Abstract
Objective
To measure the effect of statins on mortality for community based patients with ischaemic heart disease and determine whether the likely benefits are similar for women, the elderly, and patients with diabetes.
Design
Open prospective cohort study with nested case–control analysis.
Setting
1.18 million patients registered with 89 practices spread across 23 strategic health authority areas within the UK. All practices had a minimum of eight years of longitudinal data and were contributing to the UK QRESEARCH database.
Subjects
All patients with a first diagnosis of ischaemic heart disease between January 1996 and December 2003
Outcomes
Adjusted hazard ratio with 95% confidence intervals (CIs) for all cause mortality (cohort analysis) and odds ratio (OR) with 95% CI (case–control analysis) for current use of statins. Adjustments were made for current use of aspirin, β blockers, and angiotensin converting enzyme inhibitors, co‐morbidity (myocardial infarction, diabetes, hypertension, congestive cardiac failure), smoking, body mass index, and quintile of deprivation.
Results
13 029 patients had a first diagnosis of ischaemic heart disease in the study period giving an incidence rate of 3.38/1000 person years. 2266 patients with ischaemic heart disease died during the 43 460 person years of observation giving an overall mortality rate of 52.1/1000 person years (95% CI 50.0 to 54.3). In the case–control analysis, patients taking statins had a 39% lower risk of death than did patients not taking statins (adjusted OR 0.61, 95% CI 0.52 to 0.72) after use of other medication, co‐morbidity, smoking, body mass index, and deprivation were taken into account. The benefits found in this study compared favourably with those found in the randomised controlled trials, although the current study population is at higher overall risk. The benefits extend to women, patients with diabetes, and the elderly and can be seen within two years of treatment. Longer duration of usage was associated with lower OR for risk of death with a 19% reduction in risk of death with each additional year of treatment (adjusted OR 0.81, 95% CI 0.77 to 0.86 per year). Mortality was similarly reduced among patients prescribed atorvastatin (adjusted OR 0.62, 95% CI 0.48 to 0.79) and simvastatin (adjusted OR 0.62, 95% CI 0.50 to 0.76).
Conclusions
The benefits of statins found in randomised controlled trials extend to unselected community based patients. The benefits can be seen within the first two years of treatment and continue to accrue over time. Since patients in the community are likely to be at higher risk than those in trials, the potential benefits from statins are likely to be greater than expected.
Keywords: coronary heart disease, mortality, primary care, statins
Multiple randomised controlled trials have shown the benefits of statins in improving survival for patients with ischaemic heart disease.1,2,3,4,5 Although there is good evidence that statins reduce serum cholesterol effectively outside of the clinical trial setting,6 there is little information on the effect of statins on mortality in the community.
Uncritical acceptance of medical innovations or lack of evidence can result in the endorsement of ineffective or occasionally dangerous treatments.7 It can lead to the immediate withdrawal of drugs (such as rofecoxib) or limit their use (such as or hormone replacement therapy8,9). This can occur years after widespread worldwide adoption.10 While randomised trials of selected patients provide relatively unbiased evidence of effectiveness in specific targeted interventions, the application of trial results to representative populations of all patients with the disease is often inaccurate.11 A treatment that may produce an overall benefit may be ineffective or even harmful to some patients.12 Once there is clinical evidence showing benefit, it then becomes difficult, if not unethical, to perform further trials to evaluate benefits in unselected populations. Trials are usually designed to test efficacy of interventions, whereas effectiveness is important in clinical practice. Other methods are therefore needed to evaluate treatments further.
Routinely collected data from aggregated general practice databases have been used successfully to evaluate risks and benefits of treatments in the population.13,14 As a method, it has the advantage of longitudinal data, large sample size, and ability to access representative populations. Also, exposure data are collected before the outcome, thus limiting recall bias; additionally, the quality of the electronic record now surpasses that of conventional paper based systems.15
If statins really do save lives in the community setting, then we would expect to be able to measure the effect on a large population sample. If the expected reduction in mortality is not observed, then an urgent investigation in to the reasons why is warranted.
Our objective was to measure the effect of statins on survival and compare this with the benefit reported in randomised controlled trials. In addition, we determined whether the likely benefits were similar for women, the elderly, and patients with diabetes.
METHODS
Design
We conducted a prospective open cohort study with nested case–control analysis of data from UK general practices contributing to the QRESEARCH database (http://www.qresearch.org). Ethical approval was obtained from the Trent Multi‐Centre Ethics Committee.
Setting
The study was conducted in 89 general practices spread throughout 23 strategic health authority areas across the UK. Only practices with at least eight years of longitudinal data (that is, with EMIS software before 1 January 1996) were selected.
Study participants
Study participants were all patients registered with the practices from 1 January 1996 until the end of the study period (17 December 2003, the date of the last computer download). We used 1 January 1996 as our start date because this was just over 12 months after the publication of the 4S (Scandinavian simvastatin survival study).1 We assembled an open cohort selected on the basis of registration dates and dates of leaving or death. From this cohort, we identified all patients with incident ischaemic heart disease diagnosed after 1 January 1996 by using the date of first diagnosis of ischaemic heart disease recorded on computer. We excluded patients whose diagnosis was made within the first three months of registration with the general practice (to minimise information bias), patients prescribed statins before the diagnosis of ischaemic heart disease, and patients whose first diagnosis was made after death (postmortem diagnosis).
Main outcome measures
Our main outcome measure for the cohort analysis was the rate of death among patients with ischaemic heart disease taking and not taking statins. In the case–control analysis, our outcome was the adjusted odds ratio (OR) for risk of death among patients who had taken statins compared with those who had not since diagnosis of ischaemic heart disease.
Cohort analysis
We determined the incidence rate of ischaemic heart disease in the main cohort by dividing the number of new cases by the person years of observation. We then calculated the death rates by age, sex, and co‐morbidity for patients with a first diagnosis of ischaemic heart disease. We used Cox regression to investigate the effect of statins on survival of patients with incident ischaemic heart disease with statin use as a time varying covariate. The analysis time for the death rates was from the date of diagnosis of ischaemic heart disease until the first of the following occurred: the patient died, the patient was transferred out of the practice, or the study period ended. Patients who had taken statins were classified as receiving statins between the date of the first prescription and the first of the following: the statin was stopped (estimated as date of last prescription plus 90 days), the patient died, the patient was transferred out of the practice, or the study period ended. We adjusted for the potential confounding effects of age, sex, co‐morbidity (diabetes, congestive cardiac failure, hypertension, myocardial infarction, cancer), smoking, obesity, and year of diagnosis by including them as variables in the multivariate Cox regression. We allowed for clustering by general practice by defining this as a clustered variable and using a robust standard error. We checked the proportional hazards assumption graphically and with a test of proportional hazards.
Nested case–control study
Next we undertook a nested case–control analysis to determine the effects of statins and of concurrent medication on survival. All the patients with ischaemic heart disease identified in the cohort evaluation who died during the follow up period were included as cases with an index date being defined as the date of death. We used Stata (version 8.2; StataCorp, College Station, Texas, USA) to randomly select four controls for each case matched on age at diagnosis of ischaemic heart disease (five year bands), sex, and year of diagnosis of ischaemic heart disease. Controls were patients with ischaemic heart disease who were alive at the time their matched case died. We derived an index date for each control, which was the date of death of their matched case.
Case–control analysis
We used conditional logistic regression for individually matched case–control studies to derive ORs with 95% confidence intervals (CIs) for the risk of death associated with current use of statins. We reviewed the medical history and exposure data between the date of diagnosis of ischaemic heart disease and the index date for cases and controls.
To measure statin exposure, we determined the dates of the first and last scripts before the index date. We did this for all statins as a group and separately for each of the five statins (atorvastatin, cerivastatin, fluvastatin, pravastatin, and simvastatin). We coded patients as those currently taking statins (the last prescription within 90 days of the index date); those whose last prescription for statins was more than 90 days before the index date, and those not prescribed statins since diagnosis of ischaemic heart disease. We also used these dates to determine the number of months of statin usage. We coded months of usage into six groups (none and 1–12, 13–24, 25–36, 37–48, 49–60, and ⩾ 60 months). We tested for evidence of dose response undertaking a test for trend across these categories.
We adjusted for co‐morbidity (diabetes, congestive cardiac failure, hypertension, myocardial infarction, cancer) and use of β blockers, aspirin, angiotensin converting enzyme inhibitors, and calcium channel blockers all before the index date. We also adjusted for smoking status (ever smoker, never smoker, not recorded), body mass index (< 25, 25–30, or ⩾ 30 kg/m2, or not recorded) and Townsend score (in fifths). The Townsend score was calculated based on the 2001 census related data associated with the output area of the patients' postcode.
We tested for interactions between current use of statins and each of age, sex, and diabetes by including interaction terms in the models and calculating likelihood ratio tests.
We also performed analyses restricted to patients with recorded values of body mass index and smoking status. To examine possible indication bias we also carried out analyses restricted to patients without a diagnosis of cancer and to cases who survived for at least a year after the diagnosis of ischaemic heart disease and their matched controls. We also carried out an analysis restricted to patients without diabetes or congestive cardiac failure or myocardial infarction.
All the analyses were conducted with Stata (version 8.2). We selected a value of p = 0.01 (two tailed).
RESULTS
Study participants
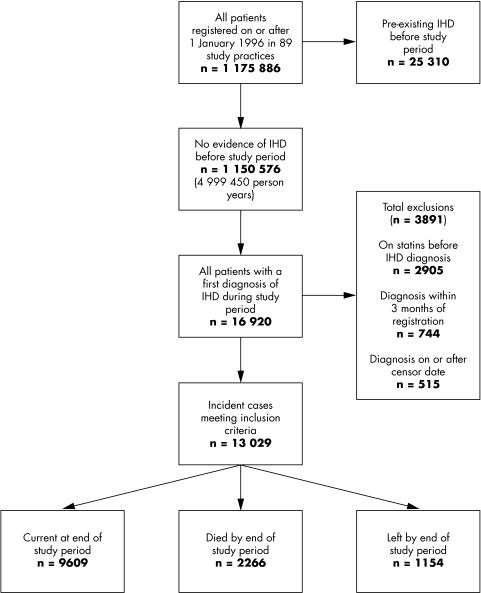
Figure 1 shows the flow of patients through the study in the 89 practices meeting the selection criteria. The cohort consisted of 1 175 886 patients registered on or after 1 January 1996 (604 781 women and 571 105 men) accumulating almost five million (n = 4 999 450) person years of observation. Of these, 25 310 patients who were recorded as having ischaemic heart disease before 1 January 1996 were not included in this analysis.
Figure 1 Flow chart of patients in the cohort. Note that exclusions are not mutually exclusive. IHD, ischaemic heart disease.
Cohort analysis
During the study period 16 920 patients had a first ever diagnosis of ischaemic heart disease. The overall incidence rate of ischaemic heart disease was 3.38/1000 person years (95% CI 3.33 to 3.44). Table 1 shows the incidence rates of ischaemic heart disease by age group and sex.
Table 1 Incidence rates of ischaemic heart disease (IHD) between 1 January 1996 and 17 December 2003.
| Cohort | Person time (years) | Number of IHD cases | Rate/1000 person years | 95% CI |
|---|---|---|---|---|
| Women | ||||
| 0–44 years | 1432317 | 157 | 0.1 | 0.1 to 0.1 |
| 45–54 years | 351396 | 538 | 1.5 | 1.4 to 1.7 |
| 55–64 years | 282339 | 1372 | 4.9 | 4.6 to 5.1 |
| 65–74 years | 227748 | 2151 | 9.4 | 9.1 to 9.9 |
| 75–84 years | 168760 | 2152 | 12.8 | 12.2 to 13.3 |
| ⩾85 years | 77685 | 901 | 11.6 | 10.9 to 12.4 |
| Total | 2540246 | 7271 | 2.9 | 2.8 to 2.9 |
| Men | ||||
| 0–44 years | 1506948 | 423 | 0.3 | 0.3 to 0.3 |
| 45–54 years | 353485 | 1343 | 3.8 | 3.6 to 4.0 |
| 55–64 years | 273984 | 2582 | 9.4 | 9.1 to 9.8 |
| 65–74 years | 189105 | 2957 | 15.6 | 15.1 to 16.2 |
| 75–84 years | 104678 | 1877 | 17.9 | 17.1 to 18.8 |
| ⩾85 years | 31005 | 467 | 15.1 | 13.8 to 16.5 |
| Total | 2459205 | 9649 | 3.9 | 3.8 to 4.0 |
CI, confidence interval.
Of the 16 920 patients with ischaemic heart disease 13 029 met our inclusion criteria. During the 43 460 person years of observation 2266 patients with ischaemic heart disease died, giving an overall mortality rate of 52.1/1000 person years (95% CI 50.0 to 54.3). Table 2 shows the death rates by age, sex, and co‐morbidity. As expected, death rates were highest among patients over 75 years, with diabetes, or with congestive cardiac failure.
Table 2 Mortality rates per 1000 person years for 13029 patients with incident IHD between 1 January 1996 and 17 December 2003.
| Cohort | Person time (years) | Number of deaths | Rate/1000 person years | 95% CI |
|---|---|---|---|---|
| Age (years) | ||||
| 0–44 | 824 | 8 | 9.7 | 4.9 to 19.4 |
| 45–54 | 3923 | 40 | 10.2 | 7.5 to 13.9 |
| 55–64 | 9270 | 156 | 16.8 | 14.4 to 19.7 |
| 65–74 | 13636 | 447 | 32.8 | 29.9 to 36.0 |
| 75–84 | 11827 | 911 | 77.0 | 72.2 to 82.2 |
| 85–94 | 3744 | 626 | 167.2 | 154.6 to 180.8 |
| ⩾95 | 235 | 78 | 331.4 | 265.4 to 413.7 |
| Total | 43460 | 2266 | 52.1 | 50.0 to 54.3 |
| Women | 18539 | 1003 | 54.1 | 50.9 to 57.6 |
| Men | 24920 | 1263 | 50.7 | 48.0 to 53.6 |
| No diabetes | 39814 | 1978 | 49.7 | 47.5 to 51.9 |
| Diabetes | 3646 | 288 | 79.0 | 70.4 to 88.7 |
| No hypertension | 30912 | 1570 | 50.8 | 48.3 to 53.4 |
| Hypertension | 12547 | 696 | 55.5 | 51.5 to 59.8 |
| No CCF | 37391 | 1546 | 41.4 | 39.3 to 43.5 |
| CCF | 6069 | 720 | 118.6 | 110.3 to 127.6 |
CCF, congestive cardiac failure.
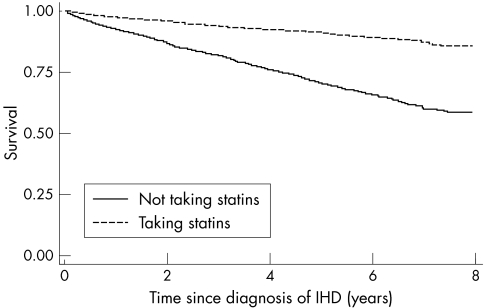
Figure 2 shows the Kaplan‐Meier survival curves for patients taking statins and those not taking statins, with use of statins treated as a time varying variable. This shows that patients taking statins have higher survival rates than those not taking statins: the six year survival rate was 89% (95% CI 88% to 90%) for patients taking statins and 66% (95% CI 64% to 67%) for patients not taking statins. Patients taking statins had a 53% lower risk of death than the patients not taking statins (adjusted hazard ratio 0.47, 95% CI 0.41 to 0.53). The hazard ratio was adjusted for sex, age, diabetes, hypertension, congestive cardiac failure, myocardial infarction, cancer, body mass index, smoking, and year of diagnosis.
Figure 2 Kaplan‐Meier plot showing survival of patients taking statins compared with patients not taking statins. Analysis time is years since diagnosis of IHD.
Case–control analysis
For the 2266 cases, we identified 9064 controls matched on age, sex, and year of diagnosis of ischaemic heart disease. The cases and controls were well matched at baseline: in both groups the median age at the index date was 80 years and 55.7% were men. The median duration of ischaemic heart disease before the index date was 20.3 months for cases and 21.0 months for controls. Overall, 445 cases (19.6% of 2,266) had been prescribed any statin compared with 2303 of the controls (25.4% of 9064) between the date of diagnosis of ischaemic heart disease and the index date. Among the cases 326 (14.4% of 2266) had received a prescription for a statin within 90 days before the index date, compared with 2079 (22.9% of 9064) of controls (table 3).
Table 3 Use of statins after diagnosis of IHD in cases (patients with IHD who died) and controls.
| Use of statins after diagnosis of IHD | Proportion of cases (n = 2266) | Proportion of controls (n = 9064) |
|---|---|---|
| Any statin | ||
| Not prescribed drug | 1821 (80.4%) | 6761 (74.6%) |
| Last script >90 days before index date | 119 (5.3%) | 224 (2.5%) |
| Last script ⩽90 days before index date | 326 (14.4%) | 2079 (22.9%) |
| Atorvastatin | ||
| Not prescribed drug | 2102 (92.8%) | 8177 (90.2%) |
| Last script >90 days before index date | 53 (2.3%) | 141 (1.6%) |
| Last script ⩽90 days before index date | 111 (4.9%) | 746 (8.2%) |
| Cerivastatin | ||
| Not prescribed drug | 2227 (98.3%) | 8897 (98.2%) |
| Last script >90 days before index date | 29 (1.3%) | 104 (1.1%) |
| Last script ⩽90 days before index date | 10 (0.4%) | 63 (0.7%) |
| Fluvastatin | ||
| Not prescribed drug | 2246 (99.1%) | 8932 (98.5%) |
| Last script >90 days before index date | 10 (0.4%) | 50 (0.6%) |
| Last script ⩽90 days before index date | 10 (0.4%) | 82 (0.9%) |
| Pravastatin | ||
| Not prescribed drug | 2238 (98.8%) | 8897 (98.2%) |
| Last script >90 days before index date | 9 (0.4%) | 51 (0.6%) |
| Last script ⩽90 days before index date | 19 (0.8%) | 116 (1.3%) |
| Simvastatin | ||
| Not prescribed drug | 2001 (88.3%) | 7699 (84.9%) |
| Last script >90 days before index date | 89 (3.9%) | 288 (3.2%) |
| Last script ⩽90 days before index date | 176 (7.8%) | 1077 (11.9%) |
Table 4 shows the unadjusted and adjusted ORs for risk of death in cases versus controls. On univariate analysis, patients who had been prescribed statins in the 90 days before their index date had a 47% lower risk of death than patients who had not been prescribed a statin (OR 0.53, 95% CI 0.46 to 0.61). When other factors such as diabetes, hypertension, congestive cardiac failure, myocardial infarction, cancer, smoking, body mass index, deprivation, and use of angiotensin converting enzyme inhibitors, aspirin, β blockers, or calcium channel blockers were included in the multivariate analysis, the risk of death changed slightly to a 39% lower risk (adjusted OR 0.61, 95% CI 0.52 to 0.72).
Table 4 Unadjusted and adjusted odds ratios (ORs) comparing cases with controls according to the timing of the last prescription during the period before the index date.
| Unadjusted OR | 95% CI | Adjusted OR* | 95% CI | p Value | |
|---|---|---|---|---|---|
| Any statin | |||||
| Not prescribed drug | 1.00 | 1.00 | |||
| Last script >90 days before index date | 1.80 | 1.42 to 2.28 | 1.24 | 0.93 to 1.65 | 0.136 |
| Last script ⩽90 days before index date | 0.53 | 0.46 to 0.61 | 0.61 | 0.52 to 0.72 | <0.001 |
| Atorvastatin | |||||
| Not prescribed drug | 1.00 | 1.00 | |||
| Last script >90 days before index date | 1.43 | 1.02 to 1.99 | 1.20 | 0.81 to 1.78 | 0.376 |
| Last script ⩽90 days before index date | 0.56 | 0.45 to 0.69 | 0.62 | 0.48 to 0.79 | <0.001 |
| Cerivastatin | |||||
| Not prescribed drug | 1.00 | 1.00 | |||
| Last script >90 days before index date | 1.12 | 0.73 to 1.72 | 1.20 | 0.72 to 2.00 | 0.480 |
| Last script ⩽90 days before index date | 0.62 | 0.32 to 1.23 | 0.59 | 0.26 to 1.37 | 0.220 |
| Fluvastatin | |||||
| Not prescribed drug | 1.00 | 1.00 | |||
| Last script >90 days before index date | 0.78 | 0.39 to 1.56 | 0.75 | 0.35 to 1.63 | 0.467 |
| Last script ⩽90 days before index date | 0.48 | 0.25 to 0.93 | 0.59 | 0.29 to 1.20 | 0.144 |
| Pravastatin | |||||
| Not prescribed drug | 1.00 | 1.00 | |||
| Last script >90 days before index date | 0.70 | 0.35 to 1.43 | 0.68 | 0.31 to 1.50 | 0.339 |
| Last script ⩽90 days before index date | 0.65 | 0.40 to 1.06 | 0.51 | 0.30 to 0.87 | 0.013 |
| Simvastatin | |||||
| Not prescribed drug | 1.00 | 1.00 | |||
| Last script >90 days before index date | 1.14 | 0.89 to 1.46 | 0.95 | 0.70 to 1.30 | 0.758 |
| Last script ⩽90 days before index date | 0.61 | 0.51 to 0.72 | 0.62 | 0.50 to 0.76 | <0.001 |
*Adjusted for co‐morbidity (diabetes, hypertension, CCF, myocardial infarction, cancer), angiotensin converting enzyme inhibitors, aspirin, β blockers, calcium channel blockers, smoking, body mass index, and deprivation (Townsend score in fifths). Adjusted odds ratios for individual statins were also adjusted for other statins.
These results for statins were similar when the analysis was restricted to patients with body mass index and smoking status recorded, when restricted to patients without a diagnosis of cancer before the index date, and when restricted to patients without a diagnosis of diabetes, congestive cardiac failure, or myocardial infarction. The results were also similar when the analysis was restricted to cases who survived for at least a year after diagnosis of ischaemic heart disease and their matched controls (adjusted OR for current use of statins compared with patients not prescribed statins since diagnosis of ischaemic heart disease 0.62, 95% CI 0.51 to 0.76).
Comparison for individual statins
When examining the effect of the individual statins, we determined a significant protective effect on risk of death for patients taking atorvastatin or simvastatin. Compared with patients who had not been prescribed the drug since diagnosis of ischaemic heart disease, the adjusted OR was 0.62 (95% CI 0.48 to 0.79) for atorvastatin and 0.62 (95% CI 0.50 to 0.76) for simvastatin. A direct comparison between atorvastatin and simvastatin by Wald's test showed no significant difference between the two drugs (p = 0.97). The magnitudes of the adjusted ORs for patients taking the other three statins (cerivastatin, fluvastatin, and pravastatin) were similar to those for atorvastatin and simvastatin but failed to reach the 0.01 significance level, due to the smaller number of patients taking these drugs.
Effect of age, sex, and diabetes on effectiveness of statins
We found no evidence of an interaction between statins and sex. The adjusted OR for current use of statins was 0.64 (95% CI 0.52 to 0.79) for men and 0.57 (95% CI 0.44 to 0.76) for women (test for interaction p = 0.94) compared with those not prescribed statins since diagnosis of ischaemic heart disease. Similarly, there was no evidence of an interaction between statins and age (test for interaction p = 0.59) with an adjusted OR for current use of statins of 0.65 (95% CI 0.51 to 0.84) for people aged less than 75 and an adjusted OR of 0.60 (95% CI 0.48 to 0.75) for people aged 75 and over. For people with diabetes the adjusted OR for current use of statins was 0.60 (95% CI 0.50 to 0.72) and for people without diabetes it was 0.68 (95% CI 0.48 to 0.97); this was also not a significant interaction (test for interaction p = 0.43). This means that the benefits of statins were not affected by age, sex, or presence of diabetes.
Duration of use of statins
We used the case–control analysis to examine the effect of duration of statin usage on risk of death. Table 5 shows the results. Longer duration of usage was associated with a lower OR for risk of death. The test for trend was significant (p < 0.001) with a 19% reduction in risk of death with each additional year of treatment (adjusted OR 0.81 95% CI 0.77 to 0.86 per year).
Table 5 Adjusted OR for duration of use of statins on survival determined by the nested case–control analysis.
| Duration (months) | Proportion of cases (n = 2266) | Proportion of controls (n = 9064) | OR* | 95% CI | p Value |
|---|---|---|---|---|---|
| No statins | 1821 (80.4%) | 6761 (74.6%) | 1.00 | ||
| 1–12 | 217 (9.6%) | 967 (10.7%) | 0.80 | 0.66 to 0.97 | 0.020 |
| 13–24 | 95 (4.2%) | 529 (5.8%) | 0.60 | 0.46 to 0.78 | <0.001 |
| 24–36 | 57 (2.5%) | 348 (3.8%) | 0.47 | 0.34 to 0.67 | <0.001 |
| 37–48 | 46 (2.0%) | 226 (2.5%) | 0.48 | 0.32 to 0.71 | <0.001 |
| 49–60 | 23 (1.0%) | 139 (1.5%) | 0.54 | 0.32 to 0.92 | 0.021 |
| >60 | 7 (0.3%) | 94 (1.0%) | 0.20 | 0.08 to 0.47 | <0.001 |
*OR adjusted for co‐morbidity (diabetes, hypertension, CCF, myocardial infarction, cancer), angiotensin converting enzyme inhibitors, aspirin, β blockers, calcium channel blockers, smoking, body mass index, and deprivation (Townsend score in fifths).
DISCUSSION
This was a large community based study to determine the effects of statins on survival of unselected patients with a first diagnosis of ischaemic heart disease in primary care. Both the cohort and nested case–control analyses in our study confirm that the benefits of statins extend to unselected patients in a non‐trial setting including the elderly,16 those with diabetes, and women. The benefits can be seen within the first two years of treatment and continue to accrue over time. The reduction in mortality is similar for the two most commonly prescribed drugs (atorvastatin and simvastatin) and probably also applies to the other statins, though this did not reach significance due to the relative low usage.
Comparison of our results with trial results
Our study was larger than the 4S,1 which randomly assigned 2221 patients to simvastatin and 2223 patients to placebo (median duration of follow up 5.3 years). We found that patients taking simvastatin had a 39% lower mortality (95% CI 25% to 50%), which is comparable with the 30% reduction reported over a shorter time in the 4S (95% CI 15% to 42%). The six year survival for treated patients in the 4S was 91.3% in the simvastatin group and 87.7% in the placebo group. These survival figures in the 4S are higher than those we report (89% with statins, 66% without statins), which suggests that the 4S trial population was a healthier population than our study population. If this is the case, then the absolute benefit of statins in reducing mortality in the community is probably greater than anticipated. Now that the patents for some statins have expired, the cost‐benefit ratio is also likely to be even more favourable.
Discussion of methods
We used a nested case–control approach in addition to the cohort analysis to examine duration response and test for interactions. Our cases and controls were well matched on age, sex, and index date, making this an appropriate environment to examine how the benefits of statins accrue over time. Our outcome (that is, whether patients died) is likely to be well recorded on the general practitioner clinical database because there is a national electronic procedure in the UK that comes into operation when a patient dies. This automatically updates the patient's health electronic record with the date of the patient's death. There was no recall bias, as the exposure data were recorded on computer before the date of death or pseudo‐death. Misclassification of exposure status is unlikely, as more than 99% of all general practitioners' repeat prescriptions are recorded on computer; at the time of the study, statins were not available over the counter. By excluding patients with a diagnosis of ischaemic heart disease within the first three months of registration with their practice, we reduced information bias that can result on registration if pre‐existing diseases are recorded as if they were new events.
In observational studies of the intended benefits of drugs, indication bias is an important issue for consideration. To examine this we repeated the analyses excluding patients with diabetes or congestive cardiac failure or myocardial infarction and found very similar results. We also examined the possibility that the benefits of statins can be exaggerated if they were less likely to be prescribed to people with cancer or with a short life expectancy after a diagnosis of ischaemic heart disease by excluding these groups from the analysis and again found the effects of statins to be similar to the effect found in the overall analysis.
Although we have adjusted for several confounding variables, residual confounding may result from misclassification of those variables and confounding by unmeasured variables. Such effects would have to be very large to account for the substantial protective effects reported here.
Validation of the QRESEARCH database
The QRESEARCH database has been validated by comparing the age–sex structure of the population with the 2001 census; birth and death rates with figures from the Office for National Statistics; prescribing rates with prescribing analysis and cost (PACT) data; consultation rates with data from the general household survey; and prevalence data for common conditions with published data and data from similar databases such as the General Practice Research Database. We found a good correspondence for all of these measures (results are available upon request). We have also compared practices taking part in regional research networks on these and other measures and found a good correspondence.17 Detailed analyses have shown good levels of completeness and consistency.18
Conclusion
The benefits of statins found in randomised controlled trials extend to unselected community based patients. Since patients in the community are likely to be at higher risk than those in trials, the potential benefits from statins are likely to be greater than expected.
ACKNOWLEDGMENTS
We thank the EMIS practices contributing to the QRESEARCH database and to Dr David Stables (EMIS Computing) for his help and expertise in establishing QRESEARCH.
Abbreviations
4S - Scandinavian simvastatin survival study
CI - confidence interval
OR - odds ratio
PACT - prescribing analysis and cost
Footnotes
Funding: None
Competing interests: None declared.
Ethical approval: Trent Multi‐Centre Research Ethics Committee.
Contributorship: JHC initiated and designed the study, obtained ethical approval, undertook the data extraction and manipulation, undertook the analysis, and drafted the paper. CC contributed to the study design and core ideas, supervised, undertook and checked the analysis, advised on interpretation, and contributed to drafting the paper.
References
- 1.Scandinavian Simvastatin Survival Group Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4S). Lancet 19943441383–1389. [PubMed] [Google Scholar]
- 2.Long‐term Intervention with Pravastatin in Ischaemic Heart Disease (LIPID) Study Group Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 19983391349–1357. [DOI] [PubMed] [Google Scholar]
- 3.Sacks F M, Pfeffer M A, Moye L A. The effects of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. The CARE study. N Engl J Med 19963351001–1009. [DOI] [PubMed] [Google Scholar]
- 4.Heart Protection Study Collaborative Group MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high risk individuals: a randomised placebo‐controlled trial. Lancet 20023607–22.12114036 [Google Scholar]
- 5.Cannon C, Braunwald E, McCabe C.et al Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 20043501495–1504. [DOI] [PubMed] [Google Scholar]
- 6.Hippisley‐Cox J, Cater R, Pringle M.et al A cross‐sectional survey of the effectiveness of lipid lowering drugs in lowering serum cholesterol in 17 general practices: how well do they work? BMJ 2003326689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haines A, Jones R. Implementing findings of research. BMJ 19943081488–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beral V. Breast cancer and hormone replacement therapy in the Million women study. Lancet 2003362419–427. [DOI] [PubMed] [Google Scholar]
- 9.Hippisley‐Cox J, Pringle M, Crown N.et al A case‐control study on the effect of hormone replacement therapy on ischaemic heart disease. Br J Gen Pract 200353191–196. [PMC free article] [PubMed] [Google Scholar]
- 10.Lagro‐Janssen A, Rosser W, Van Weel C. Breast cancer and hormone replacement therapy: up to general practice to pick up the pieces. Lancet 2003362414–415. [DOI] [PubMed] [Google Scholar]
- 11.Anon From research to practice. Lancet 1994344417–418. [PubMed] [Google Scholar]
- 12.Rothwell P. Can overall results of clinical trials be applied to all patients? Lancet 19953451616–1619. [DOI] [PubMed] [Google Scholar]
- 13.Jick H Z G L, Jick S S, Seshadri S.et al Statins and the risk of dementia. Lancet 20003561627–1631. [DOI] [PubMed] [Google Scholar]
- 14.Jick H, Kaye J, Vasilakis‐Scaramozza C.et al Risk of venous thromboembolism among users of third generation oral contraceptives compared with users of oral contraceptives with levonorgestrel before and after 1995: cohort and case‐control analysis. BMJ 20003211190–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hippisley‐Cox J, Pringle M, Cater R.et al Electronic record in primary care: regression or progression? Cross‐sectional survey. BMJ 20033261439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shepherd J, Blauw G, Murphy M.et al Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 20023601523–1630. [DOI] [PubMed] [Google Scholar]
- 17.Hammersley V, Hippisley‐Cox J, Wilson A.et al A comparison of research general practices and their patients with other practices: cross sectional survey in Trent. Br J Gen Pract 200252463–468. [PMC free article] [PubMed] [Google Scholar]
- 18.Hippisley‐Cox J, Hammersley V, Pringle M.et al How useful are general practice databases for research? Analysis of their accuracy and completeness in one research network. Health Informatics J 20041091–109. [Google Scholar]