Abstract
Objective
To investigate the spontaneous occurrence of circulating mesenchymal stem cells (MSC) and angiogenic factors in patients with ST elevation acute myocardial infarction (STEMI) treated with primary percutaneous coronary intervention (PCI).
Design
In 20 patients with STEMI, blood samples were obtained on days 1, 3, 7, 14, 21, and 28 after the acute PCI. Fifteen patients with a normal coronary angiography formed a control group. MSC (CD45−/CD34−), plasma stromal derived factor 1 (SDF‐1), vascular endothelial growth factor A (VEGF‐A), and fibroblast growth factor 2 (FGF‐2) were measured by multiparametric flow cytometry and enzyme linked immunosorbent assay (ELISA).
Results
Circulating CD45−/CD34− cells were significantly decreased on day 7 compared with day 3. Cell counts normalised one month after the acute onset of STEMI. The changes were mainly seen in patients with a large infarction. Plasma SDF‐1 increased significantly from day 3 to day 28, and VEGF‐A and FGF‐2 increased significantly from day 7 to day 28.
Conclusions
Spontaneous sequential fluctuations in MSC and the increase in vascular growth factor concentrations after STEMI suggest that the optimal time for additional stem cell therapy is three weeks after a myocardial infarction to obtain the maximum effects by stimulating endogenous growth factors on the delivered stem cells.
Keywords: stem cell, cytokines, angiogenesis, vasculogenesis, acute myocardial infarction
Acute occlusion of a major coronary artery leads to irreversible damage of cardiomyocytes and vascular structures followed by scar replacement in the ventricle. The main therapeutic focus for acute myocardial infarction has been on early reperfusion to salvage myocardial tissue and limit remodelling. Recent studies of myocardial regeneration after ST elevation acute myocardial infarction (STEMI) have concentrated on stem cell based therapy.1,2,3,4,5,6
Circulating endothelial progenitor cells derived from the bone marrow can home to and differentiate in situ in ischaemic myocardium and they are involved in myocardial vasculogenesis.1,3 In addition, improved cardiac function has been reported after intracoronary treatment with mononuclear bone marrow cells in patients with acute STEMI.4,7
The content of stem cells or progenitor cells within the mononuclear bone marrow cell fraction from bone marrow solutions used in clinical trials is only 2–3%, however, and most stem cells are haematopoietic.4,7,8 Although clinical studies have shown the feasibility and safety of haematopoietic stem cell based therapy for vasculogenesis, the differentiation of these stem cells into new cardiomyocytes has been challenged.9 This issue may call into question the mechanistic underpinnings of such clinical trials. Recently more attention has focused on the use of bone marrow derived mesenchymal stem cells (MSC).
MSC is another rare population of multipotent non‐haematopoietic CD45−/CD34− stem cells in the bone marrow.10 Although perhaps 0.001–0.01% of the nucleated cells in bone marrow are MSC, they can be isolated from the bone marrow and amplified in vitro.11 This makes it possible to produce sufficient MSC for autologous clinical cell therapy. The fact that MSC can be stimulated to lineage differentiation into either blood vessels or other tissues makes these cells particularly attractive for stem cell based therapy.3,12,13,14,15 Furthermore, it has been shown that intramyocardial injection of autologous MSC or intravenous administration of MSC can increase vasculogenesis and improve cardiac function in myocardial infarction in rats.16,17
The angiogenic factors, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor 2 (FGF‐2), seem to stimulate the development of new blood vessels.18,19,20 In addition, the stem cell homing factor, stromal derived factor 1 (SDF‐1), is considered important in the migration and trafficking of stem cells and progenitor cells into the infarcted myocardium. It can affect stem cell proliferation and mobilisation and it contributes to ischaemic neovascularisation by enhancing stem cell recruitment into ischaemic tissue.21,22
To generate and establish new clinical treatment strategies with stem cells or growth factors in patients with acute STEMI, it is essential to elucidate the natural and spontaneous occurrence of such variables in the regenerative process to find the optimal treatment period.4,7
The objective of the present study was to evaluate the possible physiological mobilisation of both MSC and endothelial progenitor cells and to investigate the natural variation of stem cell homing factor SDF‐1 and the vascular growth factors VEGF‐A and FGF‐2 in patients with acute STEMI treated with primary percutaneous coronary intervention (PCI).
METHODS
Patient population
Twenty consecutive patients with STEMI (17 men and three women; mean (SEM) age 58 (3) years) treated with PCI within 12 hours after the onset of symptoms were enrolled in the study. The control group consisted of 15 controls (11 men and four women; 55 (2) years) referred for suspicion of angina pectoris but with normal coronary angiography. Exclusion criteria were malignant disease, inflammatory disease, and severe kidney disease. This study protocol complied with the Declaration of Helsinki and was approved by the local scientific ethics committee (KF 01–112/01). All patients received oral and written information about the study and signed an informed consent form.
Blood samples
In patients with STEMI, 50 ml venous blood samples were obtained within 24 hours after admission on day 1 (baseline) and days 3, 7, 14, 21, and 28 after the onset of STEMI. In control subjects, 50 ml venous blood samples were obtained only once after coronary angiography. The blood for stem cell assessment was kept at room temperature until analysis within a few hours after drawing. Plasma was obtained by centrifugation (3000 rpm, 10 minutes at 4°C) and stored at −20°C until analysis. In the patients with STEMI, serum creatine kinase (CK) MB fraction was measured every six hours over the first 24 hours.
Enumeration of MSC by flow cytometry
Circulating putative MSC in blood was quantified as CD45− and CD34− cells by multiparametric flow cytometry as previously described.23 A panel of monoclonal antibodies and one polyclonal antibody were measured: anti‐CD45 (HI30 clone, IgG1; Becton Dickinson) and anti‐CD34 (8G12 clone, IgG1; Becton Dickinson) to exclude haematopoietic cells, and anti‐CD105 (endoglin, NI‐3A1 clone, IgG1; Ancell, Bayport, Minnesota, USA), anti‐CD106 (vascular cell adhesion molecule, 51‐10C9 clone, IgG1; Becton Dickinson), anti‐CD31 (platelet endothelial cell adhesion molecule 1 (PECAM‐1), WM59 clone, IgG1; Becton Dickinson), anti‐CD133 (haematopoietic stem cell antigen, AC133 clone, IgG1; Miltenyi Biotec), anti‐CD59 (protectin, P282(H19) clone, IgG2a; Becton Dickinson), anti‐CD146 (melanoma cell adhesion molecule, PIH12 clone, IgG1; Becton Dickinson), anti‐CD36 (glycoprotein IIIb receptor, CB38‐NL07‐EGM clone, IgG1; Becton Dickinson), anti‐CD73 (phosphatidylinositol linked, AD2 clone, IgG1; Becton Dickinson), anti‐CD166 (activated leucocyte adhesion molecule, 3A6 clone, IgG1; Becton Dickinson), anti‐CD13 (myeloid cells, aminopeptidase N, F0831 clone, IgG1; DACO), anti‐CD3 (T cell, SK7 clone, IgG1; Becton Dickinson), anti‐CXC chemokine receptor 4 (CXCR‐4, 12G5 clone, IgG2a; R&D Systems), VEGF‐R2 (KDR; R&D Systems), and anti‐CD144 (vascular endothelial cadherin, BMS158FI Polyclonalt; Bender MedSystems, Vienna, Austria).
Analytical gates were used to enumerate total number and subsets of circulating CD45−/CD34− cells. Monoclonal antibodies were conjugated with fluorescein isothiocyanate, R phycoerythrin, peridinin chlorophyll protein, or allophycocyanin. Cell suspensions were evaluated by a FACSCalibur (Becton Dickinson, San Jose, California, USA). After acquisition of at least 100 000 cells/sample, analyses were regarded as informative when adequate numbers of CD45−/CD34− events (typically 3–400) were collected in the analytical gate (fig 1).
Figure 1 Flow cytometry identification and enumeration of circulating progenitor cells. (A) Representative experiment showing the gating strategy used to exclude platelets, erythrocytes, death cells, and debris. (B, C) Gating strategy used to exclude haematopoietic cells expressing the CD45 (gate R2 indicates CD45− mononuclear bone marrow cells) and CD34 antigen. (D, E) Acquisition gate used for sampling of data and further analysis of subsets (CD105+ and CD31+). APC, allophycocyanin; FITC, fluorescein isothiocyanate; FSC‐H, forward scatter height; PerCP, peridinin chlorophyll protein; SSC‐H, side scatter height.
In vitro cell culture angiogenesis assay for MSC
MSC were isolated by the magnetic cell separation beats method with CD34 and CD45 antibodies. MSC (5000–10 000) were plated in liquid culture in a 96 well plate coated with a solidified angiogenic matrix solution (Chemicon International, Inc, Aarhus, Denmark) and maintained in an incubator at 37°C for 12 hours. Endothelial tube formation was observed and digitally photographed under an inverted light microscope at 10× magnifications.
Quantification of plasma VEGF‐A, FGF‐2, and SDF‐1
Plasma SDF‐1, VEGF‐A, and FGF‐2 concentrations were measured in duplicate by a colorimetric enzyme linked immunosorbent assay (ELISA) kit (R&D Systems). The lower limits of detection were 18 pg/ml for SDF‐1 and 10 pg/ml for VEGF‐A and FGF‐2.
Statistical analysis
All results are expressed as mean (SE). Differences between groups were analysed by the Mann‐Whitney U test for unpaired samples. Sequential changes were measured within patient group by the Wilcoxon signed ranks test for paired samples. The interaction between maximum serum CK‐MB concentration and the maximum number of MSC in peripheral blood and maximum plasma SDF‐1, VEGF‐A, and FGF‐2 concentration was examined by multivariate analysis with the general linear model. The interaction between known risk factors (age, sex, diabetes, hypertension, smoking, and hyperlipidaemia medication) before STEMI and cytokines and stem cells was analysed by a multivariate analysis by the multiple stepwise logistic regression model. All data were analysed with the SPSS statistical analysis program (SPSS version 11.0, SPSS Inc, Chicago, Illinois, USA). A value of p < 0.05 was considered significant.
RESULTS
Patient characteristics
Table 1 presents the demographic characteristics of the 20 patients with STEMI and 15 controls. Groups were comparable with respect to age, sex, and incidence of hypertension and diabetes mellitus. All STEMI patients had the standard medication and complete reperfusion with TIMI (thrombolysis in myocardial infarction) grade 3 flow after primary PCI with stent implantation. In the follow up period they were treated with standard aspirin, clopidogrel, angiotensin converting enzyme inhibitor, β blockers, and lipid lowering treatment, and they were free of ischaemic events for 28 days after the onset of acute STEMI.
Table 1 Demographic and haemodynamic characteristics of patients with ST elevation myocardial infarction (STEMI) and control subjects.
| STEMI (n = 20) | Controls (n = 15) | p Value | |
|---|---|---|---|
| Men/women | 17/3 | 11/4 | NS |
| Age (years) | 58 (3) | 55 (2) | NS |
| Smokers | 10 (50%) | 3 (20%) | NS |
| Hypertension | 7 (35%) | 3 (20%) | NS |
| Diabetes mellitus | 4 (20%) | 1 (5%) | NS |
| Hyperlipidaemia medication | 19 (95%) | 6 (40%) | NS |
| Infarct related coronary artery | |||
| LAD | 9 | NA | |
| LCx | 2 | NA | |
| RCA | 9 | NA | |
| Peak CK‐MB (mg/l) | 372 (53) | NA | |
Data are mean (SE) or number (%).
CK, creatine kinase; LAD, left anterior descending artery; LCx, left circumflex artery; NA, not applicable; NS, not significant; RCA, right coronary artery.
Variation of circulating stem cells enumerated by flow cytometry
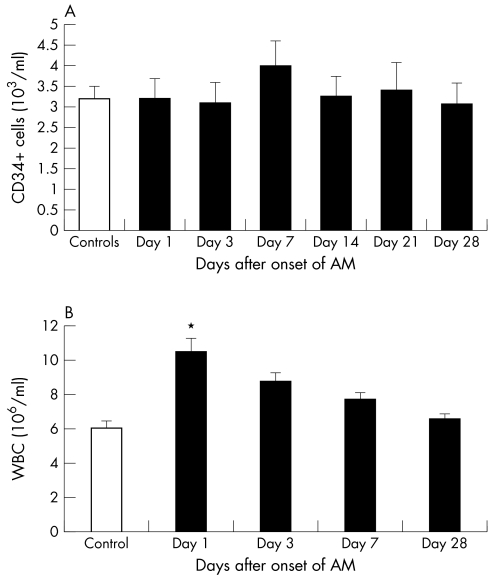
Circulating MSC (CD45−/CD34− cells) were significantly decreased in peripheral blood on day 7 compared with day 3 (fig 2A). MSC concentration in STEMI patients and controls did not differ (219 (46) × 103/ml) at any time point. Analysis of a range of subpopulations among the CD45−/CD34− cells identified significant variations for CD105+, CD31+, CD36+, and CD59+ cells indicating selective variations associated with STEMI (table 2). The endothelial CD105+ (endoglin) MSC cells decreased significantly on day 3 after the onset of STEMI compared with baseline and the control group (p < 0.05 for all) (fig 2B, table 2). Thereafter, circulating CD105+ MSC normalised on day 7 and again decreased to the minimum concentration on day 28. Circulating MSC cells with the endothelial marker CD31+ (PECAM‐1) were initially decreased significantly and then gradually normalised during the weeks after STEMI (fig 2C). Circulating CD34+ cells in patients with STEMI did not change and they were not different from controls at any time point after STEMI (fig 3A). Circulating leucocyte increased significantly only on day 1 compared with controls and then gradually decreased to the normal concentration (p < 0.05) (fig 3B).
Figure 2 (A) Absolute numbers of circulating mesenchymal stem cells (MSC) (CD45−/CD34−) and the subtypes (B) CD105+ MSC and (C) CD31+ MSC in the first month after acute ST elevation acute myocardial infarction (AMI) treated with primary percutaneous coronary intervention (PCI). *p < 0.05 day 7 versus day 3; †p < 0.05 day 3 and day 28 versus day 1.
Table 2 Analysis of a range of subpopulations among the CD45−/CD34− cells from patients after onset of STEMI and from control subjects.
| Day 1 (103/ml) | Day 3 (103/ml) | Day 7 (103/ml) | Day 28 (103/ml) | Controls (103/ml) | |
|---|---|---|---|---|---|
| CD105 (endoglin) | 4.96 (1.27) | 2.19 (0.61)*† | 4.16 (0.81) | 1.45 (0.57)*† | 4.91 (1.32) |
| CD106 (VCAM‐1) | 0.53 (0.28) | 0.55 (0.19) | 0.33 (0.13) | 0.09 (0.06) | 0.55 (0.28) |
| CD31 (PECAM‐1) | 9.95 (2.1) | 4.75 (0.78)*† | 6.98 (1.46) | 8.55 (1.59) | 10.46 (2.11) |
| CD133 | 0.36 (0.2)† | 0.09 (0.06)† | 0.37 (0.14)† | 0.19 (0.15)† | 2.38 (0.73) |
| CD59 (Protectin) | 5.02 (3.49) | 3.38 (2.28) | 1.27 (0.25)† | 1.51 (0.37)† | 4.79 (1.16) |
| CD146 (MEL‐CAM) | 0.07 (0.05) | 0.09 (0.06) | 0.27 (0.13) | 0.08 (0.08) | 0.09 (0.09) |
| CD36 (Gp IIIb receptor) | 13.98 (4.86) | 8.89 (1.36) | 7.71 (1.08)† | 8.81 (1.03) | 17.64 (5.17) |
| CD73 (PI linked) | 1.82 (0.52) | 1.26 (0.22) | 0.88 (0.11) | 1.65 (0.31) | 1.72 (0.47) |
| CD144 (endothelial) | 5.17 (3.06) | 3.59 (0.65) | 2.62 (0.52) | 3.99 (0.86) | 5.19 (1.41) |
| CD166 (ALCAM) | 0.76 (0.27) | 0.55 (0.21) | 0.74 (0.13) | 0.54 (0.15) | 0.91 (0.33) |
| CD13 (APN) | 0.44 (0.12) | 0.33 (0.11) | 0.41 (0.16) | 0.19 (0.1) | 0.67 (0.27) |
| CD3 (T cell) | 30.37 (8.4)† | 43.3 (10.11) | 23.43 (6.66)† | 35.61 (7.21) | 51.88 (13.4) |
| VEGFR‐2 | 2.59 (0.93) | 2.46 (0.47) | 1.53 (0.22) | 1.51 (0.26) | 2.38 (0.66) |
Data are mean (SE).
*p<0.05 versus day 1; †p<0.05 versus control group.
ALCAM; activated leucocyte adhesion molecule; APN, aminopeptidase N; Gp, glycoprotein; LPS, lipopolysaccharide; MEL‐CAM, melanoma cell adhesion molecule; PECAM‐1, platelet endothelial cell adhesion molecule; PI, phosphatidylinositol; VCAM‐1, vascular cell adhesion molecule; VEGFR‐2, vascular endothelial growth factor 2.
Figure 3 (A) Circulating CD34+ cells and (B) white cells (WBC) the first month after acute STEMI treated with primary PCI and in control subjects.
The SDF‐1 receptor CXCR‐4 was not included in the initial panel for CD45−/CD34− subpopulations. To verify that the receptor was present on the CD45−/CD34− cells we have additionally measured it on CD45−/CD34− cells from 25 patients with STEMI treated with primary PCI the day after STEMI. We found 11.1 (1.8) × 103 CXCR‐4 cells/ml.
MSC angiogenesis cell assay
Cultured MSC appear fibroblastic and homogeneous in size and morphology by the second culture passage (fig 4A). Twelve hours after MSC were seeded on an angiogenic culture plate, they differentiated into endothelial‐like cells and formed tubes (fig 4B). Immunohistochemical study showed von Willebrand factor positive cells located in the endothelial tube formation (fig 4C).
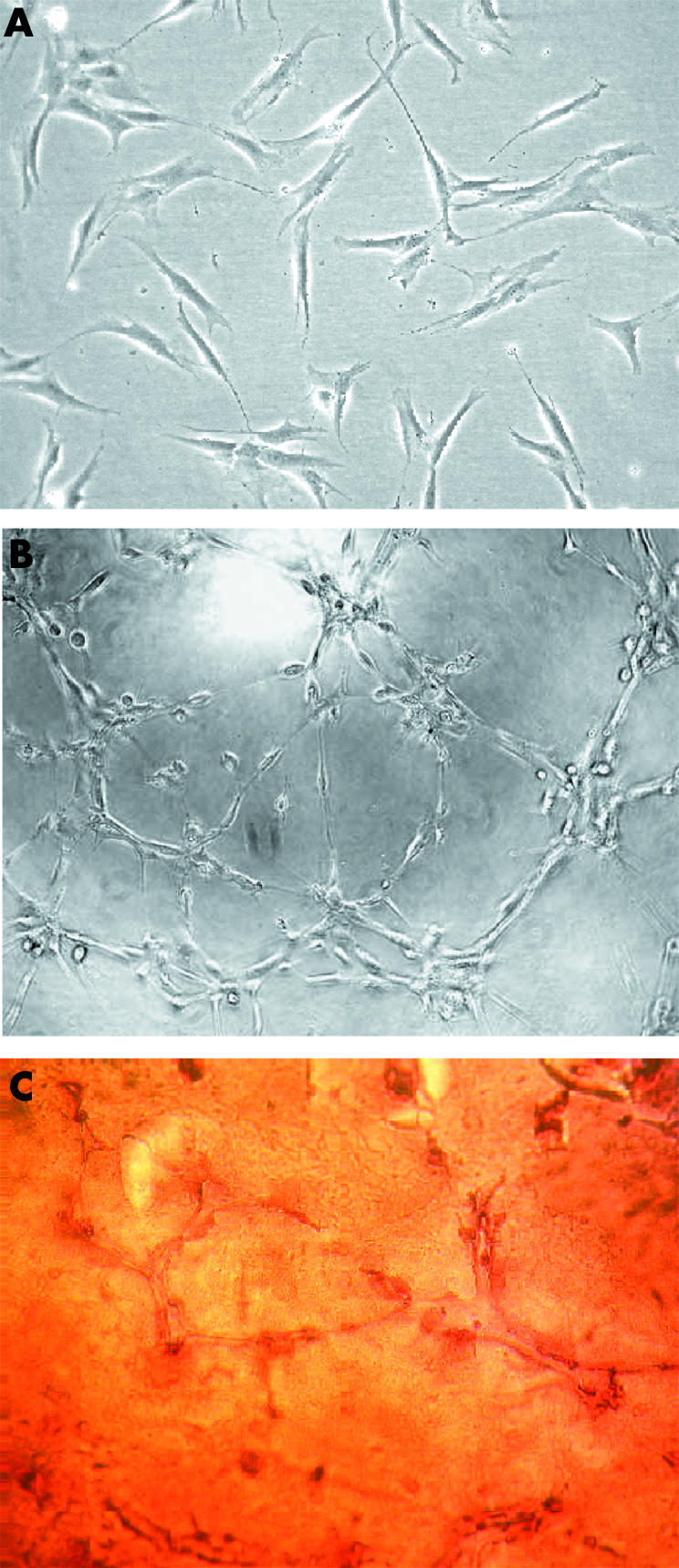
Figure 4 (A) CD45−/CD34− MSCs grew in medium containing Dulbecco's minimum essential medium and 10% EMEA‐fetal bovine serum after the second culture passage, where they appear fibroblastic and homogeneous in size and morphology. (B) The potential of the MSCs to form blood vessels was tested by cultured on Matrigel (BD Biosciences) for 12 hours. The cells differentiated into endothelial‐like cells and formed tubes. (C) Immunohistochemical verification of the endothelial von Willebrand factor in cells located in the endothelial tube formation. Original magnification 10×. EMEA, European Agency for the Evaluation of Medicinal Products.
Plasma SDF‐1, VEGF‐A, and FGF‐2
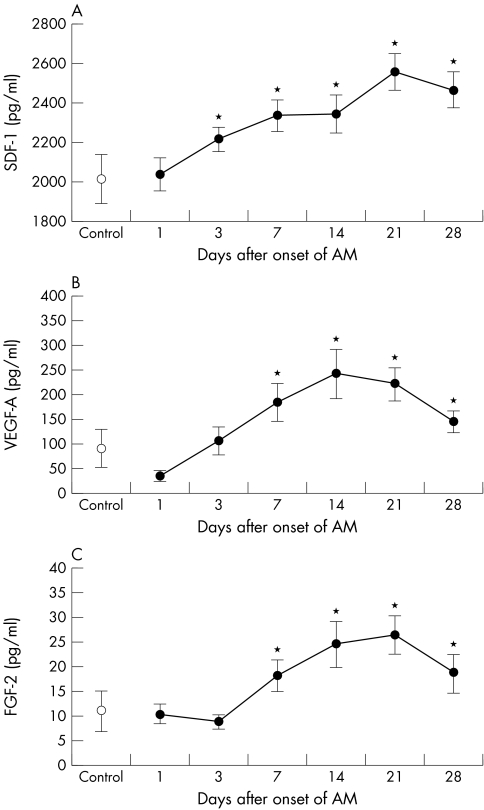
In patients with STEMI, plasma SDF‐1 increased significantly from day 3 to day 28 with the peak on day 21 compared with day 1 (p < 0.05 for all) (fig 5A). Plasma VEGF‐A tended to be reduced on day 1 compared with controls and gradually increased significantly to a maximum concentration on day 14 (244 (49) pg/ml, p < 0.05). Thereafter, VEGF‐A concentration gradually decreased but remained increased on day 28 compared with day 1 (p < 0.05 for all) (fig 5B). Significant increases in plasma FGF‐2 concentration in patients with STEMI were found from day 7 to day 28 with the peak on day 21 compared with day 1 (p < 0.05 for all) (fig 5C). There was no relation between left ventricular ejection fraction at the time of the acute infarction and plasma SDF‐1, VEGF‐A, FGF‐2, and circulating stem cell concentrations at any time point after STEMI. Maximum MSC count did not correlate with maximum plasma SDF‐1, VEGF‐A, and FGF‐2 concentrations.
Figure 5 Plasma (A) stromal derived factor 1 (SDF‐1), (B) vascular endothelial growth factor A (VEGF‐A), and (C) fibroblast growth factor 2 (FGF‐2) concentrations one month after ST elevation AMI in patients treated with primary PCI and in control subjects. *p < 0.05 versus day 1.
No correlation between any of the risk factors of the patients and circulating MSC or cytokines was found in any of the time points after STEMI. In addition, in a multivariate analysis of all variables, we did not find a significant effect of serum maximum CK‐MB concentration on maximum plasma SDF‐1, VEGF‐A, and FGF‐2 or maximum number of MSC in peripheral blood.
Impact of infarct size on concentration of MSC and growth factors
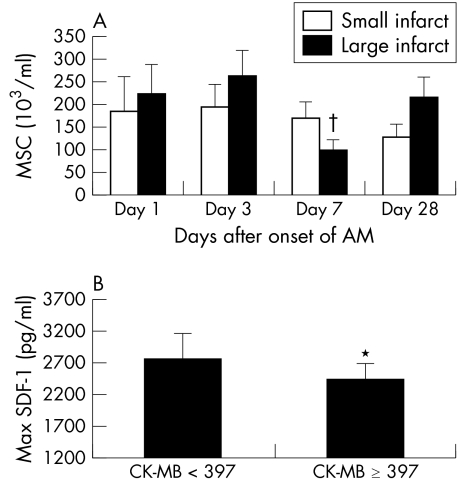
All of the patients had large infarctions in this study (table 1). To investigate the influence of infarct size, 20 patients with STEMI were divided into two groups according to their maximum serum CK‐MB concentration by using the median CK‐MB concentration for the entire group as the cut off point (397 mg/l): a large infarct group with CK‐MB ⩾ 397 mg/l (n = 11, CK‐MB 542 (54) mg/l) and a small infarct group with CK‐MB < 397 mg/l (n = 9, CK‐MB 169 (37) mg/l). In the large infarct group, circulating MSC tended to fluctuate more and there was significantly less circulating MSC on day 7 than on day 1, day 3, and day 28 (p < 0.05) (fig 6A). Maximum plasma SDF‐1 concentration was significantly lower in the large infarct group than in the small infarct group (p < 0.05) (fig 6B), whereas no significant differences of plasma VEGF‐A and FGF‐2 concentrations were seen between the groups.
Figure 6 (A) Circulating MSC and (B) maximum plasma SDF‐1 concentrations in patients with small infarcts (creatine kinase (CK) MB < 397 mg/l) and large infarcts (CK‐MB ⩾ 397 mg/l). *p < 0.05; †p < 0.05 day 7 versus day 1, day 3, and day 28 in the CK‐MB ⩾ 397 mg/l group.
DISCUSSION
The main findings of present study are evidence of acute and early changes in both circulating MSC (CD45−/CD34−) and plasma stem cell homing and vascular growth factor concentrations in patients with acute STEMI treated with primary PCI.
MSC are of non‐haematopoietic origin but are also present in the bone marrow. They are CD34 and CD45 negative. These multipotent MSC can differentiate into endothelial cells and are involved in the development of new blood vessels.13 Our in vitro studies showed that human MSC can differentiate into endothelial cells positive for von Willebrand factor and that they contribute to endothelial tube formation. Studies have shown that bone marrow derived and exogenously administrated stem cells are localised to the peri‐infarct region after myocardial infarction.2,9,15 Therefore, our finding of a decrease in circulating MSC within one week after STEMI may indicate enhanced recruitment of these MSC to the damaged myocardium. They may thereby cooperate with the increased concentration of angiogenic factors and be involved in new blood vessel formation during the subacute phase of STEMI. Therefore, the optimal time window for MSC based therapy should be during this period.
It is well known that migration is essential for stem cells or progenitor cells to invade an ischaemic tissue. SDF‐1 is of great importance for mobilisation of stem cells from bone marrow and it can enhance stem cell homing after myocardial infarction, caused by an upregulation of SDF‐1 production in hypoxic tissue.22,24,25 To verify that the SDF‐1 receptor CXCR‐4 was present on the chosen MSC in this study, we have additionally measured CXCR‐4 in 25 patients within 24 hours after an acute STEMI treated by primary PCI.
We found CXCR‐4 on a large proportion of the MSC cells. Therefore, increased plasma SDF‐1 concentration may be a pathophysiologically relevant upregulation of a chemoattractant for enhancing the recruitment of circulating stem cells or progenitor cells into the damaged myocardium after STEMI.26 Our study patients with larger infarctions had lower plasma SDF‐1 concentrations than did patients with smaller infarctions. This is in agreement with an animal study showing an initial decrease in serum SDF‐1 caused by trapping of SDF‐1 in the peri‐infarct area.22
Moreover, the tendency for circulating MSC concentrations to rise acutely after STEMI in patients with a very large infarct (CK‐MB ⩾ 397 mg/l) followed by a significant reduction in the subacute phase indicated that myocardial infarct size may influence both the mobilisation of MSC from bone marrow to peripheral blood and the homing of MSC in damaged myocardium. In addition, we found that MSC with the endothelial markers CD105 and CD31 both decreased significantly on day 3. The involvement of CD45−/CD34− and CD105+ cells in vasculogenesis and microvascular development after STEMI is supported by the results showing that human bone marrow derived CD45−/CD34− and CD105+ cells can differentiate into endothelial cells in vitro.27 These findings can be interpreted as an increased mobilisation and homing of MSC, with endothelial building potential, from bone marrow into the damaged myocardium to contribute to vasculogenesis.
Inflammation is a well known and important feature of the early phase of acute myocardial infarction. At the occlusion site in coronary vessels and in the microcirculation, inflammatory cell activation and neutrophil leucocytes induce endothelial dysfunction. Within the infarcted myocardium, cytokines and inflammatory cells mediate the acute remodelling that initiates the wound repair process. MSC have a number of surface markers including CD106 and CD166, which can interact with T cells, and they can inhibit T cell proliferation and reduce the response of responder T cells to other stimulator cells in culture.14 The changes of circulating CD45−/CD34−, CD106, and CD166+ cells one week after STEMI were not significant. On the basis of the changes of these two subpopulations of circulating MSC in the early phase of STEMI, however, we can hypothesise that part of the MSC from the bone marrow help to reduce the local inflammatory response in damaged myocardium. These cells may affect myocardial healing after acute STEMI. In opposition to this, the number of circulating MSC expressing CD3 were significantly decreased at day 1 in the STEMI group compared with the control group. Only a 15% recovery of this subtype of MSC in peripheral blood one month after STEMI was found. Inflammatory activity within coronary plaques or the myocardium may cause the consumption of circulating CD34−/CD45− and CD3+ cells. Thus, we can speculate whether circulating CD34−/CD45− and CD3+ cell populations may be predictive biomarkers for instability in patients with coronary artery disease.
The homing of MSC to the ischaemic myocardium may counteract pathological remodelling after STEMI. However, the spontaneous mobilisation of MSC from the bone marrow to the peripheral blood seems not large enough to inhibit the well known and clinically important remodelling process after myocardial infarction.
We found unchanged circulating CD34+ concentrations within the first month after myocardial infarction. Thus, our results suggest that haematopoietic CD34+ cells are less important for myocardial regeneration after myocardial infarction. This is in opposition to some studies showing that CD34+ cells seem to be rapidly mobilised to colonise ischaemic tissue after myocardial infarction, where they potentially can differentiate into mature endothelial cells and contribute to neovascularisation.3,28
The increase of VEGF‐A concentrations after acute STEMI in the present study is in accordance with previous studies.29,30 VEGF‐A peaked on day 14 and then gradually normalised, implying that endogenous VEGF was continuously synthesised and secreted due to increased VEGF mRNA expression in response to the acute myocardial infarction.31 Interestingly, we observed decreased plasma VEGF concentration within the first 24 hours after STEMI. These results disagree with those of another study showing unchanged VEGF.32 However, that study included patients with infarctions three times smaller than those in the present study; thus, a lower consumption of circulating VEGF by the infarcts may explain this difference.
The difference in study populations or in VEGF antibodies used may explain this discrepancy. Measurement of serum VEGF may cause some VEGF to be released from platelets when the blood clots.33 Furthermore, the lower plasma VEGF concentration in the acute phase may be due to increased consumption of circulating VEGF for participation in angiogenesis in the infracted myocardium. The concomitant increase of FGF‐2 peaked three weeks after STEMI. This prolonged increase in plasma VEGF‐A and FGF‐2 may stimulate a subacute angiogenic cascade in the ischaemic myocardium, lasting for a long period, and may stimulate the development of coronary collateral circulation after STEMI. In patients with chronic coronary artery disease, a correlation between circulating endothelial progenitor cells and cardiac risk factors has been described.34 We found no correlation between the measured variable and risk factors in the present patients with an acute myocardial infarction.
Some limitations of our study should be considered. We have no direct evidence that upregulated SDF‐1, VEGF‐A, and FGF‐2 induced angiogenesis in the infarcted myocardium of the patients with AMI that we studied. Since platelets greatly influence SDF‐1 plasma concentrations, the differences in the influence of antiplatelet or thrombosis treatment on SDF‐1 concentrations therefore should cause caution in further study. Experimental studies are needed to investigate the location and quantification of SDF‐1, VEGF‐A, and basic FGF mRNA in and around the infarcted myocardium to clarify the pathophysiological roles of these factors in STEMI. Such information would help to explain the clinical significance of the plasma concentrations of SDF‐1, VEGF‐A, and basic FGF in patients with acute STEMI.
In conclusion, circulating MSC with endothelial characteristics decreased in patients with acute STEMI treated by primary PCI. Plasma SDF‐1 increased, which may be important for stem cell mobilisation and homing within the first four weeks after infarction. In the same period, the vascular growth factors VEGF‐A and FGF‐2 increased. On the basis of these spontaneous sequential fluctuations in MSC and the increase in vascular growth factor concentrations after STEMI, additional stem cell therapy should be optimal three weeks after the myocardial infarction to obtain maximum effects by stimulating endogenous growth factors on the delivered stem cells.
ACKNOWLEDGEMENTS
The study was supported by the Danish Heart Association (grant no 01‐2‐5‐52‐22932), Lundbeck Foundation, and the Research Foundation at Rigshospitalet.
Abbreviations
CK - creatine kinase
CXCR‐4 - CXC chemokine receptor 4
ELISA - enzyme linked immunosorbent assay
FGF‐2 - fibroblast growth factor 2
MSC - mesenchymal stem cells
PCI - percutaneous coronary intervention
PECAM‐1 - platelet endothelial cell adhesion molecule 1
SDF‐1 - stromal derived factor 1
STEMI - ST elevation acute myocardial infarction
TIMI - thrombolysis in myocardial infarction
VEGF - vascular endothelial growth factor
Footnotes
Conflict of interest: Nothing declared.
References
- 1.Orlic D, Kajstura J, Chimenti S.et al Bone marrow cells regenerate infracted myocardium. Nature 2001410701–705. [DOI] [PubMed] [Google Scholar]
- 2.Jackson K A, Majka S M, Wang H.et al Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest 20011071395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asahara T, Masuda H, Takahashi T.et al Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 199985221–228. [DOI] [PubMed] [Google Scholar]
- 4.Schachinger V, Assmus B, Britten M B.et al Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one‐year results of the TOPCARE‐AMI trial. J Am Coll Cardiol 2004441690–1699. [DOI] [PubMed] [Google Scholar]
- 5.Jørgensen E, Ripa R S, Wang Y.et al Instent Neo‐intimal hyperplasia after stem cell mobilization by granulocyte‐colony stimulating factor: preliminary intracoronary ultrasound results from a double‐blind randomized placebo‐controlled study of patients treated with percutaneous coronary intervention for ST‐elevation myocardial infarction (STEMMI trial). Int J Cardiol. (in press) [DOI] [PubMed]
- 6.Ripa R S, Wang Y, Jørgensen E.et al Safety of bone‐marrow stem cell mobilisation induced by granulocyte‐colony stimulating factor: clinical 30 days blinded results from the stem cells in myocardial infarction (STEMMI) trial. Heart Drug 20055117–182. [Google Scholar]
- 7.Wollert K C, Meyer G P, Lotz J.et al Intracoronary autologous bone‐marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 2004364141–148. [DOI] [PubMed] [Google Scholar]
- 8.Strauer B E, Brehm M, Zeus T.et al Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in human. Circulation 20021061913–1918. [DOI] [PubMed] [Google Scholar]
- 9.Murry C E, Soonpaa M H, Reinecke H.et al Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 2004428664–668. [DOI] [PubMed] [Google Scholar]
- 10.Davani S, Deschaseaux F, Chalmers D.et al Can stem cells mend a broken heart? Cardiovasc Res 200565305–316. [DOI] [PubMed] [Google Scholar]
- 11.Koc O N, Lazarus H M. Mesenchymal stem cells: heading into the clinic. Bone Marrow Transplant 200127235–239. [DOI] [PubMed] [Google Scholar]
- 12.Reyes M, Lund T, Lenvik T.et al Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood 2001982615–2625. [DOI] [PubMed] [Google Scholar]
- 13.Reyes M, Dudek A, Jahagirdar B.et al Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest 2002109337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pittenger M F, Martin B J. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res 2004959–20. [DOI] [PubMed] [Google Scholar]
- 15.Kawamoto A, Gwon H C, Iwaguro H.et al Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 2001103634–637. [DOI] [PubMed] [Google Scholar]
- 16.Tang Y L, Zhao Q, Zhang Y C.et al Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept 20041173–10. [DOI] [PubMed] [Google Scholar]
- 17.Nagaya N, Fujii T, Iwase T.et al Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol 2004287H2670–H2676. [DOI] [PubMed] [Google Scholar]
- 18.Losordo D W, Vale P R, Hendel R C.et al Phase 1/2 placebo‐controlled, double‐blind, dose‐escalating trial of myocardial vascular endothelial growth factor 2 gene transfer by catheter delivery in patients with chronic myocardial ischemia. Circulation 20021052012–2018. [DOI] [PubMed] [Google Scholar]
- 19.Kastrup J, Jorgensen E, Ruck A.et al Direct intramyocardial plasmid VEGF‐A165 gene therapy in patients with stable angina pectoris: a randomized double‐blind placebo‐controlled study. The Euroinject one trial. J Am Coll Cardiol 200545982–988. [DOI] [PubMed] [Google Scholar]
- 20.Simons M, Annex B H, Laham R J.et al Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor‐2: double‐blind, randomized, controlled clinical trial. Circulation 2002105788–793. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi J, Kusano K F, Masuo O.et al Stromal cell‐derived factor‐1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation 20031071322–1328. [DOI] [PubMed] [Google Scholar]
- 22.Abbott J D, Huang Y, Liu D.et al Stromal cell‐derived factor‐1 alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation 20041103300–3305. [DOI] [PubMed] [Google Scholar]
- 23.Mancuso P, Burlini A, Pruneri G.et al Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood 2001973658–3661. [DOI] [PubMed] [Google Scholar]
- 24.Pillarisetti K, Gupta S K. Cloning and relative expression analysis of rat stromal cell derived factor‐1 (SDF‐1): SDF‐1 alpha mRNA is selectively induced in rat model of myocardial infarction. Inflammation 200125293–300. [DOI] [PubMed] [Google Scholar]
- 25.Hattori K, Heissig B, Tashiro K.et al Plasma elevation of stromal cell‐derived factor‐1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood 2001973354–3360. [DOI] [PubMed] [Google Scholar]
- 26.Askari A T, Unzek S, Popovic Z B.et al Effect of stromal‐cell‐derived factor 1 on stem‐cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 2003362697–703. [DOI] [PubMed] [Google Scholar]
- 27.Oswald J, Boxberger S, Jorgensen B.et al Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 200422377–384. [DOI] [PubMed] [Google Scholar]
- 28.Shintani S, Murohara T, Ikeda H.et al Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 20011032776–2779. [DOI] [PubMed] [Google Scholar]
- 29.Kranz A, Rau C, Kochs M.et al Elevation of vascular endothelial growth factor‐A serum levels following acute myocardial infarction: evidence for its origin and functional significance. J Mol Cell Cardiol 20003265–72. [DOI] [PubMed] [Google Scholar]
- 30.Kawamoto A, Kawata H, Akai Y.et al Serum levels of VEGF and basic FGF in the subacute phase of myocardial infarction. Int J Cardiol 19986747–54. [DOI] [PubMed] [Google Scholar]
- 31.Hojo Y, Ikeda U, Zhu Y.et al Expression of vascular endothelial growth factor in patients with acute myocardial infarction. J Am Coll Cardiol 20005968–973. [DOI] [PubMed] [Google Scholar]
- 32.Wojakowski W, Tendera M, Michalowska A.et al Mobilization of CD34/CXCR4+, CD34/cd117+, c.met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation 20041103213–3220. [DOI] [PubMed] [Google Scholar]
- 33.Webb N J, Bottomly M J, Watson C J.et al Vascular endothelial growth factor (VEGF) is released from platelets during blood clotting: implications for measurements of circulating VEGF levels in clinical disease. Clin Sci (Colch) 199894395–404. [DOI] [PubMed] [Google Scholar]
- 34.Vasa M, Fichtlscherer S, Aicher A.et al Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 200189e1–e7. [DOI] [PubMed] [Google Scholar]