Abstract
Objectives
To compare changes in cerebral autoregulation in response to controlled, lower body negative pressure‐induced hypotension in patients with carotid sinus syndrome (CSS) and case controls.
Design
Prospective case controlled study.
Setting
Secondary and tertiary referral falls and syncope service.
Patients
17 consecutive patients with CSS and 11 asymptomatic controls.
Interventions
Hypotension insufficient to cause syncope induced by lower body negative pressure (minimum 30 mm Hg fall in systolic blood pressure (SBP)) during concomitant transcranial Doppler ultrasonography.
Main outcome measures
Cerebral autoregulation (systolic, diastolic and mean middle cerebral arterial blood flow velocities and cerebrovascular resistance) with continuous end‐tidal carbon dioxide and haemodynamic monitoring.
Results
Cerebral autoregulatory indices differed significantly between patients with CSS and controls. Systolic, diastolic and middle cerebral arterial blood flow velocities were, respectively, 9.2 m/s (95% confidence interval (CI) 2.9 to 15.4 m/s), 4.7 m/s (95% CI 1.5 to 7.9 m/s) and 6.9 m/s (95% CI 2.5 to 11.4 m/s) slower in patients with CSS. Cerebrovascular resistance was significantly greater in patients with CSS than in controls at SBP nadir and suction release; differences were 0.9 mm Hg/m/s (95% CI 0.0 to 1.7 mm Hg/m/s) and 0.8 mm Hg/m/s (95% CI 0.0 to 1.7 mm Hg/m/s), respectively. End‐tidal carbon dioxide and systemic haemodynamic variables were similar for patients and controls at baseline and during lower body negative pressure.
Conclusions
Cerebral autoregulation is altered in patients with CSS. This difference may have aetiological implications in the differential presentation with falls and drop attacks rather than syncope.
Keywords: ageing, carotid arteries, cerebral autoregulation, cerebrovascular circulation, syncope
Carotid sinus syndrome (CSS) is among the most common causes of syncope among older people, being causal in up to one quarter of those older than 70 years.1 Recent evidence has shown that patients with CSS may present with syncope or falls,2,3,4 with cardiac pacing successfully reducing the frequency of both falls and syncope in the syndrome.2,3 Amnesia for loss of consciousness is a well recognised characteristic of the syndrome and has been reproduced in experimental studies in up to 80% of patients with witnessed loss of consciousness during carotid sinus massage‐induced asystole.4 Falls are most often attributed to amnesia for loss of consciousness during syncope in the context of unwitnessed events. One possible explanation for this amnesia for loss of consciousness is an underlying derangement of cerebral autoregulation. Although cerebral autoregulation has been measured with a variety of techniques, transcranial Doppler ultrasonography (TCD) has become the most often used approach in recent years because of its non‐invasive nature, relative simplicity and accuracy.5,6,7,8,9,10,11,12,13,14,15,16,17 TCD has been used to examine cerebral autoregulation in response to hypotension both in healthy subjects9,11,12,13,14 and in patients with vasovagal syncope,10,11,12,13,14,15 but there are few data on cerebral autoregulation in patients with CSS.16,17 To study this phenomenon without the catastrophic cerebral autoregulation derangement consequent on profound asystole and hypotension induced by carotid sinus massage in patients with CSS, a more graded fall in systemic blood pressure can be induced by lower body negative pressure (LBNP), a technique used originally to study the effects of profound orthostasis on healthy people.18 The technique is now used in the diagnosis of vasovagal syncope19,20 and avoids the unnecessary risks associated with carotid sinus massage.21 Indeed, LBNP testing has previously been used to provoke experimental hypotension to evaluate cerebral haemodynamic function in healthy subjects12,13,14 and those with vasovagal syncope,15 but never in patients with CSS.
We hypothesised that patients with CSS have different cerebral autoregulatory control from case controls. The objective of this study was thus to compare changes in cerebral autoregulation in response to controlled, LBNP‐induced hypotension in patients with CSS and asymptomatic controls.
METHODS
Participants
Consecutive patients in sinus rhythm with CSS as the sole attributable cause of symptoms (as determined by investigation according to internationally recognised guidelines22,23) presenting to our tertiary referral falls and syncope service were invited to participate in the study. CSS was of the cardioinhibitory subtype and was diagnosed in patients with recurrent events who had asystole in excess of 3 s during carotid sinus massage.19 Control subjects were of similar age, sex and co‐morbidity, had no history of falls, dizziness or syncope and were recruited through a database of control subjects. Drugs were discontinued a minimum of five half lives before testing. All participants gave fully informed, written consent. The study had approval from the Newcastle and North Tyneside local research ethics committee.
Transcranial Doppler ultrasonography
Cerebral blood flow velocity was assessed with TCD following the methods of Lipsitz et al.13 After a temporal ultrasonic bone window was confirmed, the right middle cerebral artery was insonated with a 2 MHz TCD probe (Scimed, Bristol, UK), which was fixed in place by a Mueller–Moll probe fixation device. Spectral signals were observed continuously to ensure good signal quality. Systolic (SBFV), diastolic (DBVF) and mean cerebral blood flow velocities (MBFV) were recorded continuously, with cerebrovascular resistance (CVR) derived from the formula CVR = MAP/MBFV, where MAP is mean arterial blood pressure.13 Resistance and pulsatility indices (derived measures relating SBFV, DBFV and MBFV: resistance index = SBFV − DBFV/SBFV; pulsatility index = SBFV − DBFV/MBFV) were also calculated. Because the patient was supine during the procedure, correction for hydrostatic pressure differences between heart and brain was unnecessary.
Lower body negative pressure
The lower half of the participant (to the level of the iliac crests) was enclosed in an airtight chamber with a suction engine attached, which allowed calibrated negative pressure to be applied up to 90 mm Hg. The predetermined required systolic blood pressure (SBP) nadir of 30 mm Hg was achieved by supine, graded LBNP. Beat‐to‐beat SBP, diastolic blood pressure (DBP), MAP and heart rate (surface ECG at 25 m/ms) were recorded continuously through digital photoplethysmography (Finapres, Ohmeda, Wisconsin, USA).24
Experimental procedure: static and dynamic cerebral autoregulation
Subjects lay supine in a dimly lit, quiet room at a constant temperature for 15 min before the procedure. ECG and blood pressure were monitored throughout, as was end‐tidal carbon dioxide concentration (Etco2; through a close‐fitting face mask and infrared capnography), to monitor potential confounding from the effects of swings in arterial carbon dioxide concentrations on cerebral arteriolar diameter.25,26 After insonation of the middle cerebral artery and fixation of the ultrasound probe, all baseline parameters were recorded at rest (baseline) and at the four time points described in table 1 and shown graphically in fig 1. The relatively steady‐state blood pressure changes during baseline, noise, nadir (because of the graded fall in blood pressure over several minutes) and return to baseline provide a measure of static cerebral autoregulation as described by Paulson et al,5 whereas the rapid change in blood pressure over a few seconds at overshoot is more indicative of dynamic cerebral autoregulation.5
Table 1 Definition of lower body negative pressure data points.
| Time | Definition |
|---|---|
| Baseline | Peak SBP after 15 min supine rest |
| Noise | Peak SBP with suction engine on, 5 min after baseline |
| Nadir | Lowest SBP during lower body negative pressure |
| Overshoot | Highest SBP within 10 beats of release of suction |
| Return to baseline | Steady state SBP within 60 s of overshoot |
SBP, systolic blood pressure.
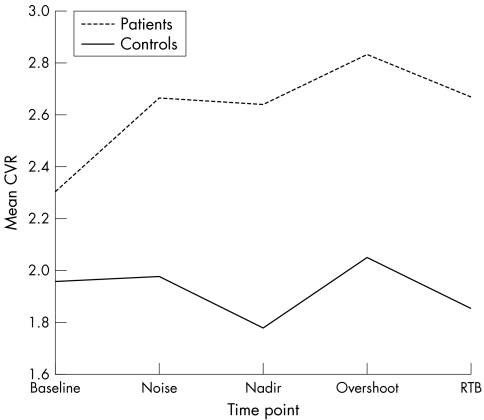
Figure 1 Cerebrovascular resistance (CVR) during lower body negative pressure in patients with carotid sinus syndrome and asymptomatic controls.
Data were recorded continuously with LabWindows software. To evaluate the beat‐to‐beat variations in systemic blood pressure, heart rate, Etco2 and cerebral blood flow velocities, an observer blinded to patient status examined the raw wave forms for each patient at each time point. The values derived at each time frame were averaged from the five beats around each point and the resulting values were averaged for each group as a whole.9,13 An independent assessor repeated this procedure in a random sample of five patients and controls to ensure agreement.
Statistical analysis
The comparison between CSS and controls was the primary objective of the research. Continuous variables (age, blood pressure) were plotted to check for outliers and wildly skewed distributions. The distribution of data was normal with the exception of RR interval responses to carotid sinus massage. Statistics are reported as mean (SD) for all comparisons with the exception of RR interval response to carotid sinus massage, which is reported as median (extreme range). Because the data are continuous variables, groups were compared at a single time point by independent sample t tests and over the five time points—baseline, noise, nadir, overshoot and return to baseline—by repeated measures analysis of variance. Data were analysed with the SPSS V.10.2 software package (SPSS Inc, Chicago, Illinois, USA).
RESULTS
Clinical characteristics
Eighteen patients with CSS completed the study but data were uninterpretable for one because of interference from an essential tremor. Of 12 controls, a suitable temporal bone ultrasound window was not obtainable for one. Age, sex or co‐morbidity did not differ between patients and controls (table 2). Patients with CSS had a mean 5.6 (SD 1.8) s asystole on carotid sinus massage.
Table 2 Clinical characteristics of patients with CSS and asymptomatic controls.
| CSS (n = 17) | Controls (n = 11) | |
|---|---|---|
| Age (years) | 76 (9.4) | 73 (7.9) |
| Sex | ||
| Women | 13 (76%) | 8 (73%) |
| Men | 4 (24%) | 3 (27%) |
| Co‐morbidity | ||
| IHD | 3 (18%) | 1 (10%) |
| Hypertension | 4 (24%) | 2 (18%) |
| COPD | 3 (18%) | 1 (10%) |
Age data are mean (SD).
COPD, chronic obstructive pulmonary disease; CSS, carotid sinus syndrome; IHD, ischaemic heart disease.
Systemic haemodynamic, Etco2 and cerebral autoregulatory responses to LBNP
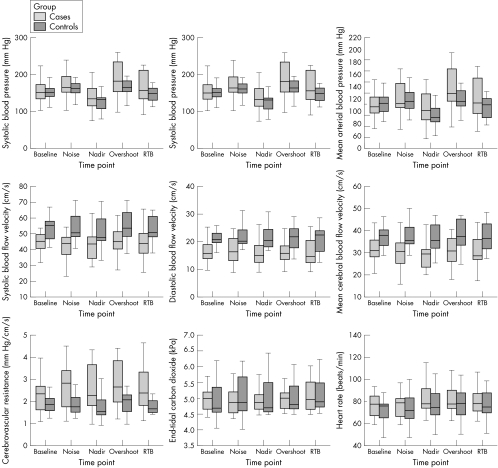
LBNP at SBP nadir was similar in both control subjects and patients (35.2 (SD 7.8) v 35.7 (SD 10.1) mm Hg, p = 0.89). No participant experienced presyncope or syncope. Figure 2 shows the distribution of responses for patients and controls for nine haemodynamic and cerebral autoregulatory indices. SBP, DBP, heart rate and Etco2 were similar for patients and controls at each time point: baseline, SBP nadir, SBP overshoot and return to baseline. MAP and heart rate followed a similar pattern, with Etco2 remaining relatively constant (fig 2, table 3).
Figure 2 Distribution of haemodynamic and cerebral autoregulatory indices. The line in the centre of each box is the median. Each box extends from the 25th centile (lower edge) to the 75th centile (upper edge) and thus indicates the interquartile range.
Table 3 Mean haemodynamic and cerebral autoregulatory indices and end‐tidal carbon dioxide in patients with CSS and asymptomatic controls.
| Variable | Baseline | Noise | Nadir | Overshoot | Return to baseline | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CSS | Control | CSS | Control | CSS | Control | CSS | Control | CSS | Control | |
| SBP (mm Hg) | 160.2 (40.6) | 154.5 (27.0) | 173.1 (38.2) | 164.6 (26.4) | 138.4 (38.2) | 125.9 (25.0) | 187.6 (48.3) | 166.8 (23.4) | 165.2 (41.5) | 147.4 (22.6) |
| p Value | 0.69 | 0.53 | 0.35 | 0.20 | 0.20 | |||||
| DBP (mm Hg) | 69.2 (19.4) | 72.4 (19.3) | 74.5 (21.6) | 73.1 (16.3) | 71.8 (21.8) | 62.9 (16.7) | 84.6 (28.8) | 72.9 (17.5) | 76.6 (24.3) | 66.8 (17.4) |
| p Value | 0.67 | 0.86 | 0.26 | 0.19 | 0.256 | |||||
| MAP (mm Hg) | 114.7 (28.6) | 113.5 (21.0) | 123.8 (28.3) | 118.9 (19.7) | 105.1 (29.0) | 94.5 (18.5) | 136.1 (34.3) | 119.9 (18.3) | 120.9 (31.2) | 107.1 (18.6) |
| p Value | 0.90 | 0.62 | 0.29 | 0.16 | 0.199 | |||||
| SBFV (mm Hg) | 45.6 (7.5) | 53.0 (8.2) | 42.9 (10.0) | 53.9 (8.8) | 42.2 (9.6) | 51.7 (10.3) | 44.6 (9.0) | 55.3 (10.4) | 44.6 (9.4) | 53.4 (8.6) |
| p Value | 0.02* | 0.006* | 0.02* | 0.022* | 0.018* | |||||
| DBFV (cm/s) | 17.5 (5.0) | 21.4 (2.8) | 17.2 (4.7) | 22.3 (4.6) | 16.9 (5.8) | 22.2 (4.7) | 16.6 (4.9) | 20.5 (6.3) | 16.6 (4.8) | 21.9 (6.8) |
| p Value | 0.026* | 0.008* | 0.018* | 0.08* | 0.021* | |||||
| MBFV (cm/s) | 31.6 (5.6) | 37.2 (5.3) | 30.1 (7.0) | 38.1 (6.3) | 29.6 (7.4) | 36.9 (7.0) | 31.4 (6.4) | 37.9 (7.9) | 30.6 (6.6) | 37.7 (6.9) |
| p Value | 0.012* | 0.005* | 0.014* | 0.025* | 0.011* | |||||
| CVR (mm Hg/m/s) | 2.2 (0.9) | 2.0 (0.5) | 2.7 (1.1) | 2.0 (0.6) | 2.7 (1.1) | 1.8 (0.7) | 2.7 (1.0) | 2.1 (0.8) | 2.7 (2.7) | 1.9 (0.7) |
| p Value | 0.26 | 0.069 | 0.027* | 0.044* | 0.045* | |||||
| Etco2 (kPa) | 5.0 (0.5) | 5.0 (0.6) | 4.9 (0.4) | 5.1 (0.7) | 4.9 (0.4) | 5.1 (0.7) | 5.0 (0.5) | 5.1 (0.5) | 5.1 (0.5) | 5.1 (0.6) |
| p Value | 0.67 | 0.35 | 0.27 | 0.94 | 0.995 | |||||
| PI | 0.90 (0.17) | 0.80 (0.08) | 0.86 (0.13) | 0.83 (0.11) | 0.87 (0.15) | 0.80 0.13) | 0.64 (0.15) | 0.94 (0.19) | 0.63 (0.15) | 0.85 (0.19) |
| p Value | 0.31 | 0.56 | 0.17 | 0.84 | 0.27 | |||||
| RI | 0.62 (0.09) | 0.59 (0.04) | 0.60 (0.06) | 0.59 (0.05) | 0.60 (0.07) | 0.57 (0.07) | 0.64 (0.07) | 0.63 (0.18) | 0.63 (0.07) | 0.69 (0.10) |
| p Value | 0.41 | 0.59 | 0.18 | 0.80 | 0.25 | |||||
| HR (beat/min) | 77.1 (11.1) | 73.2 (13.7) | 77.6 (11.8) | 73.9 (15.3) | 82.2 (15.0) | 76.6 (15.2) | 84.5 (17.3) | 78.3 (14.8) | 79.7 (12.8) | 76.9 (13.8) |
| p Value | 0.41 | 0.49 | 0.34 | 0.34 | 0.597 | |||||
Data are mean (SD).
*Significant difference.
CSS, carotid sinus syndrome patients; CVR, cerebrovascular resistance; DBFV, diastolic blood flow velocity; DBP, diastolic blood pressure; Etco2, end‐tidal carbon dioxide concentration; HR, heart rate; MAP, mean arterial blood pressure; MBFV, mean cerebral blood flow velocity; PI, pulsatility index; RI, resistance index; SBFV, systolic blood flow velocity; SBP, systolic blood pressure.
Table 4 shows the results of repeated measures analysis of variance. For each index there is an overall test of differences between time points (baseline, noise, SBP nadir, overshoot and return to baseline), an overall test of differences between groups (patients with CSS and controls) and a test of an interaction effect (the differences between time points were different for patients with CSS and controls).
Table 4 Results of repeated measures analysis of variance.
| Variable | Time points* | Groups† | Time points by group | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | df | p Value | F | df | p Value | F | df | p Value | |
| SBP | 33.2 | 4 | <0.001 | 1.02 | 1 | 0.32 | 1.13 | 4 | 0.35 |
| DBP | 6.89 | 4 | <0.001 | 0.58 | 1 | 0.45 | 3.92 | 4 | 0.01 |
| MAP | 26.2 | 4 | <0.001 | 0.94 | 1 | 0.34 | 2.29 | 4 | 0.07 |
| SBFV | 3.34 | 4 | 0.01 | 7.72 | 1 | 0.01 | 0.70 | 4 | 0.59 |
| DBFV | 0.60 | 4 | 0.66 | 7.92 | 1 | 0.01 | 0.40 | 4 | 0.81 |
| MBFV | 0.77 | 4 | 0.55 | 8.78 | 1 | 0.01 | 0.55 | 4 | 0.70 |
| CVR | 2.90 | 4 | 0.03 | 4.27 | 1 | 0.05 | 2.31 | 4 | 0.06 |
| Etco2 | 0.43 | 4 | 0.79 | 0.15 | 1 | 0.70 | 1.25 | 4 | 0.29 |
| HR | 6.30 | 4 | <0.001 | 0.74 | 1 | 0.40 | 0.50 | 4 | 0.74 |
*Time points are baseline, noise, systolic blood pressure (SBP) nadir, overshoot and return to baseline; †Patients with carotid sinus syndrome controls.
CVR, cerebrovascular resistance; DBFV, diastolic blood flow velocity; DBP, diastolic blood pressure; Etco2, end‐tidal carbon dioxide concentration; HR, heart rate; MAP, mean arterial blood pressure; MBFV, mean cerebral blood flow velocity; SBFV, systolic blood flow velocity.
For the blood pressure variables, the differences between time points were highly significant but differences between patients with CSS and controls were not. For DBP, although overall the groups did not differ, there was some evidence of an interaction between group and time point; however, post hoc t tests indicated that the difference in means was not significant at any time point.
For the blood flow velocity variables, differences between time points were not as notable (and were significant only for SBFV) but patients with CSS and controls differed significantly: the blood flow velocity was considerably less for patients with CSS (tables 3 and 4). The differences between groups were 9.2 m/s (95% confidence interval (CI) 2.9 to 15.4 m/s), 4.7 m/s (95% CI 1.5 to 7.9 m/s) and 6.9 m/s (95% CI 2.5 to 11.4 m/s) in SBFV, DBFV and MBFV, respectively. For CVR there were significant differences between time points, significant differences between patients with CSS and controls, and a significant interaction term (tables 3 and 4, fig 1). The difference between patients with CSS and controls was small at baseline (0.3, 95% CI −0.3 to 1.0) and larger at noise (0.7, 95% CI −0.1 to 1.5), SBP nadir (0.9, 95% CI 0.0 to 1.7), overshoot (0.8, 95% CI 0.0 to 1.6) and return to baseline (0.8, 95% CI 0.0 to 1.7). Pulsatility and resistance indices were similar in both groups at all five time points (table 3).
DISCUSSION
The significant differences in MBFV throughout the study and those in CVR on the SBP nadir, overshoot and return to baseline time points provide compelling evidence for disordered cerebral autoregulatory mechanisms in CSS. In the related neurally mediated disorder vasovagal syncope, analyses of several case series have shown a rise in CVR during head‐up tilt‐induced presyncope and syncope,12,24,27 but later work related this apparently paradoxical cerebral vasoconstriction to hyperventilation‐induced hypocapnia.28 Our findings echo those of Grubb et al,27 Levine et al12 and Bondar et al,14 showing significantly higher CVR in patients with CSS at SBP nadir, overshoot and return to baseline despite a highly significant average MBFV 23% lower in patients than in controls. In the presence of normocapnia (as in our study population) the converse is expected. In previous studies, cerebral blood flow velocity and carbon dioxide concentration fell during LBNP sufficiently to induce presyncope and syncope (with a potentially causal relationship between the two), but we observed neither of these changes in our study, presumably reflecting the relative modesty of the LBNP stimulus essential to our study design.
It has been argued that augmented sympathetic nervous activity during LBNP causes cerebral vasoconstriction, which overwhelms the usual mechanisms promoting vasodilatation in the face of a fall in MBFV.12,14,27 Indirect support for this contention came from a recent study of healthy young subjects examined by TCD during LBNP and ganglion blockade, in which Zhang et al29 elegantly showed that the cerebral circulation is likely to be under tonically active autonomic neural control. Although cerebral autoregulation was maintained in both patients with CSS and controls in response to LBNP, the wide differences between the groups in the variables described above suggests a baseline difference in autoregulation in those with CSS. Data are emerging showing a high prevalence of cognitive impairment and dementia in patients with CSS.30 This is attributed to small vessel disease reflected in a higher prevalence of white matter lesions in patients with CSS, with the density of white matter lesions on magnetic resonance imaging correlating with the degree of carotid sinus massage‐induced hypotension.31 We hypothesise that the abnormalities in cerebral autoregulation at baseline are due to such microvascular disease, manifesting as abnormal cerebral autoregulation. Our results raise the intriguing possibility that patients with CSS are prone to relative (and paradoxical) tonic intracerebral vasoconstriction, which predisposes them to further inappropriate vasoconstriction during CSS‐mediated vasodepression and asystole, when vasodilatation should otherwise supervene. We speculate that such paradoxical vasoconstriction preferentially affects areas intimately related to short term memory and consciousness level, with the clinical corollary being CSS presenting as falls.
Although TCD during carotid sinus massage would arguably provide a more realistic assessment, we deliberately avoided this approach because of the possibility of neurological complications during repeated carotid sinus massage21 and the confounding influence of unilateral cerebral artery occlusion on CVR in the contralateral side. Others have done so, however, and in two small, uncontrolled series Leftheriotis et al16,17 examined cerebral blood flow velocity in patients with CSS during carotid sinus massage after 10 min in the head‐up tilt position. Cerebral autoregulation failed only during a fall in systemic blood pressure below the lower limit for normal cerebral autoregulatory function—that is, 50 mm Hg32—with cerebral perfusion falling by 50% and CVR rising (non‐significantly) during carotid sinus massage, then falling rapidly immediately afterwards.16,17 These results are consistent with those presented above and with the vasovagal data of Carey et al.10
As with all TCD assessments of cerebral autoregulation, the technique is dependant on several assumptions. Firstly, middle cerebral artery cross‐sectional diameter is assumed to be constant. Secondly, as the Doppler principle depends on velocity, derivative measurement of blood flow relies on a linear relationship between flow and velocity. These factors have proved controversial, with some early criticism of the technique on the basis of the assumptions made.7,8,32 Later research comparing direct measures of internal carotid artery flow (during carotid surgical procedures) with middle cerebral artery velocity measured by TCD found that TCD flow measurements accurately mirrored changes in internal carotid arterial flow.9 Others similarly showed that changes in middle cerebral artery velocity correlated with cerebral blood flow,8,33,34 although with the caution that absolute velocity should not be used as an indicator of cerebral blood flow.8 Nonetheless, as middle cerebral arterial diameter was not measured as part of the study, this cannot be absolutely excluded. Furthermore, although the derivation of CVR from indirect measures of systemic blood pressure can be criticised, Zhang et al29 recently found a close correlation between digital photoplethysmographically measured blood pressure and intra‐arterial pressure during LBNP‐related orthostatic stress.
Summary
Cerebral autoregulation is abnormal in patients with CSS and may explain some of the clinical features of the disorder. Additional studies, with power calculations based on this work and by using TCD during more profound hypotension (induced by tilt, LBNP or carotid sinus massage‐induced asystole), may help explore this issue further.
ACKNOWLEDGEMENTS
The authors acknowledge the support of the British Heart Foundation for this work.
Abbreviations
CSS - carotid sinus syndrome
CVR - cerebrovascular resistance
DBFV - diastolic blood flow velocity
Etco2 - end‐tidal carbon dioxide concentration
LBNP - lower body negative pressure
MAP - mean arterial blood pressure
MBFV - mean cerebral blood flow velocity
SBFV - systolic blood flow velocity
SBP - systolic blood pressure
TCD - transcranial Doppler ultrasonography
Footnotes
Sources of funding: Dr Parry was supported by the British Heart Foundation.
Conflicts of interest: None declared.
References
- 1.McIntosh S, da Costa D, Kenny R A. Outcome of an integrated approach to the investigation of dizziness, falls and syncope in elderly patients referred to a ‘syncope clinic'. Age Ageing 19932253–58. [DOI] [PubMed] [Google Scholar]
- 2.Kenny R A, Richardson D A, Steen N.et al Carotid sinus syndrome: a modifiable risk factor for nonaccidental falls in older adults (SAFE‐PACE). J Am Coll Cardiol 2001381491–1496. [DOI] [PubMed] [Google Scholar]
- 3.Crilley J G, Herd B, Khurana C S.et al Permanent cardiac pacing in elderly patients with recurrent falls, dizziness and syncope, and a hypersensitive cardioinhibitory reflex. Postgrad Med J 199773415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parry S W, Baptist M, Kenny R A. Clinical characteristics in carotid sinus syndrome presenting with drop attacks versus syncope: implications for investigation and management of older subjects with atypical presentations of cardiovascular syncope. Pacing Clin Electrophysiol 200023600 A195, [Google Scholar]
- 5.Paulson O B, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev 19902161–192. [PubMed] [Google Scholar]
- 6.Aaslid R, Markwalder T‐M, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg 198257769–774. [DOI] [PubMed] [Google Scholar]
- 7.Weyland A, Stephan H, Kazmaier S.et al Flow velocity measurements as an index of cerebral blood flow. Anesthesiology 1994811401–1410. [DOI] [PubMed] [Google Scholar]
- 8.Bishop C C, Powell S, Rutt D.et al Transcranial Doppler measurement of middle cerebral artery blood flow velocity: a validation study. Stroke 198617913–915. [DOI] [PubMed] [Google Scholar]
- 9.Newell D W, Aaslid R, Lam A.et al Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke 199425793–797. [DOI] [PubMed] [Google Scholar]
- 10.Carey B J, Manktelow B N, Panerai R B.et al Cerebral autoregulatory responses to head‐up tilt in normal subjects and patients with recurrent vasovagal syncope. Circulation 2001104898–902. [DOI] [PubMed] [Google Scholar]
- 11.Aaslid R, Lindegaard K F, Sorteberg W.et al Cerebral autoregulation dynamics in humans. Stroke 19892045–52. [DOI] [PubMed] [Google Scholar]
- 12.Levine B D, Giller C A, Lane L D.et al Cerebral versus systemic hemodynamics during graded orthostatic stress in humans. Circulation 199490298–306. [DOI] [PubMed] [Google Scholar]
- 13.Lipsitz L A, Mukai S, Hamner J.et al Dynamic regulation of middle cerebral artery blood flow velocity in aging and hypotension. Stroke 2000311897–1903. [DOI] [PubMed] [Google Scholar]
- 14.Bondar R L, Kassam M S, Stein F.et al Simultaneous cerebrovascular and cardiovascular responses during presyncope. Stroke 1995261794–1800. [DOI] [PubMed] [Google Scholar]
- 15.Grubb B P, Samoil D, Kosinski D.et al Cerebral syncope: loss of consciousness associated with cerebral vasoconstriction in the absence of systemic hypotension. Pacing Clin Electrophysiol 199821652–658. [DOI] [PubMed] [Google Scholar]
- 16.Leftheriotis G, Rozak P, Dupuis J M.et al Cerebral hemodynamics during carotid massage in patients with carotid sinus syndrome. Pacing Clin Electrophysiol 1998211885–1892. [DOI] [PubMed] [Google Scholar]
- 17.Leftheriotis G, Rozak P, Dupuis J M.et al Effect of cardiac pacing on peripheral and cerebral hemodynamics in patients with carotid sinus syndrome. Am J Cardiol 199883974–977. [DOI] [PubMed] [Google Scholar]
- 18.Stevens P M. Cardiovascular dynamics during orthostasis and the influence of intravascular instrumentation. Am J Cardiol 196617211–218. [DOI] [PubMed] [Google Scholar]
- 19.Kenny R A, O'Shea D, Parry S W. The Newcastle protocols for head‐up tilt table testing in the diagnosis of vasovagal syncope, carotid sinus hypersensitivity, and related disorders. Heart 200083564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El‐Bedawi K M, Hainsworth R. Combined head‐up tilt and lower body suction: a test of orthostatic tolerance. Clin Auton Res 1994441–47. [DOI] [PubMed] [Google Scholar]
- 21.Richardson D A, Bexton R, Shaw F E.et al Complications of carotid sinus massage: a prospective series of older patients. Age Ageing 200129413–417. [DOI] [PubMed] [Google Scholar]
- 22.American Geriatrics Society Guideline for the prevention of falls in older persons. J Am Geriatr Soc 200149664–672. [PubMed] [Google Scholar]
- 23.Brignole M, Alboni P, Benditt D.et al Guidelines on management (diagnosis and treatment) of syncope. Eur Heart J 2001221256–1306. [DOI] [PubMed] [Google Scholar]
- 24.Parry S W, Seifer C M. Monitoring devices for falls and syncope. In: Kenny RA, O'Shea D, eds. Falls and syncope in elderly patients. Philadelphia, PA: WB Saunders, 2002
- 25.Novak V, Spies J M, Novak P.et al Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke 1998291876–1881. [DOI] [PubMed] [Google Scholar]
- 26.Cencetti S, Bandinelli G, Lagi A. Effect of Pco2 changes induced by head‐upright tilt on transcranial Doppler recordings. Stroke 1998281195–1197. [DOI] [PubMed] [Google Scholar]
- 27.Grubb B P, Gerard G, Roush K.et al Cerebral vasoconstriction during head‐upright tilt‐induced vasovagal syncope: a paradoxic and unexpected response. Circulation 1991841157–1164. [DOI] [PubMed] [Google Scholar]
- 28.Lagi A, Cencetti S, Corsoni V.et al Cerebral vasoconstriction in vasovagal syncope: any link with symptoms? A transcranial Doppler study. Circulation 20011042694–2698. [DOI] [PubMed] [Google Scholar]
- 29.Zhang R, Zuckerman J H, Iwasaki K.et al Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation 20021061814–1820. [DOI] [PubMed] [Google Scholar]
- 30.Pearse R, Ballard C, Hampton J.et al Prevalence and profile of cognitive impairment and dementia in carotid sinus syndrome (CSS) and carotid sinus hypersensitivity (CSH) [abstract]. Clin Auton Res 200414(Suppl)A29 [Google Scholar]
- 31.Kenny R A, Shaw F E, O'Brien J T.et al Carotid sinus syndrome is common in dementia with Lewy bodies and correlates with deep white matter lesions. J Neurol Neurosurg Psychiat 200475966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsen F S, Olsen K S, Hansen B A.et al Transcranial Doppler is valid for determination of the lower limit of cerebral blood flow autoregulation. Stroke 1994251985–1988. [DOI] [PubMed] [Google Scholar]
- 33.Kontos H A. Validity of cerebral arterial blood flow calculations from velocity measurements. Stroke 1989201–3. [DOI] [PubMed] [Google Scholar]
- 34.Daffertshofer M, Hennerici M. Cerebrovascular regulation and vasoneuronal coupling. J Clin Ultrasound 199523125–138. [DOI] [PubMed] [Google Scholar]