two copies of AURKB (fig 1C, D; data not shown). Similar results were found in the GBMs (fig 1E–H; data not shown). Finally, Aurora B expression did not correlate with p53 expression, EGFR amplification, or Ki‐67 staining (table 1).
Figure 1 Aurora B expression and amplification status in malignant gliomas cell lines and human tumors. (A, B) Immunofluorescence staining of U251 and U373, respectively, before reaching confluence, showing a predominantly nuclear staining pattern. Some cytoplasmic staining is also seen (Aurora B, red signal; 4′,6‐diamidino‐2‐phenylindole‐stained nuclei are blue). (C) Western blotting of Aurora B expression (top) and GAPDH control (bottom). Lane 1, HeLa cells (positive control); lane 2, normal human astrocytes; lanes 3 and 4, CCF‐52 and A172 (malignant gliomas cell lines expressing low levels of Aurora B); lanes 5, 6 and 7, U251, U373 and GB1, respectively (malignant gliomas cell lines that express high levels of Aurora B). (D) Fluorescence in situ hybridisation results. Left panels, top and bottom, short‐term survivor (STS) glioblastomas (GBMs) with and without amplification of aurora B (red signal; centromeric probe green); right panels, U373 (top) and U251 (bottom) with four and two copies of aurora B, respectively. (E–H), Immunohistochemical staining of tumour‐rich areas of GBMs: (E) GBM (LTS) without immunoreactivity (0); (F) GBM (STS) with 1+ staining; (G) GBM (STS) with 2+ staining; and (H) GBM (STS) with 3+ staining. Staining is predominantly nuclear, although some cytoplasmic staining is seen, which is likely to be a function of having a mixture of dividing and non‐dividing tumour cells, the ratio of which does not seem to correlate exactly with Ki‐67 levels, as shown in table 1. (E–G) 400× magnification; (H) 200× magnification.
Discussion
Auroras are a family of serine/threonine kinases involved in centrosome separation and maturation, mitotic spindle assembly and stability, chromosome condensation and segregation, and cytokinesis (http://www.ncbi.nlm.nih.gov).3 Three of these mitotic protein kinases—Auroras A, B and C—have been described. All have been found to be overexpressed in various types of cancer, in which aneuploid cells containing multiple centrosomes are seen.1,3,4,5,6
Unlike Aurora A, which is amplified in many cancers, including some malignant gliomas, and which can act as both a tumour susceptibility gene and an oncogene, the role of Aurora B in tumorigenesis and cancer progression remains to be clarified.2,3,4,5,7,9 Although some tumours, such as anaplastic or undifferentiated thyroid carcinomas and seminomas, overexpress Aurora B, it is inconsistently amplified.13,14,15,16 Furthermore, on its own, Aurora B does not transform NIH3T3 cells or cause tumours in nude mice, except in cases of Tp53 deficiency.3,4,5,13,14,15,16
GBM is the most common and lethal brain tumour in adults, with a median survival time of about 1 year, despite aggressive treatment modalities.2 However, as a range of survival times exists around this median, investigating different genetic aberrations and expression patterns in groups at the extremes of survival may provide insight into the molecular pathogenesis of these tumours and identify prognostic markers or novel targets for molecular treatment.2,10 Two small studies have looked at Aurora B expression, in just over 10 patients with GBM, with varied levels of pretreatment before tissue collection and without detailed examination of outcome, amplification status or relevant molecular data.8,9
We found that Aurora B overexpression was common in malignant glioma cell lines but was not necessarily the result of gene amplification. In our series of newly diagnosed GBM, Aurora B expression was a potent predictor of poor outcome, as 75% of STS had 1+ to 3+ levels by IHC, whereas only 7% of LTS did so (p<0.001). Gene amplification was not necessary. Interestingly, Aurora B expression did not correlate with p53 expression status, EGFR amplification or Ki‐67 levels.
Although our study is preliminary, it suggests that Aurora B may distinguish potential LTS from STS and may point the way to novel therapeutics for patients with Aurora B overexpression.3,4,5 Recent work has shown that RNA interference with Aurora B and small molecule inhibition of multiple Auroras have anti‐proliferative effects and cause regression in animal models of non‐central nervous system cancers.3,4,14 With respect to small molecule inhibitors, such as hesperidin, ZM447439 and VX‐680, more than one and in some cases all three Aurora kinases (A, B and C) are inhibited at physiologically relevant doses, although both hesperidin and ZM447439 yield cellular phenotypes principally similar to inhibition of Aurora B alone by RNA interference, which results in catastrophic and fatal mitosis. These findings, in several different cancer cell lines and implanted animal models of cancer, suggest that Aurora B inhibition may play a more dominant part in cancer treatment than blockade of the other Aurora kinases.14,17 We are currently investigating our findings in a larger, prospective series of patients with GBM to assess its ability to predict time to recurrence and overall survival.
Abbreviations
FISH - fluorescence in situ hybridisation
GBM - glioblastoma
IHC - immunohistochemistry
LTS - long‐term survivor
STS - short‐term survivor
Footnotes
Funding: This research was supported by the Melvin Burkhardt chair in neurosurgical oncology and the Karen Colina Wilson research endowment in the Brain Tumor Institute at the Cleveland Clinic Foundation.
Competing interests: None.
References
- 1.Rajagopalan H, Lenguaer C. Aneuploidy and cancer. Nature 2004432338–341. [DOI] [PubMed] [Google Scholar]
- 2.Kleihues P, Cavenee W K. eds. Pathology and genetics of tumours of the central nervous system (World Health Organization classification of tumours) 2nd edn. Lyon: IARC, 2000
- 3.Meralidi P, Honda R, Nigg E A. Aurora kinases link chromosome segregation and cell division to cancer susceptibility. Curr Opin Genet Dev 20041429–36. [DOI] [PubMed] [Google Scholar]
- 4.Giet R, Petretti C, Prigent C. Aurora kinases, aneuploidy and cancer: a coincidence or a real link? Trends Cell Biol 200515241–247. [DOI] [PubMed] [Google Scholar]
- 5.Ewart‐Toland A, Briassouli P, deKoning J P.et al Identification of stk6/STK15 as a candidate low penetrance tumor‐susceptibility gene in mouse and human. Nat Genet 200334403–412. [DOI] [PubMed] [Google Scholar]
- 6.Glotzer M. The molecular requirements for cytokinesis. Science 20053071735–1739. [DOI] [PubMed] [Google Scholar]
- 7.Klein A, Reichardt W, Jung V.et al Overexpression and amplification of STK15 in human gliomas. Int J Oncol 2004251789–1794. [PubMed] [Google Scholar]
- 8.Araki K, Nozaki K, Ueba T.et al High expression of Aurora‐B/Aurora and Ipll‐like midbody‐associated protein (AIM‐1) in astrocytomas. Neurooncol 20046753–64. [DOI] [PubMed] [Google Scholar]
- 9.Fujita M, Mizuno M, Nagasaka T.et al Aurora‐B dysfunction of multinucleated giant cells in glioma detected by site‐specific phosphorylated antibodies. J Neurosurg 20041011012–1017. [DOI] [PubMed] [Google Scholar]
- 10.Burton E, Lamborn K, Feuerstein B.et al Genetic aberrations defined by comparative genomic hybridization distinguish long‐term from typical survivors of glioblastoma. Cancer Res 2002626205–6210. [PubMed] [Google Scholar]
- 11.Ausubel F M, Brent R, Kingston R E.et alShort protocols in molecular biology, 4th edn. New York: Wiley 1999
- 12.Pack S D, Zbar B, Pak E.et al Constitutional von Hippel‐Lindau (VHL) gene deletions detected in VHL families by fluorescence in situ hybridization. Cancer Res 1999595560–5564. [PubMed] [Google Scholar]
- 13.Stukenberg P T. Triggering p53 after cytokinesis failure. J Cell Biol 2004165607–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keen N, Taylor S. Aurora‐kinase inhibitors as anticancer agents. Nat Rev Cancer 20044927–936. [DOI] [PubMed] [Google Scholar]
- 15.Sorrentino R, Libertini S, Pallante P L.et al Aurora B overexpression associates with the thyroid carcinoma undifferentiated phenotype and is required for thyroid carcinoma cell proliferation. J Clin Endocrinol Metab 200590928–935. [DOI] [PubMed] [Google Scholar]
- 16.Chieffi P, Troncone G, Caleao A.et al Aurora B expression in normal testis and seminomas. J Endocrinol 2004181263–270. [DOI] [PubMed] [Google Scholar]
- 17.Harrington E A, Bebbington D, Moore J.et al VX‐680, a potent and selective small‐molecule inhibition of the Aurora kinases, suppresses tumor growth in vivo. Nat Med 200410262–267. [DOI] [PubMed] [Google Scholar]