Abstract
Objective
To evaluate whether a well developed collateral circulation predisposes to restenosis after percutaneous coronary intervention (PCI).
Design
Prospective observational study.
Patients and setting
58 patients undergoing elective single vessel PCI in a tertiary referral interventional cardiac unit in the UK.
Methods
Collateral flow index (CFI) was calculated as (Pw − Pv)/(Pa − Pv), where Pa, Pw, and Pv are aortic, coronary wedge, and right atrial pressures during maximum hyperaemia. Collateral supply was considered poor (CFI < 0.25) or good (CFI ⩾ 0.25).
Main outcome measures
In‐stent restenosis six months after PCI, classified as neointimal volume ⩾ 25% stent volume on intravascular ultrasound (IVUS), or minimum lumen area ⩽ 50% stent area on IVUS, or minimum lumen diameter ⩽ 50% reference vessel diameter on quantitative coronary angiography.
Results
Patients with good collaterals had more severe coronary stenoses at baseline (90 (11)% v 75 (16)%, p < 0.001). Restenosis rates were similar in poor and good collateral groups (35% v 43%, p = 0.76 for diameter restenosis, 27% v 45%, p = 0.34 for area restenosis, and 23% v 24%, p = 0.84 for volumetric restenosis). CFI was not correlated with diameter, area, or volumetric restenosis (r2 < 0.1 for each). By multivariate analysis, stent diameter, stent length, > 10% residual stenosis, and smoking history were predictive of restenosis.
Conclusion
A well developed collateral circulation does not predict an increased risk of restenosis after PCI.
Keywords: collateral flow index, intravascular ultrasound, restenosis, percutaneous coronary intervention
The potential of coronary collaterals to abrogate myocardial ischaemia and limit infarction has long been established.1,2 Similarly, a well developed collateral circulation appears to predict an improved clinical outcome after percutaneous coronary intervention (PCI). When a dichotomous collateral flow index (CFI) threshold of 0.25 is used to distinguish between good and poor collateral supply, patients with inadequate collateral protection have been shown to have a four‐ to eightfold increase in the rate of death, myocardial infarction, or unstable angina after PCI compared with those with adequate collaterals.3,4 In contrast to the beneficial association between coronary collaterals and clinical sequelae, the impact of collateral flow at the time of PCI on subsequent restenosis remains controversial. Several retrospective studies have suggested that good collateral flow is a risk factor for restenosis.5,6,7 It has been postulated that this may be due to reduced antegrade flow in the target vessel caused by competitive flow through persistent collateral channels. More recent reports have failed to reproduce these findings.8,9
Much of this controversy may relate to the methods used in these studies. In early reports, the collateral circulation was characterised by coronary wedge pressure (Pw) or angiographically visible channels, which are imprecise techniques and have largely been superseded.5,6 All these studies have relied on a dichotomous angiographic definition of restenosis, which is relatively insensitive and thus may obscure potentially important associations.7,8,9 Furthermore, in many of these reports, angiographic follow up was limited to symptomatic patients, which in turn may have introduced substantial bias. We have carried out a prospective study to evaluate the potential association between collateral flow and restenosis by using ideal standard techniques to characterise both processes.
METHODS
Patients
Fifty eight consecutive patients (mean (SD) age 60 (9) years, 76% men) undergoing single vessel elective PCI were studied. Patients with high grade coronary lesions and chronic total coronary occlusions (CTOs) (TIMI (thrombolysis in myocardial infarction) grade 0 or I flow, duration of occlusion ⩾ 4 weeks) were recruited. Those who had undergone previous PCI or coronary bypass surgery were excluded from the study. The protocol was approved by the local research ethics committee and written informed consent was obtained from each participant at enrolment.
Coronary intervention
Coronary angiography and PCI were carried out through the femoral route with a 6 or 8 French guiding catheter. Patients were pretreated with aspirin and clopidogrel and unfractionated heparin was administered to maintain an activated clotting time of > 250 seconds during the procedure. An intracoronary bolus of 1 mg of isosorbide dinitrate was administered before diagnostic angiography with further boluses during the procedure. The lesion was crossed directly with a Pressurewire (Radi Medical Systems, Uppsala, Sweden) in most instances. In the case of CTOs, the lesion was crossed with a conventional guidewire and the Pressurewire was inserted through an exchange catheter. Predilatation followed by stent deployment was mandated by the protocol and patients requiring drug eluting stents were excluded from the study. PCI was performed by a single operator (SR) during the study.
Quantification of collateral supply
After calibration and equalisation to aortic pressure (Pa), the tip of a 0.014 inch pressure sensing guidewire (Pressurewire) was advanced beyond the lesion to measure distal coronary pressure. CFI was measured as previously described by simultaneous measurement of Pa, right atrial pressure (Pv), and Pw, where CFI = (Pw − Pv)/(Pa − Pv).10,11 Pw was assessed after at least 90 seconds of balloon occlusion and abolition of antegrade flow was confirmed by contrast angiography. Pa was measured through the guide catheter and Pv through the tip of a diagnostic catheter, which was inserted into the right atrium through the femoral vein. All measurements were carried out during maximum hyperaemia induced by an intravenous infusion of adenosine at 140 μg/kg/min.
Characterisation of coronary lesion
Angiograms of the target vessel were obtained in at least two orthogonal projections. Images were analysed off line by Discovery software (Quinton Instrument Co, Bothell, Washington, USA), which uses an edge detection technique (CorTrek application). Dimensions of the guide catheter were used to calibrate the system. Normal reference segments were identified proximal and distal to the lesion. Minimum lumen diameter (MLD), reference vessel diameter, percentage diameter stenosis, and lesion length were recorded in each case. Fractional flow reserve was calculated as previously described.10,11
Intravascular ultrasound
At six months' follow up, stented vessel segments were examined with a mechanical intravascular ultrasound (IVUS) catheter (Atlantis Plus 40 MHz; Boston Scientific) with automated pullback at 0.5 mm/s. Images were obtained by a Clearview or Galaxy system (Boston Scientific) and analysed off line with Indec software. The stented segment was analysed in cross sectional slices at intervals of ⩽ 0.5 mm. Lumen, stent boundaries, and external elastic lamina were delineated by a contour tracing method. A computer based contour detection program (echoPlaque) was used for automated three dimensional reconstruction of the stented segment from serial cross sectional slices. Volumetric stent obstruction was calculated as neointimal volume/stent volume × 100. Incomplete stent apposition was recorded.
Study protocol
Patients were assigned to two groups based on CFI at the time of PCI: good (CFI ⩾ 0.25) and poor (CFI < 0.25) collateral supply.3 Follow up angiography and IVUS were carried out six months after PCI. Three dichotomous classifications of restenosis were examined: neointimal volume ⩾ 25% of stent volume on IVUS; minimum lumen area (MLA) ⩽ 50% of stent area on IVUS; and MLD ⩽ 50% of reference vessel diameter on quantitative coronary angiography (QCA). In patients with restenosis, target vessel revascularisation (TVR) was based on clinical status. Investigators were blinded to CFI and patient characteristics during IVUS and QCA analysis.
Statistical analysis
Data are presented as mean (SD), unless otherwise specified. Between group categorical variables were compared by a χ2 test (with Yates' correction where appropriate) and continuous variables by a Mann‐Whitney test, at a significance level of 5%. Baseline variables found to correlate with restenosis in a univariate analysis (p < 0.10) were assessed by a multiple logistic regression model. Analyses were carried out with StatView 5.0 software (SAS Institute, Cary, North Carolina, USA).
Previous studies have shown binary angiographic restenosis rates of 85% and 45% in patients with good and poor collateral flow, respectively.7 Anticipating lower restenosis rates on account of systematic follow up (30% in the poor collateral group), a sample size of 50 would have 80% power (α = 5%) to detect the magnitude of difference found by Wahl et al.7 With the use of volumetric IVUS analysis, a sample of 50 provides sufficient power to detect even smaller differences in restenosis rates than previously shown.12
RESULTS
CFI was 0.22 (11) at the time of PCI; 33 patients had poor and 25 had good collateral supply. Compared with those with poor collaterals, patients with good collaterals had more severe coronary stenoses at baseline (90 (11)% v 75 (16)%, p < 0.001) and included a higher proportion of CTOs (48% v 6%, p < 0.001). Left ventricular ejection fraction was higher in patients with poor collaterals (68 (10)% v 60 (11)%, p = 0.002). Baseline characteristics were otherwise similar in both groups (table 1).
Table 1 Clinical and lesion characteristics.
| Poor collaterals (CFI <0.25) | Good collaterals (CFI ⩾0.25) | p Value | |
|---|---|---|---|
| Number | 33 | 25 | |
| Age (years) | 59 (9) | 60 (9) | 0.71 |
| Men | 73 | 80 | 0.74 |
| Smoking history | 0.07 | ||
| Non‐smoker | 43 | 28 | |
| Former smoker | 30 | 60 | |
| Current smoker | 27 | 12 | |
| Hypertension | 58 | 40 | 0.29 |
| Diabetes mellitus | 18 | 4 | 0.22 |
| Previous myocardial infarction | 21 | 40 | 0.21 |
| LV ejection fraction (%) | 68 (10) | 60 (11) | 0.002 |
| Target vessel | 0.36 | ||
| LAD | 49 | 56 | |
| Right coronary artery | 24 | 32 | |
| Circumflex artery | 27 | 12 | |
| Reference vessel diameter (mm) | 2.85 (0.45) | 2.85 (0.72) | 0.70 |
| Baseline diameter stenosis (%) | 75 (16) | 90 (11) | <0.001 |
| Chronic total occlusions | 6 | 48 | <0.001 |
| Stent length (mm) | 19.1 (9.3) | 25.9 (16.8) | 0.30 |
| Stent diameter (mm) | 3.36 (0.42) | 3.25 (0.64) | 0.35 |
| Final diameter stenosis (%) | 9 (11) | 10 (10) | 0.74 |
Data are mean (SD) or number (%). Categorical variables were compared by χ2 and continuous variables by Mann‐Whitney tests.
LAD, left anterior descending artery.
Follow up was completed in 50 patients, 189 (32) days after PCI. Eight patients, who were asymptomatic at follow up, declined further investigation. The patients who declined follow up had a higher final MLD (3.1 (0.6) v 2.5 (0.5) mm, p = 0.003) but otherwise their baseline characteristics were similar to those of the group who underwent follow up angiography. CFI in the two groups was 0.22 (0.12) and 0.22 (0.11), respectively (p = 0.85).
Angiographic follow up
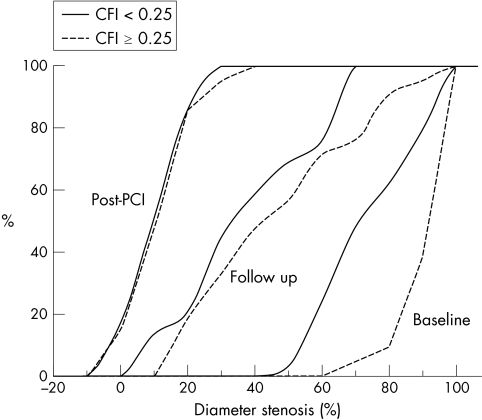
At six months, MLD on QCA was 42 (23)% of reference vessel diameter, with no significant differences between poor and good collateral groups (38 (21)% v 47 (26)%, p = 0.33) (figs 1 and 2). The binary in‐stent restenosis rate was 35% in patients with poor collaterals and 43% in those with good collaterals (p = 0.76). Baseline CFI was similar in patients who had binary angiographic restenosis and those who did not (0.21 (0.12) v 0.23 (0.11), p = 0.68). The degree of lesion stenosis at baseline was positively correlated with CFI (r2 = 0.33, p < 0.001) but CFI and the degree of restenosis at six months were not correlated (r2 = 0.03, p = 0.27).
Figure 1 Angiographic restenosis. Cumulative distribution curves for percentage diameter stenosis in patients with good (dashed line) and poor (full line) collateral flow, before and immediately after percutaneous coronary intervention (PCI) and at six months' follow up. Patients with good collateral flow had more severe stenoses at baseline but diameter stenosis after PCI and at follow up did not differ significantly. CFI, collateral flow index.
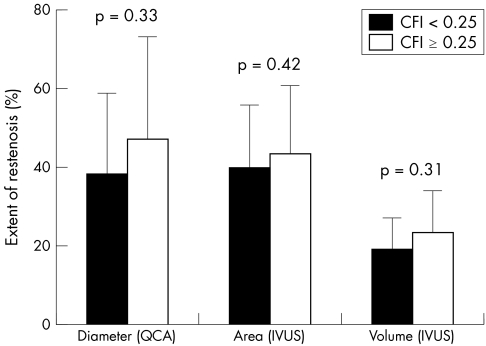
Figure 2 Extent of restenosis on quantitative coronary angiography (QCA) (percentage diameter stenosis) and intravascular ultrasound (IVUS) (percentage area stenosis and percentage volume stenosis) in poor and good collateral groups (filled and open bars, respectively) was not significantly different.
IVUS follow up
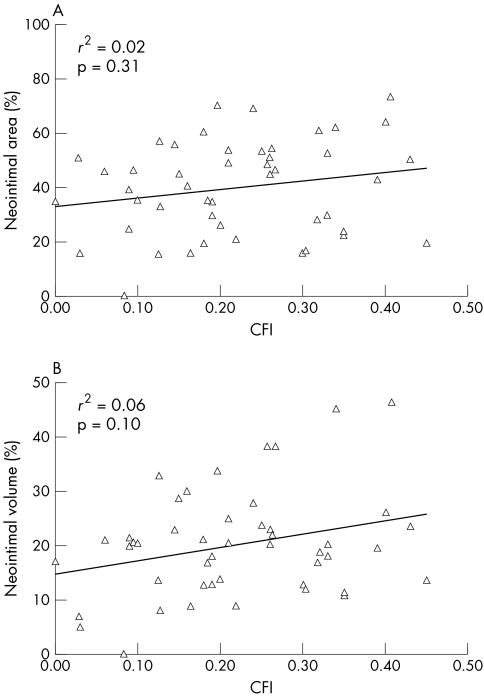
At six months, MLA on IVUS was 6.0 (2.8) mm2, with an MLA ⩽ 4 mm2 in 12% and 40%, respectively, of the poor and good collateral groups (p = 0.06). The binary restenosis rate (for MLA ⩽ 50% stent area) was 27% and 45% in the poor and good collateral groups, respectively (p = 0.34). Even a more conservative threshold (MLA ⩽ 60% stent area) did not show significant differences in restenosis rates between poor and good collateral groups (12% v 20%, p = 0.71). Furthermore, area stenosis at follow up and CFI at baseline were not correlated (r2 = 0.02, p = 0.31) (fig 3A). On volumetric analysis, neointimal volume was 51 (59) mm3 versus 62 (48) mm3 (p = 0.22) and degree of stent obstruction was 19 (8)% versus 23 (11)% (p = 0.31) in the poor and good collateral groups, respectively (fig 2). The binary volumetric restenosis rate was 23% in the poor collateral and 24% in the good collateral group (p = 0.84). Baseline CFI was similar in patients who had binary volumetric restenosis and those who did not (0.25 (0.10) v 0.22 (0.12), p = 0.37). Volumetric stent obstruction and CFI at baseline were not correlated (r2 = 0.06, p = 0.10) (fig 3B).
Figure 3 Restenosis on IVUS in relation to CFI. (A) Maximum neointimal area (in relation to stent area) and (B) neointimal volume (in relation to stent volume) had no correlation to baseline CFI.
Fractional flow reserve at follow up
The haemodynamic severity of restenosis assessed by fractional flow reserve did not differ significantly between patients with poor and good collateral supply (0.83 (0.07) v 0.86 (0.08), respectively, p = 0.41). There was a trend towards a positive correlation between fractional flow reserve at follow up and CFI at baseline (r2 = 0.17, p = 0.08).
Predictors of restenosis
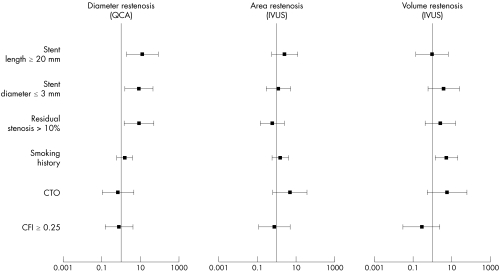
By univariate analysis, stent length ⩾ 20 mm, stent diameter ⩽ 3 mm, and residual stenosis > 10% were predictive of binary QCA restenosis; baseline stenosis severity and smoking history were predictive of binary volumetric restenosis; and baseline stenosis severity, CTO, and stent length ⩾ 20 mm were predictive of binary area restenosis. CFI was not a predictor of restenosis on either univariate analysis or multivariate logistic regression analysis (fig 4).
Figure 4 Predictors of restenosis. Logistic regression model (data are presented as odds ratios and 95% confidence intervals). CTO, chronic total coronary occlusion.
Target vessel revascularisation
The six month TVR rate was 7% in the whole cohort, with two patients undergoing further PCI and two undergoing coronary artery bypass surgery. CFI at the time of PCI was 0.27 (0.14) in patients who had subsequent TVR, but the difference in TVR rates in the poor and good collateral groups was not significant (1 of 33 (3%) v 3 of 25 (12%), p = 0.42). On univariate analysis, stent diameter ⩽ 3 mm was weakly related to TVR (r = 0.29, p = 0.03), but none of these variables was predictive of TVR in a multivariate regression model.
DISCUSSION
Despite substantial technical advances in recent years, restenosis after PCI continues to be the major limitation of this procedure. It affects about 30% of coronary lesions treated with bare metal stents, with rates approaching 60% after recanalisation of CTOs.13,14 Interestingly, collateral flow at the time of PCI has emerged as one of many factors that may predict the development of subsequent restenosis. Although a biologically plausible mechanism has not been documented, it has been proposed that collateral flow may have a causal role in promoting restenosis, in a manner analogous to the progression of atherosclerosis in native coronary arteries proximal to insertion of an aortocoronary bypass graft.15,16 Alternatively, collateral flow may merely be a marker for another established risk factor for restenosis, such as the morphology or chronicity of a coronary lesion.17 Robust methods of characterising both the collateral circulation and the restenotic process are central to unravelling this putative association.
Many of the methodological difficulties posed by characterising the collateral circulation according to Pw or angiographically visible channels have been overcome by the use of CFI, which is now the accepted clinical standard for quantifying collateral flow.5,6,10,18 However, even recent studies that have used CFI have yielded conflicting results. Wahl et al7 reported that patients with symptomatic restenosis had a higher CFI at the time of PCI (0.26 (0.14) v 0.12 (0.09), p < 0.0001). The actual incidence of restenosis in this population is unclear, as follow up was available in only one third of the patients studied. Conversely, Werner et al9 found that patients with restenosis had similar baseline CFIs to those who did not. This study was restricted to patients with CTOs and as such was notable for the excellent collateral flow observed in all patients (with relatively few possessing a CFI < 0.25), which limits the comparison of restenosis between good and poor collateral groups. In addition to the methodological issues listed above, some of the contradictory results may have arisen from the reliance of these studies on a dichotomous angiographic threshold for detection of restenosis. Although ⩾ 50% diameter stenosis has traditionally been the working definition of restenosis, the limitations of angiography in delineating neointimal hyperplasia and the superiority of IVUS are well established.19,20,21 The improved sensitivity of IVUS allows detection of more subtle differences in restenosis rates in small samples, particularly when three dimensional volumetric measurements are performed.12,22
By using CFI to determine collateral support and IVUS to document restenosis, we have shown that the degree of collateral flow at the time of PCI is not predictive of subsequent restenosis. In particular, we found similar binary restenosis rates in patients with poor and good collateral flow when we used three different classifications of restenosis based on IVUS and QCA. Furthermore, we observed no correlation between baseline collateral flow and any of the continuous parameters reflecting neointimal hyperplasia. On the other hand, we found several established risk factors to be predictive of in‐stent restenosis in the current study, including residual diameter stenosis after PCI and the use of smaller and longer stents.13,23,24
It seems clear that a well developed collateral circulation should not be considered a risk factor for restenosis after PCI. On the other hand, we have shown that good collateral flow tends to be associated with higher grade coronary lesions and CTOs, which do predict an increased likelihood of restenosis.17 The concept of competitive flow through collateral channels after PCI is simplistic, as flow down these conduits is passive and likely to be minimal once the pressure gradient across the collateral bed is abolished. However, these channels may remain recruitable if this pressure gradient is later re‐established due to acute coronary occlusion, which may underlie the improved clinical outcome observed in well collateralised patients.3,4 Altogether, a good collateral circulation can probably be regarded as a favourable indicator rather than a contraindication to PCI.
The principal limitation of our study was the relatively small sample size. However, several features of this population make them particularly suitable for prospective evaluation of the potential association between restenosis and collateral flow. Firstly, the inclusion strategy was devised to ensure that a broad spectrum of collateral support was represented in the study population. This was achieved by recruiting patients with non‐occlusive lesions as well as CTOs, given that about two thirds of patients with non‐occlusive lesions have poor collaterals and most of those with CTOs have good collateral supply.9,25 The statistical constraints imposed by low numbers were further ameliorated by using IVUS, which is a sensitive and reproducible technique for evaluating restenosis. The lower left ventricular ejection fraction in the well collateralised group may be accompanied by a degree of microvascular dysfunction. Theoretically, this dysfunction can limit the induction of maximum hyperaemia during infusion of adenosine, which in turn may affect assessment of CFI. However, given the relatively normal left ventricular function in both groups, the actual impact of this difference is likely to be minimal.
Conclusion
A well developed collateral circulation does not predict an increased risk of restenosis after PCI.
ACKNOWLEDGEMENTS
Divaka Perera was funded by a fellowship from the Guy's and St Thomas' Charitable Foundation, London, UK
Abbreviations
CFI - collateral flow index
CTO - chronic total coronary occlusion
IVUS - intravascular ultrasound
MLA - minimum lumen area
MLD - minimum lumen diameter
Pa - aortic pressure
PCI - percutaneous coronary intervention
Pv - right atrial pressure
Pw - coronary wedge pressure
QCA - quantitative coronary angiography
TIMI - thrombolysis in myocardial infarction
TVR - target vessel revascularisation
Footnotes
Competing interests: None declared
References
- 1.Reimer K A, Jennings R B. The “wavefront phenomenon” of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest 197940633–644. [PubMed] [Google Scholar]
- 2.Habib G B, Heibig J, Forman S A.et al Influence of coronary collateral vessels on myocardial infarct size in humans: results of phase I thrombolysis in myocardial infarction (TIMI) trial. The TIMI investigators. Circulation 199183739–746. [DOI] [PubMed] [Google Scholar]
- 3.Pijls N H, Bech G J, el Gamal M I.et al Quantification of recruitable coronary collateral blood flow in conscious humans and its potential to predict future ischemic events. J Am Coll Cardiol 1995251522–1528. [DOI] [PubMed] [Google Scholar]
- 4.Billinger M, Kloos P, Eberli F R.et al Physiologically assessed coronary collateral flow and adverse cardiac ischemic events: a follow‐up study in 403 patients with coronary artery disease. J Am Coll Cardiol 2002401545–1550. [DOI] [PubMed] [Google Scholar]
- 5.Urban P, Meier B, Finci L.et al Coronary wedge pressure: a predictor of restenosis after coronary balloon angioplasty. J Am Coll Cardiol 198710504–509. [DOI] [PubMed] [Google Scholar]
- 6.Probst P, Baumgartner C, Gottsauner‐Wolf M. The influence of the presence of collaterals on restenoses after PTCA. Clin Cardiol 199114803–807. [DOI] [PubMed] [Google Scholar]
- 7.Wahl A, Billinger M, Fleisch M.et al Quantitatively assessed coronary collateral circulation and restenosis following percutaneous revascularization. Eur Heart J 2000211776–1784. [DOI] [PubMed] [Google Scholar]
- 8.Lee C W, Hong M K, Choi S W.et al Influence of coronary collateral flow on restenosis following primary angioplasty for acute myocardial infarction. Catheter Cardiovasc Interv 200255477–481. [DOI] [PubMed] [Google Scholar]
- 9.Werner G S, Bahrmann P, Mutschke O.et al Determinants of target vessel failure in chronic total coronary occlusions after stent implantation: the influence of collateral function and coronary hemodynamics. J Am Coll Cardiol 200342219–225. [DOI] [PubMed] [Google Scholar]
- 10.Pijls N H, van Son J A, Kirkeeide R L.et al Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation 1993871354–1367. [DOI] [PubMed] [Google Scholar]
- 11.Perera D, Biggart S, Postema P.et al Right atrial pressure: can it be ignored when calculating fractional flow reserve and collateral flow index? J Am Coll Cardiol 2004442089–2091. [DOI] [PubMed] [Google Scholar]
- 12.Mehran R, Mintz G S, Hong M K.et al Validation of the in vivo intravascular ultrasound measurement of in‐stent neointimal hyperplasia volumes. J Am Coll Cardiol 199832794–799. [DOI] [PubMed] [Google Scholar]
- 13.Cutlip D E, Chauhan M S, Baim D S.et al Clinical restenosis after coronary stenting: perspectives from multicenter clinical trials. J Am Coll Cardiol 2002402082–2089. [DOI] [PubMed] [Google Scholar]
- 14.Bell M R, Berger P B, Bresnahan J F.et al Initial and long‐term outcome of 354 patients after coronary balloon angioplasty of total coronary artery occlusions. Circulation 1992851003–1011. [DOI] [PubMed] [Google Scholar]
- 15.Aldridge H E, Trimble A S. Progression of proximal coronary artery lesions to total occlusion after aorta‐coronary saphenous vein bypass grafting. J Thorac Cardiovasc Surg 1971627–11. [PubMed] [Google Scholar]
- 16.Cashin W L, Sanmarco M E, Nessim S A.et al Accelerated progression of atherosclerosis in coronary vessels with minimal lesions that are bypassed. N Engl J Med 1984311824–828. [DOI] [PubMed] [Google Scholar]
- 17.Meier B. Total coronary occlusion: a different animal? J Am Coll Cardiol 199117(6 suppl B)50B–7B. [DOI] [PubMed] [Google Scholar]
- 18.Eeckhout E, van Melle G, Stauffer J C.et al Can early closure and restenosis after endoluminal stenting be predicted from clinical, procedural, and angiographic variables at the time of intervention? Br Heart J 199574592–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann R, Mintz G S, Dussaillant G R.et al Patterns and mechanisms of in‐stent restenosis: a serial intravascular ultrasound study. Circulation 1996941247–1254. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann R, Mintz G S, Popma J J.et al Overestimation of acute lumen gain and late lumen loss by quantitative coronary angiography (compared with intravascular ultrasound) in stented lesions. Am J Cardiol 1997801277–1281. [DOI] [PubMed] [Google Scholar]
- 21.Kozuma K, Regar E, Bruining N.et al Sensitivity and specificity of QCA in detecting coronary arterial remodeling after intracoronary brachytherapy: a comparison to serial volumetric three‐dimensional intravascular ultrasound analysis. Can we detect positive remodeling by luminography? J Invasive Cardiol 200315636–640. [PubMed] [Google Scholar]
- 22.Prati F, Pawlowski T, Sommariva L.et al Intravascular ultrasound and quantitative coronary angiography assessment of late in‐stent restenosis: in vivo human correlation and methodological implications. Catheter Cardiovasc Interv 200257155–160. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg S L, Loussararian A, De Gregorio J.et al Predictors of diffuse and aggressive intra‐stent restenosis. J Am Coll Cardiol 2001371019–1025. [DOI] [PubMed] [Google Scholar]
- 24.Mercado N, Boersma E, Wijns W.et al Clinical and quantitative coronary angiographic predictors of coronary restenosis: a comparative analysis from the balloon‐to‐stent era. J Am Coll Cardiol 200138645–652. [DOI] [PubMed] [Google Scholar]
- 25.Pohl T, Seiler C, Billinger M.et al Frequency distribution of collateral flow and factors influencing collateral channel development: functional collateral channel measurement in 450 patients with coronary artery disease. J Am Coll Cardiol 2001381872–1878. [DOI] [PubMed] [Google Scholar]