Abstract
Background
Prognostic factors in predicting outcomes in patients with head and neck squamous‐cell carcinoma (HNSCC) are limited to the clinical–pathological parameters, including lymph node metastasis, location, grade and stage of the disease.
Aim
To determine whether the expression of these proteins has a value in predicting patient outcome.
Methods
Ezrin, maspin and nm23‐H1 immunohistochemistry in tissue samples of 120 patients with HNSCC were evaluated using the microarray technique.
Results
In determining the association among each of the three proteins and the clinical–pathological parameters, low maspin expression was the only one found to be significantly associated with high tumour grade (p = 0.007); all others showed no significant associations. In univariate analysis, patients with tumours expressing high ezrin had a shorter disease‐free survival (DFS) of 51% than those with low ezrin expression (DFS 84%; p = 0.08). In multivariate analysis, tumours with the combination of loss of maspin and low histological grade had longer DFS (83%) compared with those with high maspin and high histological grade (DFS 42%; p = 0.08).
Conclusion
Our study is the first to determine the value of ezrin and maspin in HNSCC in a large series of patients with long follow‐up. Ezrin and maspin seem to have a potential prognostic value in patients with HNSCC but results should be confirmed with further studies.
Head and neck squamous‐cell carcinoma (HNSCC) is the fifth most common malignancy worldwide, and it claims >7800 lives/year in the US alone.1 Despite different treatment modalities, patients with HNSCC still have a poor prognosis, with a 5‐year disease‐free survival (DFS) of about 30–50%.1 The prognostic factors are mostly confined to the histopathological and clinical parameters such as grade, stage, pattern of invasion, location and lymph node metastasis.2,3,4,5 Recently, new techniques, such as tumour molecular gene profiling using complementary DNA (cDNA) microarray, provide us with in‐depth knowledge of HNSCC pathogenesis, where hundreds of oncogenes, tumour suppressor genes, cell‐cycle regulators and cell adhesion proteins have been implicated in tumour progression and responsiveness to radiation therapy.6,7 Ezrin is one of the genes that is consistently increased with disease progression of HNSCC. Ezrin is a member of the ezrin/radixin/moesin family of cytoskeletal proteins that is involved in the regulation of several cytoskeletal‐related functions such as cell adhesion, cell survival and cell motility.8 Even though overexpression of ezrin was associated with aggressive tumour behaviour, including prostate adenocarcinoma, breast cancer, astrocytoma, uvula melanoma and malignant soft tissue sarcomas, its value in HNSCC is widely unknown.9,10,11,12,13,14 Another good candidate gene in tumorigenesis is the tumour suppressor gene maspin (mammary serine protease inhibitor). Maspin is a tumour suppressor protein of a serine protease inhibitor family that regulates the degradation of the extracellular matrix and inhibits tumour cells from invasion and distant metastasis.15,16 Down regulation of maspin has been documented in numerous malignancies including breast and prostate cancers.17,18,19,20 Only three scientific works discussing the value of maspin in HNSCC have been documented in the literature, two of which were in the oral cavity.21,22,23 nm23‐H1 is another suppressor gene for tumour metastasis that has been identified by screening the cDNA libraries. Deregulation of nm23‐H1 expression has been associated with disease progression and adverse disease outcome in HNSCC.24,25,26,27,28,29 However, the value of nm23‐H1 is still controversial, and this study is a good opportunity to shed some light on this issue.
In this study, we aimed to determine the utility of ezrin, maspin and nm23‐H1 in predicting the outcome of patients with HNSCC after curative treatment with radiotherapy with or without chemotherapy.
Patients and methods
Patient population
A retrospective study of patients with HNSCC seen at Geneva University Hospital, Geneva, Switzerland, covering 9 years (1992–2000) was conducted. Criteria for inclusion were patients with no prior treatment, histological diagnosis and material adequate for analysis. Tumours of a nasopharyngeal origin were excluded from the study. In all, 120 patients were identified and selected for the present analysis. Table 1 summarises the patient and tumour characteristics.
Table 1 Summary of patient population and tumour characteristics.
| Median age (years) | 60 (range 35–82) |
| Sex (M:F) | 96:24 |
| Tumour sublocation | |
| Oral cavity | 3 (2.5) |
| Oropharynx | 80 (66.7) |
| Hypopharynx | 21 (17.5) |
| Larynx | 16 (13.3) |
| TN classification and stages (UICC, 1997) | |
| T1–2 | 48 (40) |
| T3–4 | 72 (60) |
| N0 | 53 (44.2) |
| N+ | 67 (55.8) |
| Stages I–II | 24 (20) |
| Stages III–IV | 96 (80) |
| Histological grading | |
| Well differentiated (G1) | 43 (35.8) |
| Moderately differentiated (G2) | 59 (49.2) |
| Poorly differentiated (G3) | 18 (15) |
F, female; M, male; TN, tumour–node; UICC, international union against cancer.
Values are n (%) unless otherwise mentioned.
After the initial diagnosis, patients were treated with radical radiotherapy with or without chemotherapy. All patients were regularly followed up by the otolaryngologist and the radio‐oncologist. Treatment modalities and follow‐up data were retrieved from the registry at the Division of Radiation Oncology, Geneva University Hospital. The median (range) follow‐up for the surviving patients was 63 (14–119) months.
Treatment
All patients received the same accelerated radiotherapy schedule using a concomitant boost technique.30 The planned total dose was 69.9 Gy, delivered in 41 fractions over a period of 38 days. The basic course was given to a total dose of 50.4 Gy over 5.5 weeks. The boost to sites of initial macroscopic tumour involvement consisted of a dose of 19.5 Gy and was given as a second daily fraction, starting the last day of the second week of the basic treatment. According to our institutional policy, 14 (12%) patients underwent a planned neck dissection before radiotherapy; either radical or modified radical, whereas in one patient, the involved lymph node was excised.31 Otherwise, surgery was reserved for salvage of locoregional failures.
Chemotherapy was given to 32 (27%) patients, usually for patients presenting with T3–4 or N2–3 tumours if their medical condition was judged good enough to tolerate multimodality treatment. All patients received ⩾1 cycles of cisplatin with or without 5‐fluorouracil‐based chemotherapy concomitantly with radiotherapy.
Tissue microarray and immunohistochemistry
The tissues analysed consisted of the initial (pretreatment) biopsy specimens. Paraffin‐wax‐embedded tissues from patients' samples were used. The tissue microarray was constructed as described and recommended previously by Kononen et al.32 After carefully choosing the morphologically representative region on the chosen individual paraffin‐wax‐embedded blocks (donor blocks), a core tissue biopsy specimen of 0.6 mm was punched and transferred to the donor paraffin‐wax‐embedded block (recipient block). One section was stained with haematoxylin and eosin to evaluate the presence of the tumour by light microscopy.
From the formalin‐fixed, paraffin‐wax‐embedded tissue microarray, 4 μm sections were processed for immunohistochemical analysis (IHC). Endogenous peroxidase was blocked with 0.3% hydrogen peroxidase for 5 min. Antigen retrieval was carried out in citrate buffer for 3 min in a steamer. Then, sections were incubated with monoclonal antibodies: ezrin (1:100, BD Pharmingen, California, USA) maspin Ab‐1 (1:50, Labvision, California, USA) and nm23‐H1 (1:50, Novocastra, Newcastle, UK) at room temperature. A subsequent reaction was performed with the biotin‐free horse radish peroxidase‐labelled polymer of the Envision plus detection system (Dakocytomation, California, USA). Diaminobenzidine complex was used as chromogen. In negative controls, normal goat serum was used instead of the primary antibody, resulting in a lack of detectable staining. The IHC slides were evaluated semiquantitatively by a pathologist (PM‐F) who was not aware of the original histological diagnosis. The IHC slides were evaluated twice at 3‐week intervals. The individual scores were reviewed, and whenever a discrepancy was noted between the first and the second interpretations, the pathologist decided on the final scoring. Normal squamous mucosa was negative for ezrin and positive for maspin and nm23‐H1. Immunoreactivity was cytoplasmic for ezrin and nm23‐H1, and nuclear and cytoplasmic for maspin. For the three antibodies, the tumours were divided into two categories: group I (negative/weak) and group II (moderate/strong).
Statistical analysis
Fisher's exact test was used to compare proportions. The actuarial overall survival and DFS rates were calculated using the Kaplan–Meier method. For comparison between curves, the log rank test was used. Multivariate analyses based on Cox proportional hazards standard model were used to identify the most significant factors related to outcomes; p⩽0.05 was considered significant. All analyses were performed with the StatView V.5.0.1 software.
Results
Overall results
At the last follow‐up, 42 patients were alive and 78 had died; 45 patients presented with some component of failure (27 with locoregional disease and 18 with distant metastases either alone or associated with locoregional failure). At 5 years, the actuarial DFS was 56% (95% confidence interval (CI) 46% to 66%) and the overall survival was 36% (95% CI 27% to 45%).
Protein expressions and clinicopathological associations
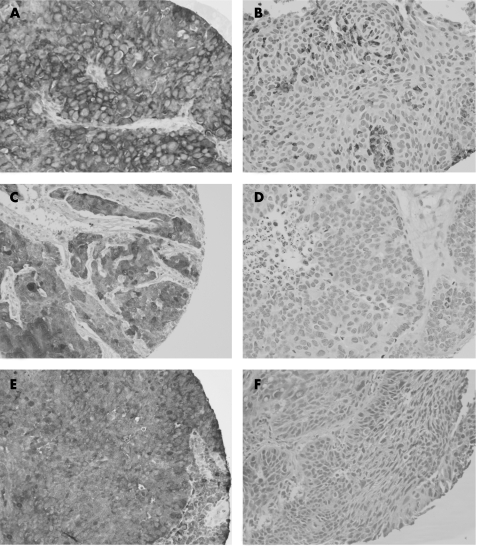
Ezrin was strongly expressed in 93 cases and decreased and/or lost in 15 cases. Maspin was strongly expressed in 65 cases and decreased and/or lost in 54 cases. nm23‐H1 was strongly expressed in 60 cases and was decreased in 53 cases. Figure 1A–E shows examples of strong and weak expressions of ezrin, maspin and nm23‐H1. Loss and/or decrease of maspin expression was seen in 42/77 high‐grade HNSCC (G2–G3) and 12/42 low‐grade HNSCC (fig 1). Among all three proteins, maspin was the only one found to have a significant association with histological grade (p = 0.007). Meaning that poorly differentiated tumours are more likely to show a loss or decrease of maspin expression, in comparison to those low‐grade or well‐differentiated tumours. There was no association between ezrin, maspin and nm23‐H1 markers, and any of the other clinical parameters, including tumour sublocation, T and N classification.
Figure 1 (A) Tumour cells showing strong immunoreactivity in a cytoplasmic pattern. (B) Tumour cells showing loss of ezrin reactivity. (C) Tumour cells showing strong maspin reactivity with a cytoplasmic and nuclear pattern. (D) Tumour cells showing a total loss of maspin immunoreactivity. (E) nm23‐H1 is strongly positive in tumour cells with a cytoplasmic pattern. (F) An example of a case with nm23‐H1‐negative expression.
Univariate and multivariate analyses
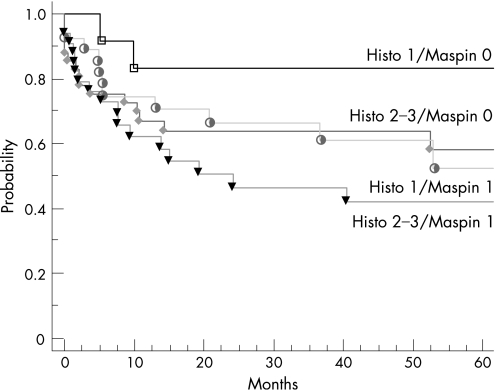
In the univariate analysis, advanced T (p = 0.06) and N categories (p = 0.002) were associated with a trend or a significantly lower 5‐year DFS. Patients with tumours expressing a high level of ezrin tend to have worse prognosis than those with loss and/or decrease of ezrin expression (DFS rate 51% v 84%, respectively; p = 0.08). If we take subcategories of patients, such as patients with tumours, showing a combination of loss of maspin and low histological grade, then these patients seemed to have longer DFS than those having the inverse (83% v 43%, respectively; figs 1 and 2).
Figure 2 Disease‐free survival according to expression of maspin and histological grade.
In the multivariate analysis, we constructed models in which each protein was introduced individually along with the main clinical factors (T and N classification) stratified by histological grade to observe their effect on DFS (table 2). When maspin was introduced, a trend to significant effect was observed (p = 0.06), whereas N and T categories retained their significance with p = 0.009 and p = 0.05, respectively. When ezrin was introduced, only the N category retained its significant effect (p = 0.004), whereas a trend to significant effect was observed for ezrin (p = 0.069). The T category failed to retain any significance, with p = 0.11. When nm23‐H1 was introduced, only the N category retained its significance (p = 0.002), whereas the effects of nm23‐H1 and the T category were not significant (p = 0.89 and p = 0.14, respectively).
Table 2 Multivariate analysis of the value of ezrin, maspin and nm23‐H1 protein expressions in predicting patients' outcome.
| Variables | DFS | ||
|---|---|---|---|
| RR | 95% CI | p Value | |
| Model 1 | |||
| T category: T1–2/T3–4 | 0.528 | 0.276 to 1.009 | 0.053 |
| N category: N0/N1–3 | 0.409 | 0.208 to 0.806 | 0.0097 |
| Maspin: absent or present | 0.552 | 0.297 to 1.027 | 0.06 |
| Model 2 | |||
| T category: T1–2/T3–4 | 0.59 | 0.306 to 1.138 | 0.11 |
| N category: N0/N1–3 | 0.356 | 0.17 to 0.717 | 0.0039 |
| Ezrin: absent or present | 0.266 | 0.63 to 1.111 | 0.069 |
| Model 3 | |||
| T category: T1–2/T3–4 | 0.61 | 0.316 to 1.117 | 0.14 |
| N category: N0/N1–3 | 0.337 | 0.167 to 0.68 | 0.0024 |
| nm23‐H1: absent or present | 0.961 | 0.524 to 1.765 | 0.899 |
DFS, disease‐free survival; N, nodal status; T, tumour size.
All models have been constructed by stratifying by histological grade (1 v 2–3)
Discussion
Ezrin is involved in signalling events that regulate cell survival, proliferation and migration. The literature determining the value of ezrin in patients with HNSCC is lacking, which makes the comparison of our results to others unfeasible. In our study, ezrin protein expression was not associated with tumour grade, stage or lymph node status in patients with HNSCC. The same results were seen in soft tissue tumours, where no correlation was found between ezrin protein expression and the clinical or histological parameters.14 Further, in this study, low ezrin expression was strongly associated with significantly better DFS in univariate analysis, but ezrin did not maintain its value in multivariate analysis. Similar findings were found in cutaneous melanoma and ovarian serous carcinoma, where a strong association of ezrin expression with better patients' survival was only seen in univariate analysis.12,13 Ezrin was found to be essential for metastasis in breast cancer cell lines, and ezrin expression was higher in metastasis tumours in comparison to the matching primary tumours. The above data are strong evidence of the role ezrin has in the late process of tumour progression and metastasis.9 In our study, we did not evaluate this issue, but it will be of interest to study the difference between ezrin expression in primary tumours and their matching metastasis in future work.
Maspin is a tumour suppressor protein that seemed to be down regulated in numerous types of cancer, including HNSCC, prostate and breast cancers, and up regulated in pancreatic and gastric adenocarcinomas. Considering head and neck carcinoma, there are three reports evaluating maspin: two of them focused on squamous‐cell carcinoma of the oral cavity and the third on squamous‐cell carcinoma of the larynx.21,22,23 We found an association between loss of maspin protein expression and high tumour grade. However, maspin was not associated with any of the remaining clinical and pathological parameters, including age, sex, lymph node status, tumour‐node‐metastasis staging and tumour location. The same results were also seen in a study by Yasumatu et al,21 where loss of maspin was only associated with tumour grade in lingual carcinoma, and that by Marioni et al23 on laryngeal carcinoma, where a significant trend was also observed. Our result is somewhat different from that of Xia et al,22 who found an association between maspin and lymph node status in oral carcinoma but not with any of the other parameters. As for patients' prognosis, in our study, the combination of maspin loss and low histological grade was associated with longer DFS in univariate and multivariate analyses. Our results are in accordance with those of lingual and oral carcinoma, but in those studies no multivariate analysis was performed. Marioni et al23 reported that maspin nuclear staining correlated with shorter DFS. We did not have any case with only nuclear pattern, but all our cases showed cytoplasmic and nuclear staining. Thus, we could not confirm or disagree with Marioni et al's results, but maspin nuclear expression was not mentioned in the two other studies on HNSCC.21,22 Notably, the maspin pattern of expression (cytoplasmic and nuclear) was confirmed by the in vitro study.19 Finally, it is pertinent to mention that the study by Marioni et al was small as only 21 patients were evaluated, and these results could be of interest if confirmed with a larger group of patients.
The nm23‐H1 gene (NME1) is a metastasis suppressor gene. Down regulation of nm23‐H1 has been seen in bladder, liver, breast, stomach and ovarian carcinomas. In head and neck carcinoma, loss of nm23‐H1 was associated with tumour grade, increased incidence of lymph node metastasis and distant metastasis, and thus poor prognosis.24,25,28 In addition, previous studies showed no association between the loss of nm23‐H1 expression age, sex and stage, and no correlation between the loss of nm23‐H1 protein expression and DFS in multivariate analysis was identified.25,27,28 In our study, we did not find a correlation between nm23‐H1 expression and clinical–histological parameters. Finally, nm23‐H1 had no effect on patients' prognosis in univariate and multivariate analyses. It has been suggested that nm23‐H1 expression might increase cisplatin chemosensitivity and improve survival in patients with oesophageal squamous‐cell carcinoma.33 However, this was not the case in our study.
In summary, evaluating the three tumour and metastasis suppressor proteins (ezrin, maspin and nm23‐H1) in HNSCC, we found that ezrin and maspin could have a potential use in predicting patients' outcome after treatment. Patients with tumours expressing a high ezrin protein level are more likely to have shorter DFS. On the other hand, in multivariate analysis, patients with tumours showing loss of maspin and low histological grade have better DFS. Our study is a preliminary one and should be confirmed with further studies, but it is still important in showing the potential use of these proteins in the management of patients with HNSCC.
Take‐home message
Ezrin and Mapin seemed to have predictive values in HNSCC and further studies are warranted.
Acknowledgements
We thank Mr Jeff Groth for his technical help and Mr Doug Nixon for his illustration skills.
Abbreviations
DFS - disease‐free survival
HNSCC - head and neck squamous‐cell carcinoma
IHC - immunohistochemical analysis
Footnotes
Competing interests: None declared.
References
- 1.Landis S H, Murray T, Bolden S.et al Cancer statistics. CA Cancer J Clin 1999498–31. [DOI] [PubMed] [Google Scholar]
- 2.Wiernik G, Millard P R, Haybittle J L. The predictive value of histological classification into degrees of differentiation of squamous cell carcinoma of the larynx and hypopharynx compared with the survival of pateints. Histopathology 199119411–417. [DOI] [PubMed] [Google Scholar]
- 3.Crissman J D, Liu W Y, Gluckman J L.et al Prognostic value of histopathologic parameters in squamous cell carcinoma of the oropharynx. Cancer 1984542995–3001. [DOI] [PubMed] [Google Scholar]
- 4.DeStefani A, Magnano M, Bussi M.et al Identification of clinical, biological and prognostic factors in recurring squamous cell carcinoma of the head and neck. Acta Otorhinolaryngol Ital 199717219–224. [PubMed] [Google Scholar]
- 5.Pera E, Moreno A, Galindo L. Prognostic factors in laryngeal cancer: a multifactorial study of 416 cases. Cancer 198658928–934. [DOI] [PubMed] [Google Scholar]
- 6.Beldin T J, Singh B, Smith R V.et al Molecular profiling of tumor progression in head and neck cancer. Arch Otolaryngol Head Neck Surg 200513110–18. [DOI] [PubMed] [Google Scholar]
- 7.Hanna E, Shrieve D C, Ratanatharathorn V.et al A novel alternative approach for prediction of radiation response of squamous cell carcinoma of head and neck. Cancer Res 2001612376–2380. [PubMed] [Google Scholar]
- 8.Srivastava J, Elliot B E, Louvard D.et al Src‐dependent ezrin phosphorylation in adhesion‐mediated signaling. Mol Biol Cell 2005161481–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott B E, Meens J A, Sengupta S K.et al The membrane cytoskeletal crosslinker ezrin is required for metastasis of breast cracinoma cells. Breast Cancer Res 20057365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valdman A, Fang X, Pang S T.et al Ezrin expression in prostate cancer and benign prostatic tissue. Eur Urol 200548852–857. [DOI] [PubMed] [Google Scholar]
- 11.Pang S T, Fang X, Valdman A.et al Expression of ezrin in prostatic intraepithelial neoplasia. Urology 200463609–612. [DOI] [PubMed] [Google Scholar]
- 12.Ilmonen S, Vaheri A, Asko‐Seljavaara S.et al Ezrin in primary cutaneous melanoma. Mod Pathol 200518503–510. [DOI] [PubMed] [Google Scholar]
- 13.Moilanen J, Lassus H, Leminen A.et al Ezrin immunoreactivity in relation to survival in serous ovarian carcinoma patients. Gynecol Oncol 200390273–281. [DOI] [PubMed] [Google Scholar]
- 14.Weng W ‐ H, Ahlen J, Astrom K.et al Prognostic impact of immunohistochemical expression of ezrin in highly malignant soft tissue sarcomas. Clin Cancer Res 2005116198–6204. [DOI] [PubMed] [Google Scholar]
- 15.Blacque O E, Worrall D M. Evidence for a direct interaction between the tumor suppressor serpin, maspin, and types I and II collagen. J Biol Chem 20022710783–10788. [DOI] [PubMed] [Google Scholar]
- 16.Ngamkitidechakul C, Warejcka D J, Burke J M.et al Sufficiency of the reactive site loop maspin for induction of cell‐matrix adhesion and inhibition of cell invasion. J Biol Chem 200327831796–31806. [DOI] [PubMed] [Google Scholar]
- 17.Son H J, Sohn T ‐ S, Song S Y.et al Maspin expression in human gastric adenocarcinoma. Pathol Int 200252508–513. [DOI] [PubMed] [Google Scholar]
- 18.Mass N, Hojo T, Ueding M.et al Expression of the tumor suppressor gene maspin in human pancreatic cancers. Clin Cancer Res 20017812–817. [PubMed] [Google Scholar]
- 19.Sood A K, Fletcher M S, Gruman L M.et al The paradoxical expression of maspin in ovarian carcinoma. Clin Cancer Res 200282924–2932. [PubMed] [Google Scholar]
- 20.Mohsin S K, Zhang M, Clark G M.et al Maspin expression in invasive breast cancer: association with other prognostic factors. J Pathol 2003199432–435. [DOI] [PubMed] [Google Scholar]
- 21.Yasumatsu R, Nakashima T, Hirakawa N.et al Maspin expression in stage I and II oral tongue squamous cell carcinoma. Head Neck 200123962–966. [DOI] [PubMed] [Google Scholar]
- 22.Xia W, Lau Y ‐ K, Hu M C ‐ T.et al High tumoral maspin expression is associated with improved survival of patients with oral squamous cell carcinoma. Oncogene 2000192398–2403. [DOI] [PubMed] [Google Scholar]
- 23.Marioni G, Blandamura S, Giacomelli L.et al Nuclear expression of maspin is associated with a lower recurrence rate and a longer disease‐free interval after surgery for squamous cell carcinoma of the larynx. Histopathology 200546576–582. [DOI] [PubMed] [Google Scholar]
- 24.Chow N ‐ H, Liu H ‐ S, Chan S ‐ H. The role of nm23‐H1 in the progression of transitional cell bladder cancer. Clin Cancer Res 200063595–3599. [PubMed] [Google Scholar]
- 25.Gunduz M, Ayhan A, Gullu I.et al Nm23 protein expression in larynx cancer and the relationship with metastasis. Eur J Cancer 1997332338–2341. [DOI] [PubMed] [Google Scholar]
- 26.Lee C S, Redshaw A, Boag G. nm23‐H1 protein immunoreactivity in laryngeal carcinoma. Cancer 1996772246–2250. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y ‐ F, Chow K ‐ C, Chang S ‐ Y.et al Prognostic significance of nm23‐H1 expression in oral squamous cell carcinoma. Br J Cancer 2004902186–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavelic K, Kapitanovic S, Radosevic S.et al Increased activity of nm23‐H1 gene in squamous cell carcinoma of the head and neck is associated with advanced disease and poor prognosis. J Mod Med 200078111–118. [DOI] [PubMed] [Google Scholar]
- 29.Muzio L L, Mignogna M D, Pannone G.et al The NM23 gene and its expression in oral squamous cell carcinoma. Oncol Rep 19994747–751. [DOI] [PubMed] [Google Scholar]
- 30.Allal A S, de Pree C, Dulguerov P.et al Avoidance of treatment interruption: an unrecognized benefit of accelerated radiotherapy in oropharyngeal carcinomas? Int J Radiat Oncol Biol Phys 19994541–45. [DOI] [PubMed] [Google Scholar]
- 31.Allal A S, Dulguerov P, Bieri S.et al A conservation approach to pharyngeal carcinoma with advanced neck disease: optimizing neck management. Head Neck 199921217–222. [DOI] [PubMed] [Google Scholar]
- 32.Kononen J, Bubendorf L, Kallioniemi A.et al Tissue microarrays for high‐throughput molecular profiling of tumor specimens. Nat Med 19984844–847. [DOI] [PubMed] [Google Scholar]
- 33.Wang L S, Chow K C, Lien Y C.et al Prognostic significance of nm23‐H1 expression in esophageal squamous cell carcinoma. Eur J Cardiothorac Surg 200426419–424. [DOI] [PubMed] [Google Scholar]