Abstract
Objective
To investigate the prevalence of anaemia and its influence on mortality among hospitalised patients with congestive heart failure (CHF) with preserved left ventricular systolic function (LVSF).
Method and results
210 patients with preserved LVSF admitted to the cardiology department of a tertiary hospital for CHF between 1 January 2000 and 31 December 2002 were analysed. Anaemic patients, who constituted 46% of the whole group, were older (75 v 72 years, p = 0.036); were in hospital longer (mean (SD) 13 v 11 days, p = 0.007); had a higher prevalence of ischaemic heart disease (54% v 35%, p = 0.009), left bundle branch block (12% v 4%, p = 0.018), and kidney failure (56% v 34%, p = 0.003); and had faster erythrocyte sedimentation rates (mean (SD) 50 v 26 mm in the first hour, p < 0.001), a tendency to lower serum cholesterol concentration (mean (SD) 4.65 v 5.22 mmol/l, p = 0.073), and smaller body mass index (mean (SD) 27 v 29 kg/m2, p = 0.126) than their non‐anaemic counterparts. Kaplan‐Meier analysis showed the anaemic group to have significantly poorer survival (p = 0.0001), with a one year survival rate of 72.2% versus 90.5% in the non‐anaemic group. Multivariate analysis showed anaemia to be the most powerful independent predictor of mortality, increasing the risk of death by a factor of 2.7 (p = 0.007).
Conclusion
Anaemia is a very prevalent condition in hospitalised patients with CHF with preserved LVSF and is independently associated with higher mortality. Appropriately designed randomised studies are needed to determine whether the prevention or treatment of anaemia can improve survival of these patients.
Keywords: anaemia, congestive heart failure, preserved systolic function, prognosis
Congestive heart failure (CHF) is a major health problem in Western countries. With an annual incidence of about 1% and a high mortality rate, it is found in about 16 million people. The identification of modifiable factors affecting the risk of CHF related mortality or morbidity may lead to the development of improved strategies for the clinical management of these patients.
It has recently been found that mild or moderate anaemia is widespread among patients with CHF1,2,3,4,5,6 and, reciprocally, that severe chronic anaemia is associated with increased risk of CHF.7,8,9 Furthermore, although current clinical guidelines do not cite anaemia as a determinant of the clinical situation and prognosis of CHF or its prevention as an objective for treatment,10,11 a recent analysis of patients in the SOLVD (studies of left ventricular dysfunction) identified low haemoglobin concentration as a potent predictor of mortality independently of kidney dysfunction.12 SOLVD and several other studies found that patients with CHF with anaemia had a poorer clinical situation, a greater risk of rehospitalisation, and shorter survival than did non‐anaemic patients.1,4,5,6,12,13 However, almost all this information has concerned patients with CHF with deteriorated left ventricular systolic function (LVSF); very little has been published on the prevalence and implications of anaemia among patients with CHF with preserved LVSF, who constitute 30–50% of all patients with CHF.14,15,16 The prognosis of patients with CHF with preserved LVSF, the determinants of their clinical evolution, and the best way to treat them are largely unexplored.
In this study we investigated the prevalence of anaemia and its influence on mortality among patients who had been hospitalised with CHF and had preserved LVSF.
PATIENTS AND METHODS
Study groups
Between 1 January 2000 and 31 December 2002, 630 patients were admitted to the cardiology service of our tertiary hospital satisfying modified Framingham criteria for CHF—namely, the presence of two or more major symptoms and signs (paroxysmal nocturnal dyspnoea, orthopnoea, rales, jugular vein distension, third heart sound, and radiological signs of pulmonary congestion or cardiomegaly) or one major symptom or sign together with two or more minor symptoms or signs (effort dyspnoea, peripheral oedema, hepatomegaly, and pleural effusion). Of these 630 patients, 547 were examined echocardiographically while hospitalised (for patients admitted more than once, only the data acquired during the first admission during the study period were considered for this study). For 536 of these 547 it was possible to determine left ventricular ejection fraction (LVEF), which was ⩾ 50% in 242 patients. The patients enrolled in the study were the 210 who had preserved LVSF (LVEF ⩾ 50%) and whose blood had been analysed, including haemograms, on admission before application of any other diagnostic tests or treatment. Informed consent was obtained from all patients who entered into the study.
The study group defined as above was divided into anaemic and non‐anaemic subgroups, anaemia being identified by the World Health Organization definition with plasma haemoglobin concentration < 130 g/l for men or < 120 g/l for women.17
Variables studied
Apart from demographic data, information was collected on cardiovascular risk factors, CHF aetiology (see definitions in our previous publication16), clinical characteristics, the results of laboratory and other tests (blood analyses, chest radiographs, ECG, echocardiogram, and coronary angiography), the treatment prescribed on release from hospital, and the duration of hospitalisation. Specialised cardiologists performed echocardiography during the first few days of hospitalisation, and LVEF was determined by the modified Simpson method. Two cardiologists with long experience treating patients with CHF collected all of the data, such as how patients were selected for inclusion in the study.
Survival data were obtained by searching the general archives of the hospital and by means of a telephone survey during May 2003.
Statistical analyses
Data for categorical or dichotomous variables are expressed as percentages and were compared by χ2 tests. Data for continuous variables are expressed as mean (SD) and were compared by Student's t tests. Survival curves were constructed by the Kaplan‐Meier method and pairwise comparisons were performed by using the two sample log rank test. Factors that were independently and significantly associated with survival were identified by Cox's proportional hazards model in a backward stepwise regression analysis followed by a secondary Cox analysis in which the independent variables were those identified as significant in the first analysis. The resulting regression coefficients were used to estimate relative risk and the corresponding 95% confidence interval (CI). The validity of the assumption of proportional hazards was supported by the results of calculating log‐log survival plots for each variable with age and sex controlled. The criterion for significance was p < 0.05.
RESULTS
Characteristics of the whole study group
Tables 1 and 2 list the characteristics of the patients enrolled in the study. About 48% were men, and mean age was 73 years. Almost 70% were hypertensive. The underlying cardiopathy was diagnosed as ischaemic heart disease in 43% of patients and as valve disease in 33%. At admission, 37% of patients exhibited atrial fibrillation and 65% were in New York Heart Association (NYHA) functional class III or IV. Mean plasma haemoglobin concentration was 127 g/l and anaemia was present in 46% of the study population. About 43% exhibited moderate to severe kidney failure, as reflected by a glomerular filtration rate < 60 ml/min/1.73 m2, which was estimated by the MDRD (modification of diet in renal disease) equation (186 × serum creatinine−1.154 × age−0.203 × 1.210 (if black) × 0.742 (if female)).
Table 1 Clinical characteristics and treatment of 210 patients hospitalised for congestive heart failure (CHF) who had preserved left ventricular systolic function (LVSF) (left ventricular ejection fraction (LVEF) ⩾50%).
| Variable | Whole group (n = 210) | Non‐anaemic (n = 113) | Anaemic (n = 97) | p Value* |
|---|---|---|---|---|
| Age (years) | 73.2 (10.4) | 71.8 (9.6) | 74.8 (11.0) | 0.036 |
| Hospitalisation (days) | 11.9 (7.5) | 10.7 (5.7) | 13.4 (8.9) | 0.007 |
| Men | 48.1% | 46.9% | 49.5% | 0.782 |
| Risk factors | ||||
| Hypertension | 68.6% | 70.8% | 66.0% | 0.652 |
| Hyperlipidaemia | 41.9% | 45.1% | 38.1% | 0.480 |
| Diabetes mellitus | 21.0% | 18.6% | 23.7% | 0.394 |
| Smoking | 23.1% | 25.7% | 19.6% | 0.409 |
| Aetiology | ||||
| Ischaemic heart disease | 43.3% | 34.5% | 53.6% | 0.009 |
| Valve disease | 33.3% | 35.4% | 30.9% | |
| Other cardiopathy | 23.4% | 30.1% | 15.5% | |
| Clinical status at admission | ||||
| BMI >25 kg/m2 | 72.9% | 80.9% | 64.1% | 0.126 |
| NYHA class III or IV | 64.8% | 63.7% | 66.0% | 0.773 |
| Jugular vein distension | 35.7% | 30.1% | 42.5% | 0.600 |
| Rales | 78.1% | 77.9% | 78.4% | 0.867 |
| Third sound | 2.4% | 0.9% | 4.1% | 0.184 |
| Hepatomegaly | 17.1% | 15.9% | 18.6% | 0.714 |
| Peripheral oedema | 34.3% | 28.3% | 41.2% | 0.058 |
| Radiographic signs at admission | ||||
| Alveolar oedema | 6.7% | 6.2% | 6.9% | 1.000 |
| Pleural effusion | 15.2% | 15.0% | 15.5% | 1.000 |
| Cardiomegaly | 71.9% | 71.7% | 72.2% | 1.000 |
| ECG at admission | ||||
| Left bundle branch block | 7.6% | 3.5% | 12.4% | 0.018 |
| Sinus rhythm | 47.6% | 46.0% | 49.5% | 0.578 |
| Atrial fibrillation | 36.7% | 43.4% | 28.9% | 0.044 |
| Coronary angiography done | 33.8% | 37.2% | 29.9% | 0.307 |
| Treatment at discharge | ||||
| ACE inhibitors | 50.5% | 54.9% | 44.8% | 0.199 |
| ARBs | 5.0% | 6.2% | 3.4% | 0.518 |
| β Blockers | 34.0% | 36.3% | 31.0% | 0.456 |
| Spironolactone | 9.5% | 8.8% | 10.3% | 0.809 |
| Diuretics | 66.0% | 58.4% | 75.9% | 0.011 |
| Digoxin | 16.0% | 18.6% | 12.4% | 0.331 |
| Anticoagulants | 33.0% | 40.7% | 23.7% | 0.010 |
| Antiaggregants | 54.0% | 49.6% | 59.8% | 0.156 |
| Calcium antagonists | 35.0% | 32.7% | 37.9% | 0.459 |
| Nitrates | 37.5% | 33.6% | 42.5% | 0.239 |
Data are mean (SD) or percentage.
*Anaemic versus non‐anaemic groups.
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; NYHA, New York Heart Association.
Table 2 Laboratory results for 210 patients hospitalised for CHF who had preserved LVSF (LVEF ⩾50%).
| Variable | Whole group (n = 210) | Non‐anaemic (n = 113) | Anaemic (n = 97) | p Value* |
|---|---|---|---|---|
| Haemoglobin (g/l) | 127 (21) | 142 (14) | 109 (13) | <0.001 |
| Packed cell volume (%) | 37.9 (6.5) | 42.1 (4.5) | 33.1 (4.9) | <0.001 |
| Leucocytes (×109/l) | 8.28 (4.59) | 7.95 (3.42) | 8.66 (5.66) | 0.268 |
| ESR (mm in first hour) | 37.1 (27.9) | 25.7 (20.7) | 50.1 (29.5) | <0.001 |
| Creatinine (μmol/l) | 91.6 (45.8) | 76.3 (30.5) | 106.8 (61.0) | <0.001 |
| GFR (ml/min/1.73 m2) | 67.4 (34.4) | 71.3 (23.6) | 62.8 (43.6) | 0.073 |
| GFR <60 ml/min/1.73 m2 (%) | 42.9 | 33.6 | 55.7 | 0.003 |
| Glucose (g/l) | 1.33 (0.58) | 1.35 (0.57) | 1.31 (0.61) | 0.669 |
| Cholesterol (mmol/l) | 4.96 (1.04) | 5.22 (1.03) | 4.65 (0.974) | 0.073 |
*Anaemic versus non‐anaemic groups.
ESR, erythrocyte sedimentation rate; GFR, glomerular filtration rate.
Characteristics of the anaemic and non‐anaemic subgroups
The mean plasma haemoglobin concentration in the anaemic group was 109 g/l, compared with 142 g/l in the non‐anaemic group. Although there was practically no difference between the two groups as regards their clinical situation, tables 1 and 2 show a number of differential characteristics. The anaemic group was significantly older (mean 75 years v 72 years); was in hospital longer (mean 13 v 11 days); had a higher prevalence of ischaemic heart disease (54% v 35%), left bundle branch block (12% v 4%), and kidney failure (56% v 34%); and had faster erythrocyte sedimentation rates (mean 50 v 26 mm in the first hour), lower serum cholesterol concentration (mean 4.65 v 5.22 mmol/l), and smaller body mass index (mean 27 v 29 kg/m2); the differences in cholesterol and body mass index were not significant. Anaemic patients also had a lower prevalence of atrial fibrillation (29% v 43%) and a correspondingly lower prevalence of prescription of anticoagulants (24% v 41%), but prescription of diuretics was more frequent in the anaemic group (76% v 58%).
Survival
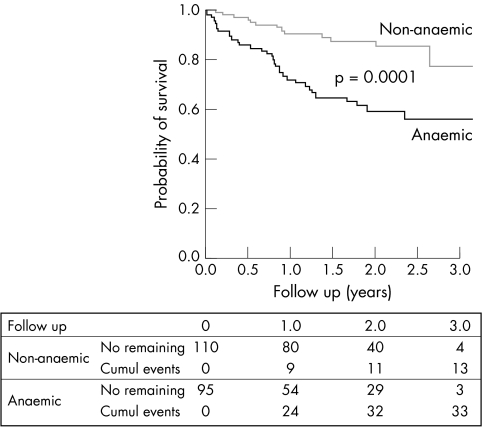
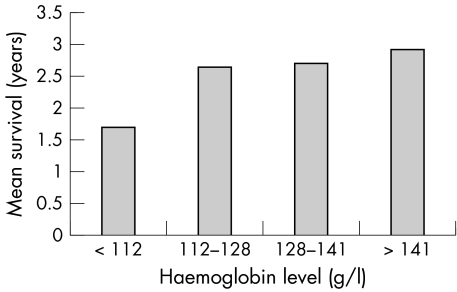
Survival data were obtained for 205 of the patients in the study, 95 of them in the anaemic group and 110 in the non‐anaemic group. Mean follow up time among these 205 was 1.5 years. By the end of the study, 46 of the 205 had died (33 anaemic and 13 non‐anaemic patients). Kaplan‐Meier analysis showed the anaemic group to have significantly poorer survival (p = 0.0001), with a one year survival rate of 72.2% and a mean survival time of 2.2 years (95% CI 1.9 to 2.5 years) compared with 90.5% and 2.9 years (95% CI 2.7 to 3.1 years), respectively, in the non‐anaemic group (fig 1). A log rank test also showed a positive correlation between plasma haemoglobin concentration and the one year survival rate, which for successive quarters of the distribution of haemoglobin concentrations was 63.7%, 85.4%, 84.2%, and 95.2% (p < 0.0001); fig 2 shows the reflection of this correlation in the corresponding mean survival times. Multivariate analysis of age, sex, aetiology, diabetes, NYHA class, kidney failure, and angiotensin converting enzyme inhibitor prescription as well as anaemia (table 3) showed anaemia to be the most powerful independent predictor of mortality, increasing the risk of death by a factor of 2.7 (p = 0.007).
Figure 1 Kaplan‐Meier survival curves for patients with congestive heart failure (CHF) with preserved left ventricular systolic function (left ventricular ejection fraction (LVEF) ⩾50%), with and without anaemia.
Figure 2 Mean survival of patients with CHF with preserved left ventricular systolic function (LVEF ⩾50%) in each quarter of the distribution of haemoglobin concentrations.
Table 3 Variables included in the multivariate analysis as possible predictors of mortality among patients with CHF with preserved LVSF (LVEF ⩾50%).
| Variable | Relative risk (95% CI) | p Value |
|---|---|---|
| Age* | 1.042 (1.003 to 1.082) | 0.035 |
| Ischaemic aetiology | 1.417 (0.708 to 2.836) | 0.599 |
| Male sex | 1.618 (0.823 to 3.179) | 0.163 |
| Diabetes mellitus | 0.764 (0.343 to 1.701) | 0.510 |
| NYHA class IV | 1.112 (0.589 to 2.101) | 0.744 |
| ACE inhibitors | 0.513 (0.263 to 1.002) | 0.051 |
| GFR >60 ml/min/1.73 m2 | 0.628 (0.328 to 1.203) | 0.161 |
| Anaemia* | 2.647 (1.308 to 5.357) | 0.007 |
*Significant independent predictors.
CI, confidence interval.
DISCUSSION
The above results show that anaemia is very common among our patients with CHF with preserved LVSF, and is a powerful predictor of death for these patients, independently of their age, sex, NYHA class, or CHF aetiology and whether they have kidney failure. Haemoglobin concentration was directly correlated with one year survival rate, which for the highest haemoglobin concentrations was better than 95%. The anaemic group had a higher prevalence of ischaemic heart disease and analytical data reflecting greater metabolic and proinflammatory activity. These findings open new avenues to a better understanding of the physiopathology of this clinical form of CHF and to its better therapeutic management. Although the influence of anaemia on prognosis has been established for patients with kidney failure and for patients with CHF with deteriorated systolic function, it has not been extensively investigated for patients with CHF with preserved LVSF.18,19
As far as we know, anaemia in patients with CHF with preserved LVSF has previously been investigated only by Brucks et al,20 who studied its influence on survival and the risk of hospitalisation among 137 outpatients with LVEF ⩾ 50%. They found the prevalence of anaemia to be similar to that observed in the present study, and that anaemic patients exhibited greater deterioration of left ventricular diastolic function (as evaluated by Doppler echocardiography) than did non‐anaemic patients and had a greater risk of hospitalisation for cardiovascular events. Although the survival rate of anaemic patients was not significantly less than that of non‐anaemic patients, anaemia was one of only two independent predictors of hospitalisation‐free survival, the other being age. In the present study, in which the survival rates of the anaemic and non‐anaemic groups did differ significantly, anaemia and age were also the only independent predictors of outcome, in this case survival. The poorer survival of our patients may have been due to their being older than those studied by Brucks et al,20 to their having been hospitalised, and to the longer follow up time in the present study.
Although the high prevalence of anaemia among our patients suggests that it might have been a determinant of the CHF crisis for which they were hospitalised, in a recent analysis of the causes of hospitalisation for CHF of patients with preserved LVSF anaemia was not among the causes identified (it must be pointed out that it was possible to identify the cause in only about half the cases considered).21 It is likewise tempting to speculate that correction of anaemia in these patients would contribute to improving their clinical stability, cardiac function, and survival, but we know of no study of the effects of correcting anaemia among patients with CHF with preserved LVSF.
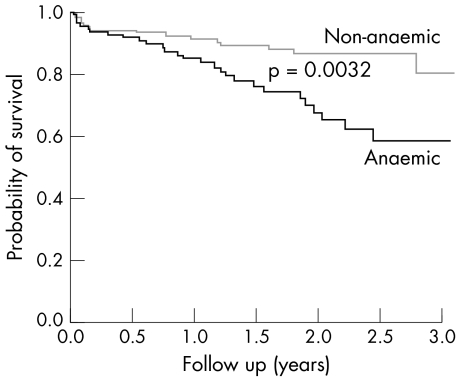
Some recent epidemiological studies have related low plasma haemoglobin and packed cell volume to higher mortality among patients with CHF with deteriorated LVSF.1,4,5,6,12 Their findings are further supported by our data for the 264 patients with CHF with LVEF < 50% and haemograms who were admitted during the study period; those with anaemia had a one year survival rate of 84.7% and a mean survival time of 2.3 years (95% CI 2.1 to 2.6 years) compared with 91.3% and 2.9 years (95% CI 2.7 to 3.0 years), respectively, for those without anaemia (fig 3). The Framingham study also found anaemia to be a risk factor for the development of CHF.22 Thus, anaemia seems both to predict the evolution of CHF—which the present study corroborates for CHF with preserved LVSF—and to favour its development, but the mechanisms underlying these relations have not been elucidated.
Figure 3 Kaplan‐Meier survival curves for patients with CHF with deteriorated left ventricular systolic function (LVEF <50%) with and without anaemia.
Although anaemic patients with CHF, like other patients with chronic anaemia, have high plasma concentration of erythropoietin,23 anaemia in CHF, as in other chronic diseases, may be a consequence of a proinflammatory state and may be mediated by high concentrations of circulating cytokines.24,25 Malnutrition may also play a part, since anaemic patients with CHF have been reported to have low plasma albumin concentration and body mass index.6,8,26 In this study, our anaemic patients tended to have a lower body mass index and plasma cholesterol concentration and higher erythrocyte sedimentation rates and leucocyte counts than the non‐anaemic patients. As in the case of CHF with deteriorated LVSF, these findings may indicate a relation between anaemia and a hypercatabolic, proinflammatory state characteristic of severe, advanced disease stages and may explain the influence of anaemia on mortality (although Horwich et al6 found the influence of anaemia on the prognosis of CHF to be independent of body mass index and albumin concentration).
It has been reported that a significant proportion of patients with CHF have anaemia associated with haemodilution and that the prognosis of these patients is worse than that of patients with CHF with true anaemia.27 In the present study we did not distinguish between haemodilution anaemia and true anaemia, but the possibility of haemodilution being present in a significant number of patients is suggested by most of our patients having been hospitalised for congestion.
Physiopathologically, anaemia and myocardial ischaemia in CHF may be directly related. Ischaemic heart disease may make the heart more vulnerable to anaemia and in patients with CHF anaemia may favour myocardial ischaemia, myocardial stunning, apoptosis, and necrosis. On the other hand, treatment with antiaggregants, which are indicated for patients with ischaemic heart disease, may contribute to anaemia development due to gastrointestinal blood loss. These hypotheses are in keeping with our finding that the prevalence of ischaemic heart disease was higher among anaemic than non‐anaemic patients (54% v 35%); so, although CHF was no more severe among patients with or without anaemia, our results provide the first indication of a possible relation between anaemia and ischaemic heart disease among patients with CHF with preserved LVSF. In view of the poorer prognosis of our anaemic patients, it is perhaps worth drawing attention to a report that blood transfusion treatment of patients with myocardial infarction with packed cell volume less than 33% reduced the 30 day death rate.28
Kidney failure is known to affect the survival of patients with CHF and has a central role in the physiopathology of anaemia.29,30 Although anaemia correlates with plasma markers of kidney failure and with glomerular filtration rate, however, several studies have found anaemia to increase the risk of death among patients with CHF independently of kidney disease and other predictors.4,6,7,8,12 In keeping with this, our results suggest that among patients with CHF with preserved LVSF anaemia and kidney failure are related (plasma creatinine concentration being significantly higher in the anaemic group), but kidney failure was not an independent predictor of mortality.
Limitations of the study
Since all our patients had been hospitalised, caution must be exercised in extrapolating our findings to all patients with CHF with preserved LVSF. Moreover, our mortality data do not distinguish between different causes of death; we have no information on the number or causes of repeat hospitalisations during follow up. The haemoglobin concentration and packed cell volume considered are those determined at the time of hospitalisation, no data being available that allow evaluation of the influence of changes in these parameters during follow up.
Conclusions
A significant proportion of patients who are hospitalised for CHF and have preserved LVSF are anaemic. Their anaemia probably has a variety of origins and is a powerful predictor of mortality. Our findings suggest the need for further investigation of the causes, prevention, and treatment of anaemia in patients with CHF with preserved LVSF and for appropriately designed randomised studies to determine whether the prevention or treatment of anaemia reduces mortality among these patients.
Abbreviations
CHF - congestive heart failure
CI - confidence interval
LVEF - left ventricular ejection fraction
LVSF - left ventricular systolic function
MDRD - modification of diet in renal disease
NYHA - New York Heart Association
SOLVD - studies of left ventricular dysfunction
Footnotes
Competing interests: None declared.
References
- 1.Mozaffarian D, Nye R, Levy W C. Anemia predicts mortality in severe heart failure: the prospective randomized amlodipine survival evaluation (PRAISE). J Am Coll Cardiol 2003411933–1939. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg D S, Wexler D, Blum M.et al The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol 2000351737–1744. [DOI] [PubMed] [Google Scholar]
- 3.Tanner H, Moschovitis G, Kuster G M.et al The prevalence of anemia in chronic heart failure. Int J Cardiol 200286115–121. [DOI] [PubMed] [Google Scholar]
- 4.Ezekowitz J A, Ezekowitz J A, McAlister F A.et al Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new‐onset heart failure. Circulation 2003107223–225. [DOI] [PubMed] [Google Scholar]
- 5.Kosborod M, Smith G L, Radford M J.et al The prognostic importance of anemia in patients with heart failure. Am J Med 2003114112–119. [DOI] [PubMed] [Google Scholar]
- 6.Horwich T B, Fonarow G C, Hamilton M A.et al Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol 2002391780–1786. [DOI] [PubMed] [Google Scholar]
- 7.Silverberg D S, Wexler D, Blum M.et al The cardio renal anemia syndrome: correcting anemia in patients with resistant congestive heart failure can improve both cardiac and renal function and reduce hospitalizations. Clin Nephrol 200360(suppl 1)S93–102. [PubMed] [Google Scholar]
- 8.Felker G M, Adams K F, Gattis W A.et al Anemia as a risk factor and therapeutic target in heart failure. J Am Coll Cardiol 200444959–966. [DOI] [PubMed] [Google Scholar]
- 9.Sarnak M J, Tighiouart H, Manjunath G.et al Anemia as a risk factor for cardiovascular disease in the atherosclerosis risk in communities (ARIC) study. J Am Coll Cardiol 20024027–33. [DOI] [PubMed] [Google Scholar]
- 10.Hunt S A, Baker D W, Chin M H.et al ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee to revise the 1995 guidelines for the evaluation and management of heart failure). J Am Coll Cardiol 2001382101–2113. [DOI] [PubMed] [Google Scholar]
- 11.Remme W J, Swedberg K. Task force for the diagnosis and treatment of chronic heart failure, European Society of Cardiology. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J 2001221527–1560. [DOI] [PubMed] [Google Scholar]
- 12.Al‐Ahmad A, Rand W M, Manjunath G.et al Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol 200138955–962. [DOI] [PubMed] [Google Scholar]
- 13.Felker G M, Gattis W A, Leimberger J D.et al Usefulness of anemia as a predictor of death and rehospitalization in patients with decompensated heart failure. Am J Cardiol 200392625–628. [DOI] [PubMed] [Google Scholar]
- 14.Banerjee P, Banerjee T, Khand A.et al Diastolic heart failure: neglected or misdiagnosed? J Am Coll Cardiol 200239138–141. [DOI] [PubMed] [Google Scholar]
- 15.Hogg K, Swedberg K, McMurray J J. Heart failure with preserved left ventricular systolic function. J Am Coll Cardiol 200443317–327. [DOI] [PubMed] [Google Scholar]
- 16.Varela‐Roman A, Grigorian L, Barge E.et al Heart failure in patients with preserved and deteriorated left ventricular ejection fraction: long term prognosis. Heart 200591489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization Nutritional anaemias: report of a WHO scientific group. World Health Organ Tech Rep Ser 19684053–37. [PubMed] [Google Scholar]
- 18.Foley R N, Parfrey P S, Harnett J D.et al The impact of anemia on cardiomyopathy, morbidity, and mortality in end‐stage renal disease. Am J Kidney Dis 19962853–61. [DOI] [PubMed] [Google Scholar]
- 19.Harnett J D, Kent G M, Foley R N.et al Cardiac function and hematocrit level. Am J Kidney Dis 199525S3–S7. [DOI] [PubMed] [Google Scholar]
- 20.Brucks S, Little W C, Chao T.et al Relation of anemia to diastolic heart failure and the effect on outcome. Am J Cardiol 2004931055–1057. [DOI] [PubMed] [Google Scholar]
- 21.Klapholz M, Maurer M, Lowe A M.et al Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction. J Am Coll Cardiol 2004431432–1438. [DOI] [PubMed] [Google Scholar]
- 22.Kannel W. Epidemiology and prevention of cardiac failure: Framingham study insights. Eur Heart J 1987823–26. [DOI] [PubMed] [Google Scholar]
- 23.Volpe M, Tritto C, Testa U.et al Blood levels of erythropoietin in congestive heart failure and correlation with clinical, hemodynamic, and hormonal profiles. Am J Cardiol 199474468–473. [DOI] [PubMed] [Google Scholar]
- 24.Voulgari P V, Kolios G, Papadopoulos G K.et al Role of cytokines in the pathogenesis of anemia of chronic disease in rheumatoid arthritis. Clin Immunol 199992153–160. [DOI] [PubMed] [Google Scholar]
- 25.Bolger A P, Haehling S, Doehner W.et al Anemia and inflammation in chronic heart failure. J Card Fail 2003933 [abstract] [Google Scholar]
- 26.Hussein S J, Jain R, Shlipak M G.et al Chronic heart failure is not an independent cause of anemia. J Card Fail 2003919 [abstract] [Google Scholar]
- 27.Androne A S, Katz S D, Lund L.et al Hemodilution is common in patients with advanced heart failure. Circulation 2003107226–229. [DOI] [PubMed] [Google Scholar]
- 28.Wu C H, Rathore S S, Wang Y.et al Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med 20013451230–1236. [DOI] [PubMed] [Google Scholar]
- 29.Dries D L, Exner D V, Domanski M J.et al The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol 200035681–689. [DOI] [PubMed] [Google Scholar]
- 30.Hillege H L, Girbes A R, de Kam P J.et al Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 2000102203–210. [DOI] [PubMed] [Google Scholar]