Abstract
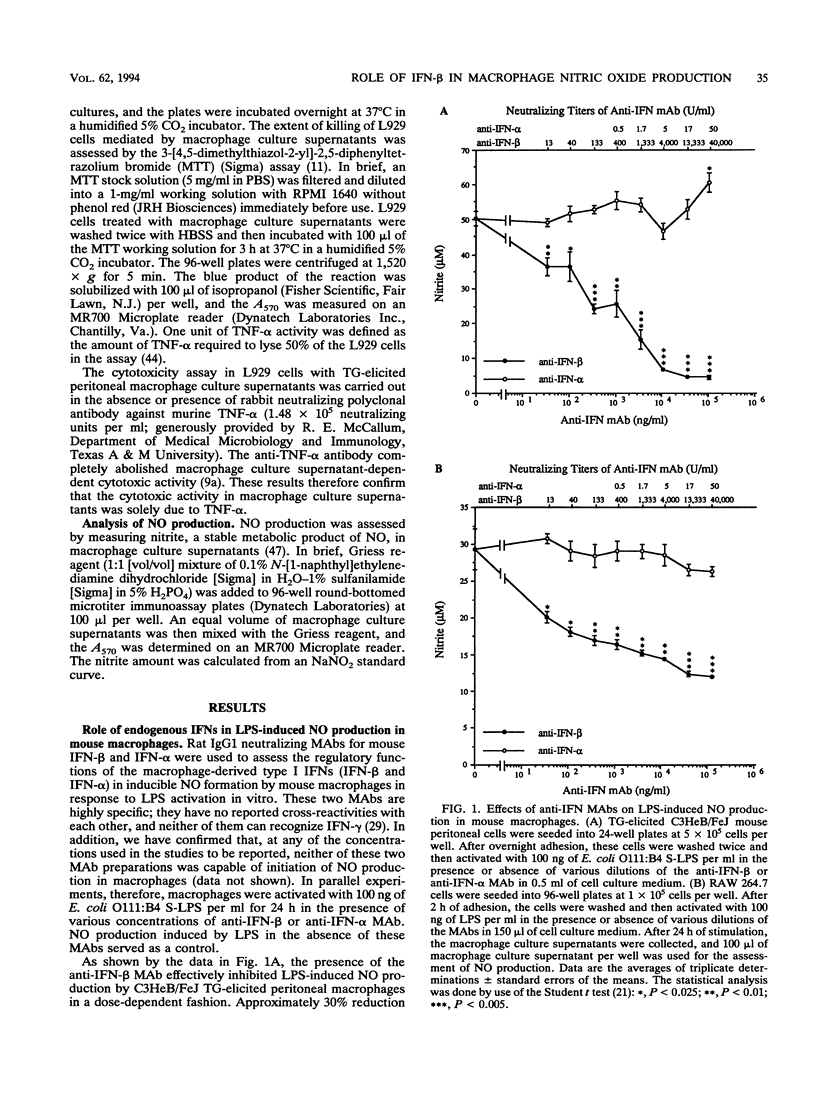
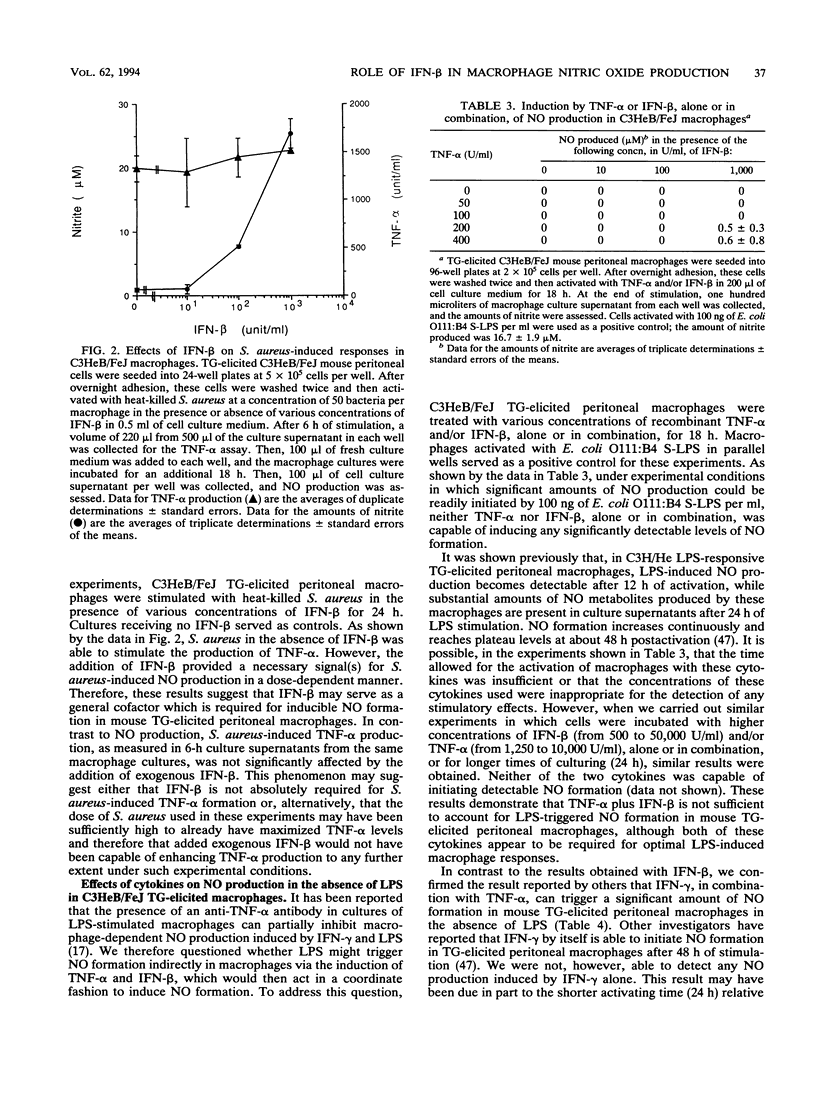
Bacterial lipopolysaccharide and some cytokines can activate macrophages to secrete nitric oxide. Macrophage-derived nitric oxide is a key cytotoxic factor for microbicidal and tumoricidal processes. We report here that a monoclonal antibody specific for beta interferon inhibited lipopolysaccharide-induced nitric oxide production in thioglycolate-elicited C3HeB/FeJ peritoneal macrophages and macrophage-like cell line RAW 264.7. In addition, exogenous added beta interferon enabled lipopolysaccharide-hyporesponsive thioglycolate-elicited C3H/HeJ peritoneal macrophages to produce nitric oxide in response to lipopolysaccharide. These data support the concept that beta interferon provides an essential signal(s) for lipopolysaccharide-triggered nitric oxide production by mouse macrophages. Heat-killed Staphylococcus aureus, a gram-positive bacterium which was unable to initiate nitric oxide production in thioglycolate-elicited C3HeB/FeJ peritoneal macrophages in vitro, promoted nitric oxide formation in the presence of beta interferon, suggesting that beta interferon may be a general cofactor necessary for bacterium-derived stimulus-induced nitric oxide production in these macrophages. However, neither beta interferon nor tumor necrosis factor alpha, alone or in combination, triggered nitric oxide production in thioglycolate-elicited mouse peritoneal macrophages, demonstrating that these macrophage-derived cytokines, while necessary, were not sufficient by themselves for the induction of nitric oxide production in these cells. On the other hand, gamma interferon and tumor necrosis factor alpha acted together to induce nitric oxide production in vitro in the absence of lipopolysaccharide in thioglycolate-elicited mouse peritoneal macrophages, indicating that these two types of interferons provided different signals during the activation of these macrophages.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. B., Franzblau S. G., Vavrin Z., Hibbs J. B., Jr, Krahenbuhl J. L. L-arginine-dependent macrophage effector functions inhibit metabolic activity of Mycobacterium leprae. J Immunol. 1991 Sep 1;147(5):1642–1646. [PubMed] [Google Scholar]
- Adams L. B., Hibbs J. B., Jr, Taintor R. R., Krahenbuhl J. L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990 Apr 1;144(7):2725–2729. [PubMed] [Google Scholar]
- Amber I. J., Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Cytokines induce an L-arginine-dependent effector system in nonmacrophage cells. J Leukoc Biol. 1988 Jul;44(1):58–65. doi: 10.1002/jlb.44.1.58. [DOI] [PubMed] [Google Scholar]
- Auguet M., Lonchampt M. O., Delaflotte S., Goulin-Schulz J., Chabrier P. E., Braquet P. Induction of nitric oxide synthase by lipoteichoic acid from Staphylococcus aureus in vascular smooth muscle cells. FEBS Lett. 1992 Feb 3;297(1-2):183–185. doi: 10.1016/0014-5793(92)80356-l. [DOI] [PubMed] [Google Scholar]
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Belardelli F., Gessani S., Proietti E., Locardi C., Borghi P., Watanabe Y., Kawade Y., Gresser I. Studies on the expression of spontaneous and induced interferons in mouse peritoneal macrophages by means of monoclonal antibodies to mouse interferons. J Gen Virol. 1987 Aug;68(Pt 8):2203–2212. doi: 10.1099/0022-1317-68-8-2203. [DOI] [PubMed] [Google Scholar]
- Benoit P., Maguire D., Plavec I., Kocher H., Tovey M., Meyer F. A monoclonal antibody to recombinant human IFN-alpha receptor inhibits biologic activity of several species of human IFN-alpha, IFN-beta, and IFN-omega. Detection of heterogeneity of the cellular type I IFN receptor. J Immunol. 1993 Feb 1;150(3):707–716. [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Stuehr D. J., Ferrari F. K., Simmons R. L. Evidence that activation of Kupffer cells results in production of L-arginine metabolites that release cell-associated iron and inhibit hepatocyte protein synthesis. Surgery. 1989 Aug;106(2):364–372. [PubMed] [Google Scholar]
- Curran R. D., Billiar T. R., Stuehr D. J., Hofmann K., Simmons R. L. Hepatocytes produce nitrogen oxides from L-arginine in response to inflammatory products of Kupffer cells. J Exp Med. 1989 Nov 1;170(5):1769–1774. doi: 10.1084/jem.170.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986 May 22;89(2):271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- Di Rosa M., Radomski M., Carnuccio R., Moncada S. Glucocorticoids inhibit the induction of nitric oxide synthase in macrophages. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1246–1252. doi: 10.1016/0006-291x(90)91583-e. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Ding A. H., Sanchez E., Srimal S., Nathan C. F. Macrophages rapidly internalize their tumor necrosis factor receptors in response to bacterial lipopolysaccharide. J Biol Chem. 1989 Mar 5;264(7):3924–3929. [PubMed] [Google Scholar]
- Ding A., Nathan C. F., Graycar J., Derynck R., Stuehr D. J., Srimal S. Macrophage deactivating factor and transforming growth factors-beta 1 -beta 2 and -beta 3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-gamma. J Immunol. 1990 Aug 1;145(3):940–944. [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Drapier J. C., Wietzerbin J., Hibbs J. B., Jr Interferon-gamma and tumor necrosis factor induce the L-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol. 1988 Oct;18(10):1587–1592. doi: 10.1002/eji.1830181018. [DOI] [PubMed] [Google Scholar]
- Fast D. J., Shannon B. J., Herriott M. J., Kennedy M. J., Rummage J. A., Leu R. W. Staphylococcal exotoxins stimulate nitric oxide-dependent murine macrophage tumoricidal activity. Infect Immun. 1991 Sep;59(9):2987–2993. doi: 10.1128/iai.59.9.2987-2993.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flebbe L. M., Chapes S. K., Morrison D. C. Activation of C3H/HeJ macrophage tumoricidal activity and cytokine release by R-chemotype lipopolysaccharide preparations. Differential effects of IFN-gamma. J Immunol. 1990 Sep 1;145(5):1505–1511. [PubMed] [Google Scholar]
- Flebbe L., Vukajlovich S. W., Morrison D. C. Immunostimulation of C3H/HeJ lymphoid cells by R-chemotype lipopolysaccharide preparations. J Immunol. 1989 Jan 15;142(2):642–652. [PubMed] [Google Scholar]
- Gazzinelli R. T., Oswald I. P., Hieny S., James S. L., Sher A. The microbicidal activity of interferon-gamma-treated macrophages against Trypanosoma cruzi involves an L-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-beta. Eur J Immunol. 1992 Oct;22(10):2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- Gessani S., Belardelli F., Pecorelli A., Puddu P., Baglioni C. Bacterial lipopolysaccharide and gamma interferon induce transcription of beta interferon mRNA and interferon secretion in murine macrophages. J Virol. 1989 Jun;63(6):2785–2789. doi: 10.1128/jvi.63.6.2785-2789.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. J., Meltzer M. S., Hibbs J. B., Jr, Nacy C. A. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol. 1990 Jan 1;144(1):278–283. [PubMed] [Google Scholar]
- Harbrecht B. G., Billiar T. R., Stadler J., Demetris A. J., Ochoa J. B., Curran R. D., Simmons R. L. Nitric oxide synthesis serves to reduce hepatic damage during acute murine endotoxemia. Crit Care Med. 1992 Nov;20(11):1568–1574. doi: 10.1097/00003246-199211000-00015. [DOI] [PubMed] [Google Scholar]
- Havell E. A., Spitalny G. L. Endotoxin-induced interferon synthesis in macrophage cultures. J Reticuloendothel Soc. 1983 May;33(5):369–380. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Westenfelder C., Taintor R., Vavrin Z., Kablitz C., Baranowski R. L., Ward J. H., Menlove R. L., McMurry M. P., Kushner J. P. Evidence for cytokine-inducible nitric oxide synthesis from L-arginine in patients receiving interleukin-2 therapy. J Clin Invest. 1992 Mar;89(3):867–877. doi: 10.1172/JCI115666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawade Y. An analysis of neutralization reaction of interferon by antibody: a proposal on the expression of neutralization titer. J Interferon Res. 1980 Fall;1(1):61–70. doi: 10.1089/jir.1980.1.61. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Salter M., Brooks S. L., Moncada S. Anti-inflammatory glucocorticoids inhibit the induction by endotoxin of nitric oxide synthase in the lung, liver and aorta of the rat. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1042–1048. doi: 10.1016/0006-291x(90)91551-3. [DOI] [PubMed] [Google Scholar]
- Kosaka H., Wishnok J. S., Miwa M., Leaf C. D., Tannenbaum S. R. Nitrosation by stimulated macrophages. Inhibitors, enhancers and substrates. Carcinogenesis. 1989 Mar;10(3):563–566. doi: 10.1093/carcin/10.3.563. [DOI] [PubMed] [Google Scholar]
- Kwon N. S., Stuehr D. J., Nathan C. F. Inhibition of tumor cell ribonucleotide reductase by macrophage-derived nitric oxide. J Exp Med. 1991 Oct 1;174(4):761–767. doi: 10.1084/jem.174.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay R. J., Russell S. W. Protein changes associated with stages of activation of mouse macrophages for tumor cell killing. J Immunol. 1986 Aug 15;137(4):1392–1398. [PubMed] [Google Scholar]
- Miwa M., Stuehr D. J., Marletta M. A., Wishnok J. S., Tannenbaum S. R. Nitrosation of amines by stimulated macrophages. Carcinogenesis. 1987 Jul;8(7):955–958. doi: 10.1093/carcin/8.7.955. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Morrison D. C., Leive L. Fractions of lipopolysaccharide from Escherichia coli O111:B4 prepared by two extraction procedures. J Biol Chem. 1975 Apr 25;250(8):2911–2919. [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor. Sci Am. 1988 May;258(5):59-60, 69-75. doi: 10.1038/scientificamerican0588-59. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Ruff M. R., Gifford G. E. Purification and physico-chemical characterization of rabbit tumor necrosis factor. J Immunol. 1980 Oct;125(4):1671–1677. [PubMed] [Google Scholar]
- Stuehr D. J., Griffith O. W. Mammalian nitric oxide synthases. Adv Enzymol Relat Areas Mol Biol. 1992;65:287–346. doi: 10.1002/9780470123119.ch8. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Synthesis of nitrite and nitrate in murine macrophage cell lines. Cancer Res. 1987 Nov 1;47(21):5590–5594. [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. N., Fertsch D. Macrophages from endotoxin-hyporesponsive (Lpsd) C3H/HeJ mice are permissive for vesicular stomatitis virus because of reduced levels of endogenous interferon: possible mechanism for natural resistance to virus infection. J Virol. 1987 Mar;61(3):812–818. doi: 10.1128/jvi.61.3.812-818.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zembowicz A., Vane J. R. Induction of nitric oxide synthase activity by toxic shock syndrome toxin 1 in a macrophage-monocyte cell line. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2051–2055. doi: 10.1073/pnas.89.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Morrison D. C. Lipopolysaccharide-induced selective priming effects on tumor necrosis factor alpha and nitric oxide production in mouse peritoneal macrophages. J Exp Med. 1993 Feb 1;177(2):511–516. doi: 10.1084/jem.177.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Morrison D. C. Pertussis toxin-sensitive factor differentially regulates lipopolysaccharide-induced tumor necrosis factor-alpha and nitric oxide production in mouse peritoneal macrophages. J Immunol. 1993 Feb 1;150(3):1011–1018. [PubMed] [Google Scholar]
- al-Ramadi B. K., Meissler J. J., Jr, Huang D., Eisenstein T. K. Immunosuppression induced by nitric oxide and its inhibition by interleukin-4. Eur J Immunol. 1992 Sep;22(9):2249–2254. doi: 10.1002/eji.1830220911. [DOI] [PubMed] [Google Scholar]



