In addition to its role as the main regulator of erythropoiesis, erythropoietin has a wide range of protective, antiapoptotic activities in vitro and in vivo, particularly in the brain and, as more recently shown, in the heart.1
Of note, erythropoietin is not exclusively produced by fetal liver and adult kidney but is expressed also in the brain, where it can be induced by hypoxia or ischaemia. Several studies underline the potential role of erythropoietin in mediating hypoxic–ischaemic preconditioning in the brain.1 On the other hand, the heart has never been described as a site for erythropoietin production. However, hypoxia inducible factor 1 (HIF‐1), the main transcriptional regulator of erythropoietin, is induced by hypoxia in myocardial cells in vivo and in vitro.1 HIF‐1 has recently been reported to mediate hypoxic preconditioning in the heart.2 We investigated whether, as in the brain, erythropoietin is produced in mouse ischaemic heart.3
MATERIALS AND METHODS
After permanent coronary artery occlusion (CAO), CD1 mice were killed.4 For immunohistochemistry, the portion of heart below the ligature was formalin fixed, paraffin embedded, cut into 5 µm thick sections, and processed as previously described (antierythropoietin: H‐162, 1:100; Santa Cruz Biotechnology, Santa Cruz, California, USA).5 For RNA preparation, infarcted and non‐infarcted areas, distinguishable by their difference in colour, were separated and frozen in liquid nitrogen. Total RNA was extracted, subjected to reverse transcription, and amplified by real time polymerase chain reaction (PCR)with the SYBR Green PCR master mix (Applied Biosystems, Foster City, California, USA), according to standard procedures (primers erythropoietin 5′‐GAGGCAGAAAATGTCACGATG‐3′ and 5′‐CTTCCACCTCCATTCTTTTCC‐3′; 18S (internal control) 5′‐TCAAGAACGAAAGTCGGAGGTT‐3′ and 5′‐GGACATCTAAGGGCATCACAG‐3′). Data were expressed as erythropoietin mRNA units, calculated with the comparative threshold cycle method according to Applied Biosystems' guidelines, with a cDNA from mouse kidney used as a calibrator.
RESULTS
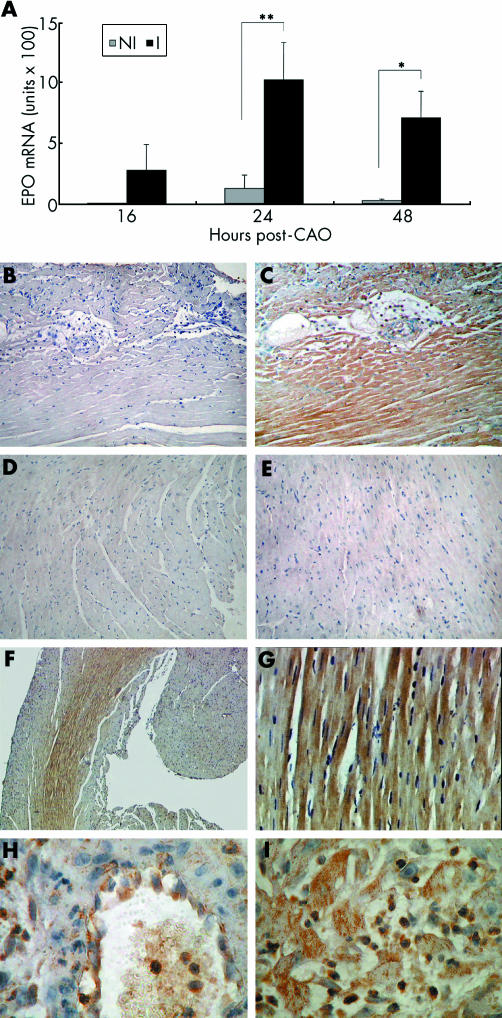
By reverse transcription PCR, erythropoietin was undetectable in heart of control mice (not shown), as reported.1 The mRNA concentration was very low in only two of seven non‐infarcted areas 24 hours after CAO. In contrast, we found erythropoietin mRNA in infarcted areas 24 hours and 48 hours after CAO. At 16 hours only two of five infarcted areas expressed measurable erythropoietin mRNA, which was therefore not significantly different from non‐infarcted heart (fig 1A). Eythropoietin was undetectable at earlier time points (two, four, and eight hours after CAO, n = 2 each time point) and 72 hours after CAO (n = 3), and at all time points in sham operated animals (n = 2 to 3 at each time point; not shown). Eythropoietin expression was therefore increased in infarcted areas 24 to 48 hours after CAO.
Figure 1 (A) Erythropoietin (EPO) mRNA in mouse non‐infarcted (NI) and infarcted (I) heart 16 (n = 5), 24 (n = 7), and 48 (n = 6) hours after coronary artery occlusion (CAO). Results are mean (SEM). Undetectable EPO mRNA was assigned the value of the limit of detection of the assay, calculated by assigning the value of 40 to the threshold cycle of erythropoietin amplification and normalising for the threshold cycle of 18S. Undetectable mRNA values ranged between 0.02 and 0.6 units × 100, depending on the size of the sample (input cDNA). *p < 0.05 v NI; **p < 0.01 v NI, paired Student's t test. (B–I) Representative images of EPO immunohistochemistry in mouse heart showing sections stained (B) without or (C–I) with EPO antibody, sections 48 hours after (B, C) CAO or (D) sham operation, (E) septum 48 hours after CAO, and sections (F, G) 24 hours and (H, I) 48 hours after CAO. Original magnification (B–E) 200×, (F) 50×, (G) 400×, and (H, I) 1000×.
Serial transverse sections prepared from hearts 48 hours after CAO were stained without (fig 1B) or with (fig 1C) erythropoietin antibody. Strong erythropoietin immunoreactivity was present only in fig 1C, showing the specificity of the signal. Eythropoietin was not detectable 48 hours after sham operation (fig 1D) or in the septum 48 hours after CAO (fig 1E). Low (50×; fig 1F) and higher magnifications (400×; fig 1G) show a strong erythropoietin signal 24 hours after CAO in the myocardium nearest to the infarct (fig 1F), both in cytoplasm and in sarcolemma of viable cardiomyocytes (fig 1G). Forty eight hours after CAO in the infarcted region of the left ventricle several infiltrating and circulating mononuclear cells were positive for erythropoietin (1000×; fig 1H and fig 1I). In fig 1H, the endothelial lining of a venula shows strong erythropoietin immunoreactivity.
DISCUSSION
This study shows that erythropoietin is expressed in ischaemic mouse heart 24 and 48 hours after permanent CAO. Our data in the heart parallel the results obtained by Bernaudin et al3 in the brain, where erythropoietin was induced after focal permanent cerebral ischaemia in mice.
Cardiomyocytes can produce erythropoietin, as they can express HIF‐1 in hypoxic conditions.1 In this regard, we found that the rat cardiomyoblast cell line H9c2 expresses erythropoietin mRNA, and a trend for hypoxia increased erythropoietin mRNA was observed in these cells (not shown). A contribution of infiltrating mononuclear cells and of endothelial cells, as suggested by the immunohistochemistry results, cannot be excluded. Of note, in cerebral ischaemia post‐ischaemic erythropoietin immunoreactivity was localised to endothelial cells, microglia, and macrophage‐like cells.3
In conclusion, this is the first study showing that the ischaemic heart can produce erythropoietin. Owing to the cardioprotective effects of erythropoietin, our results suggest the possibility of a local mechanism of erythropoietin mediated protection in the ischaemic heart.1
ACKNOWLEDGEMENTS
Supported by the Kenneth S Warren Institute and by the Fondazione CARIPLO, Milan, Italy. This work emanates from the European Vascular Genomics Network (http://www.evgn.org), a Network of Excellence supported by the European Community's Sixth Framework Programme for Research Priority 1 “Life sciences, genomics and biotechnology for health” (Contract No. LSHM‐CT‐2003‐503254).
Footnotes
Competing interests: None declared.
References
- 1.Bogoyevitch M A. An update on the cardiac effects of erythropoietin cardioprotection by erythropoietin and the lessons learnt from studies in neuroprotection. Cardiovasc Res 200463208–216. [DOI] [PubMed] [Google Scholar]
- 2.Cai Z, Manalo D J, Wei G.et al Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia‐reperfusion injury. Circulation 200310879–85. [DOI] [PubMed] [Google Scholar]
- 3.Bernaudin M, Marti H H, Roussel S.et al A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab 199919643–651. [DOI] [PubMed] [Google Scholar]
- 4.Galli D, Innocenzi A, Staszewsky L.et al Mesoangioblasts, vessel‐associated multipotent stem cells, repair the infarcted heart by multiple cellular mechanisms: a comparison with bone marrow progenitors, fibroblasts, and endothelial cells. Arterioscler Thromb Vasc Biol 200525692–697. [DOI] [PubMed] [Google Scholar]
- 5.Grasso G, Sfacteria A, Passalacqua M.et al Erythropoietin and erythropoietin receptor expression after experimental spinal cord injury encourages therapy by exogenous erythropoietin. Neurosurgery 200556821–827. [DOI] [PubMed] [Google Scholar]