Abstract
Objective
To characterise prospectively by magnetic resonance imaging (MRI) changes in right ventricular (RV) volume, function, and mass after transcatheter closure of atrial septal defects (ASDs) and to evaluate the course of pulmonary pressure and functional class criteria.
Methods
In 20 patients with secundum‐type ASD and dilated RV diameter, MRI was performed to quantify RV end diastolic (RVEDV) and end systolic volumes (RVESV), RV mass, tricuspid annular diameter, and RV ejection fraction before and 6 and 12 months after transcatheter closure of the ASD. RV systolic pressure was measured during follow up by transthoracic echocardiography.
Results
Functional class improved in the majority of patients after ASD closure. RVESV (from 81 (18) ml/m2 to 53 (15) ml/m2, p < 0.001), RVEDV (from 127 (17) ml/m2 to 99 (18) ml/m2, p < 0.001), and RV mass (from 79 (10) g to 63 (8) g, p < 0.01) decreased significantly during follow up, although tricuspid annular diameter did not. RV ejection fraction improved (by 9% compared with baseline, p < 0.05) and RV systolic pressure decreased significantly (from 33 (8) mm Hg to 24 (6) mm Hg, p < 0.001) after closure.
Conclusion
MRI studies showed significant improvement of RV volumes, mass, and function after transcatheter closure of ASDs. Restoration of the RV leads to decreased pulmonary pressure resulting in a better functional class in the majority of patients.
Keywords: atrial septal defect, transcatheter closure, right ventricle, magnetic resonance imaging
Atrial septal defects (ASDs) are among the most common congenital heart lesions found in adult life.1 Most infants and children with ASD are asymptomatic and physical findings may be unimpressive, making survival into adulthood normal. Long term exposure to chronic right heart volume overload leads to dilatation of the right atrium and right ventricle (RV) and in certain cases, due to an increased pulmonary flow, to deleterious effects such as pulmonary hypertension and right heart failure.2 Patients may present with symptoms of fatigue, dyspnoea, recurrent lower respiratory tract infection, palpitations, and thromboembolic events. It has been accepted that closure of the interatrial defect is the treatment of choice for an ASD with significant pulmonary to systemic flow ratio, especially if RV volume overload is present, even if the patient has few or no symptoms.1,3
In recent years transcatheter closure of interatrial defects was introduced into clinical practice and became, next to the surgical approach, a secure, efficient, and cost effective option for defect management.4,5,6 In a study comparing surgical versus transcatheter closure of ASDs, the transcatheter approach proved superior. Myocardial function, both systolic and diastolic, was impaired by surgical closure but preserved after transcatheter occlusion.7
Conflicting data exist as to whether the course after ASD closure leads to improved RV performance or only prevents further deterioration.3,7,8,9,10,11,12,13,14 For example, Eidem et al11 described no significant change in RV performance in patients after surgical closure of ASDs; however, Salehian et al14 reported that transcatheter closure of ASDs was associated with improvement of left ventricular (LV) and RV function.
Divergent results regarding RV volume and function after closure may be caused by different ASD closure techniques and limitations of echocardiography in the quantitative assessment of the RV.
The objective of this study was to clarify whether, after percutaneous ASD closure, RV volume and mass are restored and RV function improves. Changes in RV end diastolic (RVEDV) and end systolic volumes (RVESV), RV ejection fraction (RVEF), RV mass, and tricuspid annular diameter (TAD) were analysed by magnetic resonance imaging (MRI), the most robust technique for morphological evaluation of the RV.15,16 Lastly, we sought to evaluate the course of pulmonary pressure and functional class criteria.
METHODS
Study population
Twenty consecutive patients referred for transcatheter closure of a secundum‐type ASD were enrolled in this study. Before undergoing MRI, all patients gave written informed consent in accordance with ethical guidelines followed in our institution. To be eligible, patients had to be 18 years or older and echocardiographic estimation had to show a dilated RV diameter. None of the patients had additional coronary artery disease, valvular heart disease, or pulmonary disease. The study protocol consisted of clinical assessments, evaluation of New York Heart Association (NYHA) functional class criteria, and transthoracic (TTE) and transoesophageal echocardiography (TOE) and MRI examinations before and six and 12 months after transcatheter ASD closure. Patients also had a complete haemodynamic evaluation by cardiac catheterisation just before closure of the defect.
Transthoracic echocardiography
Two dimensional colour Doppler TTE was performed before and six and 12 months after ASD closure with a Sonos 5500 ultrasound system (Agilent Technologies). The examination focused on the measurement of RV end diastolic diameter and RV systolic pressure (RVSP). Two measurements of the RV were made in the apical four chamber view: maximum RV long axis dimension, defined as the distance between the RV apex and the mid point of the tricuspid valve; and RV short axis dimension, defined as the maximum dimension from the right septal surface to the free wall perpendicular to the long axis. RVSP was estimated by use of the maximum velocity of the tricuspid regurgitant jet. The systolic pressure gradient between the RV and right atrium was calculated by the modified Bernoulli equation. Right atrial pressure was estimated by examination of the jugular venous pulse as described previously.17,18 Adding the transtricuspid gradient to the right atrial pressure (10 (5) mm Hg) predicted the RVSP.19 All patients were in sinus rhythm at the time of their examinations.
Magnetic resonance imaging
MRI was performed for determination of RV volumes, function, and mass and TAD. From ventricular apex to base ECG triggered, breath hold, segmented k space cine gradient echo sequences (1.5 T, Somatom Vision; Siemens) were obtained in the short axis view. Parameters were as follows: echo time, 3.8 ms; repeat time equivalent to the RR interval; slice thickness, 10 mm; field of view, 35 × 35 cm; read matrix, 256; phase matrix, 128; 16 frames (typical temporal resolution of 50 ms); flip angle, 40°; and phase encode grouping, 6–10. Eight to 12 short axis slices were needed to encompass the entire RV. Manual tracing of epicardial and endocardial borders of contiguous short axis slices at end diastole (first cine phase of the R wave triggered acquisition) and end systole (image phase with smallest cavity area) allowed for calculation of RV mass, RVEDV, and RVESV, from which RVEF was derived. TAD was measured as the maximum diameter of the tricuspid annulus in a stack of cine gradient echo sequences in the four chamber view. For RV mass calculation, the myocardial volume was multiplied by the specific density of myocardium (taken as 1.05 g/cm3).20 The interventricular septum was treated as part of the LV and excluded from RV mass measurements. The moderator band and evident trabeculations were included in the mass and excluded from the RV volume. The scans were analysed with the investigator blinded to previous results. All patients underwent MRI in the morning, with no oral intake between midnight and scanning. Volumes and TAD were normalised to body surface area. As normal values we used RVEDV of 48.0–107.5 ml/m2, RVESV of 13.1–47.2 ml/m2, RVEF of 49.1–74.1%, TAD of 13–20 mm/m2, and RV mass of 33–80 g.16,21,22,23,24
Haemodynamic study and ASD closure
Haemodynamic study and percutaneous closure were performed under sedation and local anaesthesia. The size, location, and relation of the ASD to the surrounding structures were assessed by continuous TOE. Pulmonary to systemic flow ratio was calculated by oximetry according to the Fick principle. The Amplatzer septal occluder (AGA Corp) or the Cardia persistent foramen ovale/ASD device (Cardia Inc, Burnsville, Minnesota, USA) was used to close the ASD as previously described.25,26 Postinterventional treatment included oral aspirin (100 mg/day) for 12 months, clopidogrel (75 mg/day) for six weeks, and low dose heparin for the first three days (2 × 5000 IU subcutaneously) after intervention.
Statistical analysis
The Wilcoxon matched pairs test was used to compare pre‐ versus six or 12 month post‐ASD closure variables. A value of p < 0.05 was considered significant.
RESULTS
The ASD was closed in 14 patients with an Amplatzer device (median device size 26 (6 )mm) and in six patients with a PFO‐Star device (median device size 22 (5) mm). At TTE and TOE performed peri‐interventionally and after six and 12 months, no residual shunt was noted in the entire study group. Major complications did not occur. The mean age of the 20 patients (12 women, eight men) was 43 (13) years (range 19–65). No patient was lost to clinical follow up. Table 1 shows patients' characteristics, along with baseline TTE, TOE, and MRI findings.
Table 1 Baseline clinical and haemodynamic data.
| Age at closure (years) | 43 (13) |
| Women | 12 (60%) |
| NYHA functional class I | 12 (60%) |
| NYHA functional class >I | 8 (40%) |
| Stretch balloon size (mm) | 24 (6) |
| Qp:Qs ratio ⩾2:1 | 9 (45%) |
| Qp:Qs ratio <2:1 | 11 (55%) |
| Median Amplatzer device size (mm) | 26 (6) |
| Median PFO‐Star device size (mm) | 22 (5) |
| RVSP at TTE (mm Hg) | 33 (8) |
| RVSP >30 mm Hg | 8 (40%) |
| RVEDD at TTE (mm) | 36 (4) |
| RVEDV at MRI (ml/m2 BSA) | 127 (17) |
| RVESV at MRI (ml/m2 BSA) | 81 (18) |
| RVEF at MRI (%) | 37 (9) |
| TAD at MRI (mm/m2 BSA) | 23 (7) |
BSA, body surface area; MRI, magnetic resonance imaging; NYHA, New York Heart Association; Qp:Qs, pulmonary to systemic blood flow; RVEDD, right ventricular end diastolic diameter; RVEDV, right ventricular end diastolic volume; RVEF, right ventricular ejection fraction; RVESV, right ventricular end systolic volume; RVSP, right ventricular systolic pressure; TAD, tricuspid annular diameter; TTE, transthoracic echocardiography.
Mean interval from the transcatheter closure procedure to six months' follow up was 6.4 months (range 5.6–9.2 months) and to 12 months' follow up it was 12.8 months (range 11.2–14.8 months). TOE showed sufficient ASD closure in all patients.
Functional status
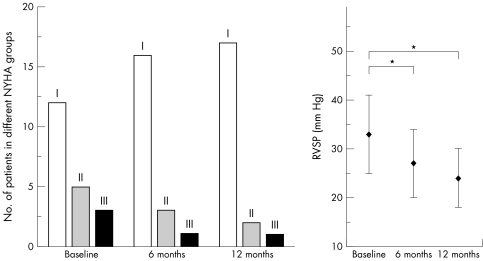
Before ASD closure 12 patients were in NYHA class I, five were in class II, and three were in class III. The patients' conditions improved during follow up, leading to a better functional class in five of eight symptomatic patients (fig 1).
Figure 1 Distribution of patients in New York Heart Association (NYHA) classes at baseline and during follow up after atrial septal defect (ASD) closure (left). Right ventricular systolic pressure (RVSP) decreased significantly after transcatheter closure (right). *p < 0.001.
Echocardiography
Before ASD closure all patients had a dilated RV end diastolic diameter (36 (4) mm) as measured by two dimensional TTE (inclusion criterion). Baseline RVSP was 33 (8) mm Hg, which decreased significantly after six months to 27 (7) mm Hg and after 12 months to 24 (6) mm Hg (p < 0.001). Before closure six patients had moderate tricuspid regurgitation (TR). In 14 patients minimal to mild TR was seen. At the six month follow up 17 patients had minimal to mild TR and in three patients moderate TR persisted. TTE at 12 months after ASD closure showed moderate TR in two patients and minimal to mild TR in 18 patients. No patient had worsening of TR.
Magnetic resonance imaging
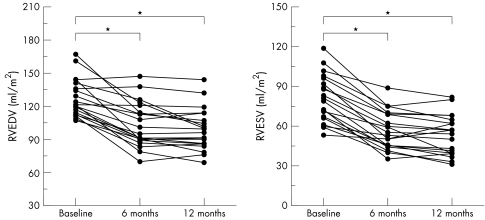
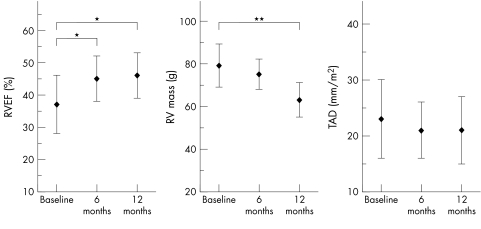
Baseline measurements showed enlarged RV volumes in all patients and dilated TAD in 13 (65%) patients: RVEDV 127 (17) ml/m2, RVESV 81 (18) ml/m2, RVEF 37 (9)%, RV mass 79 (10) g, and TAD 23 (7) mm/m2 (see methods for normal values). At six months after closure RVEDV was significantly reduced to 103 (20) ml/m2 and RVESV to 57 (14) ml/m2 (both p < 0.001) (figs 2 and 3), RVEF was significantly increased by 8%. RV mass and TAD changed slightly but not significantly compared with baseline MRI measurements. Follow up after 12 months assessed a further reduction of RVEDV to 99 (18) ml/m2 and of RVESV to 53 (15) ml/m2 (p < 0.001) (fig 3), an improvement of RVEF by 9% compared with baseline measurements (p < 0.05), a significant reduction of RV mass to 63 (8) g (p < 0.01), and a slight decrease of TAD (21 (6) mm/m2, not significant; fig 4).
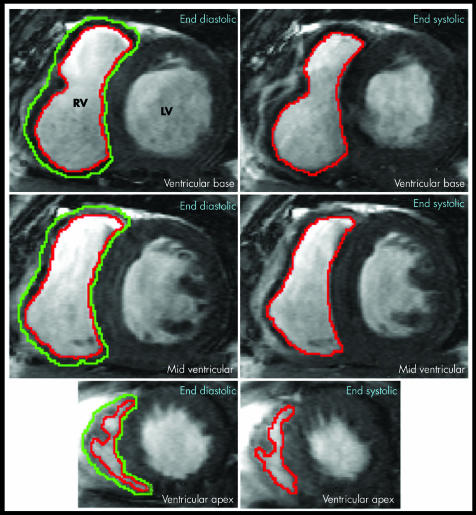
Figure 2 Short axis apical end diastolic (left) and end systolic (right) images of both ventricles from the base to the apex in a patient with ASD and right ventricular (RV) dilatation. The epicardial (green) and endocardial (red) boundaries of the RV are traced for calculation of RV end diastolic volume (RVEDV), RV end systolic volume (RVESV), and RV mass. LV, left ventricle.
Figure 3 Changes in RVEDV (left) and RVESV (right) after transcatheter closure of an ASD. *p < 0.001.
Figure 4 Changes in RV ejection fraction (RVEF), RV mass, and tricuspid annular diameter (TAD) after transcatheter closure of an ASD. *p < 0.05; **p < 0.01.
DISCUSSION
This is the first study to show changes of RV volumes, function, and mass by MRI after ASD closure. RV volumes and function improved significantly within six months after transcatheter ASD closure in adults. Volume was further reduced and RVEF was improved up to 12 months, although the major changes occurred within the first six months after closure. Significant regression of RV hypertrophy was observed at the 12 month follow up investigation. Pulmonary pressure decreased significantly over time and patients' conditions improved during follow up, leading to a better functional class in five of eight symptomatic patients. At baseline 65% of patients had a dilated TAD. During follow up after ASD closure we saw no significant changes in TAD. Dilated RVs seem to be reshaped quickly within six months and regression of RV mass was evident within 12 months. TAD, however did not follow the changes of the RV suggesting that fibrous cardiac tissue is less prone to remodelling. Secondary TR seemed to be resolved with regression of RV volume load. We hypothesise that regression of TR during follow up is the result of RV restoration and not the result of structural changes of the valve annulus. We believe this is the case because MRI measurements of TAD did not alter significantly after ASD closure.
Despite the observed RV volume reductions, 30% of patients were still outside the normal range for RV volumes after 12 months.
Before this study, data were conflicting as to whether the course after ASD closure leads to improved RV performance or only prevents further deterioration.7,9,11,12,13 After closure the diameter of the dilated RV seems to decrease, but objective measures of improved RV volumes and function had been lacking. Some authors described partial resolution up to normalisation of echocardiographically measured RV diameter within various intervals after closure.3,8,10 However, other authors reported that ASD closure is relatively less effective in resolving RV enlargement and dysfunction especially after surgical repair in children.9,12
These divergent results regarding RV volume and function after ASD closure may be caused by different closure and imaging techniques. Whereas surgical closure of ASDs seems to result in persisting RV dysfunction up to several years, percutaneous ASD closure seems to lead to a faster resolution of RV dilatation.3,7,9,11,12,13,27,28 This delay of RV recovery after surgical closure can be explained by the trauma of operation, the use of a cardioplegic solution, adverse inflammatory responses, and transiently impaired cardiac output that accompany cardiopulmonary bypass.29,30,31,32,33
Two studies have compared RV haemodynamic parameters after surgical closure versus percutaneous closure of ASDs. One study showed normalisation of RV volume and RVEF early after ASD closure, irrespective of whether this was achieved surgically or by transcatheter closure.8 However, Dhillon et al7 showed that RV function, both systolic and diastolic, is impaired by surgical closure but preserved after transcatheter device closure. These findings may support the percutaneous approach to closure of anatomically suitable ASDs.
Another reason for divergent results regarding RV performance after ASD closure is the limited accuracy of two dimensional echocardiography in quantifying RV parameters.34 RV volume and function are more difficult to estimate than are similar LV measurements mainly because the configuration of the RV is far more complex and not well approximated by simple geometric formulas.35 In the present study MRI as a non‐invasive imaging technique was chosen for the evaluation of RV function because of its potential to obtain RV geometry and function with good accuracy and reproducibility.16,36,37,38,39 To our knowledge, this is the first study investigating RV function, mass, and volume by MRI after transcatheter ASD closure to give detailed information about postinterventional RV changes. According to studies on the safety of MRI in patients with cardiovascular implants, these closure devices are not affected by MRI six months after implantation.40,41
Our data suggest that the abolition of left to right shunting after ASD closure decreases RV filling, thereby reducing pulmonary volume overload and pulmonary pressure and leading to a better functional class. Giardini et al42, who focused on LV haemodynamic function after ASD closure, postulated that LV filling would be augmented due to an abolished paradoxical movement of the interventricular septum; thus, increasing LV stroke volumes led to an improvement in peak oxygen pulse. Also Salehian et al14 reported that transcatheter closure of secundum‐type ASDs was associated with improved LV performance and RV function; in contrast, Eidem et al11 did not find improved RV function.
We cannot draw firm conclusions from this study on whether RV resolution is caused by passive changes in ventricular dynamics after shunt abolition or by cellular and molecular remodelling. However, the reduction in RV mass, indicated by the present data, suggests that the RV myocardial structural is actively altered. We speculate that after ASD closure both processes—active RV myocardial structural alteration and passive RV volume change by abolition of the interatrial shunt—contribute to the restoration of RV volume and the improvement of RVEF.
Various MRI imaging options and orientations for RV volume and mass measurements have been described in the literature. For example, Alfakih et al43 compared two orientations for RV volumetry. They found that datasets acquired in the axial orientation seem to have lower intraobserver and interobserver variability than the short axis orientation. To assess RV mass some authors recommend imaging planes perpendicular to the RV outflow tract and turbo inversion recovery imaging. However, compared with gradient echo images no significant difference between masses was found and repeatability of analysis was equally good with both methods.44 Optimal MRI visualisation and quantification of RV volumes, mass, and function have to be addressed.
Conclusion
MRI studies showed significant improvement of RV volumes, mass, and function after transcatheter closure of ASD. Restoration of the RV after closure leads to decreased pulmonary pressure resulting in a better functional class in the majority of patients.
Abbreviations
ASD - atrial septal defect
LV - left ventricular
MRI - magnetic resonance imaging
NYHA - New York Heart Association
RV - right ventricular
RVEDV - right ventricular end diastolic volume
RVEF - right ventricular ejection fraction
RVESV - right ventricular end systolic volume
RVSP - right ventricular systolic pressure
TAD - tricuspid annular diameter
TOE - transoesophageal echocardiography
TR - tricuspid regurgitation
TTE - transthoracic echocardiography
References
- 1.Moss A J, Adams F H.Heart disease in infants, children, and adolescents including the fetus and young. 5th ed. Baltimore: Williams & Wilkins, 199560–70, 689-701.
- 2.Graham T P., Jr Ventricular performance in congenital heart disease. Circulation 1991842259–2274. [DOI] [PubMed] [Google Scholar]
- 3.Brochu M C, Baril J F, Dore A.et al Improvement in exercise capacity in asymptomatic and mildly symptomatic adults after atrial septal defect percutaneous closure. Circulation 20021061821–1826. [DOI] [PubMed] [Google Scholar]
- 4.Pedra C A, Pedra S R, Esteves C A.et al Transcatheter closure of secundum atrial septal defects with complex anatomy. J Invasive Cardiol 200416117–122. [PubMed] [Google Scholar]
- 5.Fischer G, Stieh J, Uebing A.et al Experience with transcatheter closure of secundum atrial septal defects using the Amplatzer septal occluder: a single centre study in 236 consecutive patients. Heart 200389199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruch L, Parsi A, Grad M O.et al Transcatheter closure of interatrial communications for secondary prevention of paradoxical embolism: single‐center experience. Circulation 20021052845–2848. [DOI] [PubMed] [Google Scholar]
- 7.Dhillon R, Josen M, Henein M.et al Transcatheter closure of atrial septal defect preserves right ventricular function. Heart 200287461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger F, Jin Z, Ishihashi K. Comparison of acute effects on right ventricular haemodynamics of surgical versus interventional closure of atrial septal defects. Cardiol Young 19999484–487. [DOI] [PubMed] [Google Scholar]
- 9.Ning S B, Fazal H, Cook D. Right ventricular size and ventricular septal motion after repair of atrial septal defect in children. Can J Surg 198427395–398. [PubMed] [Google Scholar]
- 10.Thomson J D R, Aburawi E H, Watterson K G.et al Surgical and transcatheter (Amplatzer) closure of atrial septal defects: a prospective comparison of results and cost. Heart 200287466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eidem B W, O'Leary P W, Tei C.et al Usefulness of the myocardial performance index for assessing right ventricular function in congenital heart disease. Am J Cardiol 200086654–658. [DOI] [PubMed] [Google Scholar]
- 12.Meyer R A, Korfhagen J C, Covitz W.et al Long‐term follow‐up study after closure of secundum atrial septal defect in children: an echocardiographic study. Am J Cardiol 198250143–148. [DOI] [PubMed] [Google Scholar]
- 13.Veldtman G R, Razack V, Siu S.et al Right ventricular form and function after percutaneous atrial septal defect device closure. J Am Coll Cardiol 2001372108–2113. [DOI] [PubMed] [Google Scholar]
- 14.Salehian O, Horlick E, Schwerzmann M.et al Improvements in cardiac form and function after transcatheter closure of secundum atrial septal defects. J Am Coll Cardiol 200545499–504. [DOI] [PubMed] [Google Scholar]
- 15.Constantine G, Shan K, Flamm S D.et al Role of MRI in clinical cardiology [review]. Lancet 20043632162–2171. [DOI] [PubMed] [Google Scholar]
- 16.Grothues F, Moon J C, Bellenger N G.et al Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J 2004147218–223. [DOI] [PubMed] [Google Scholar]
- 17.Perloff J K.Physical examination of the heart and circulation, 3rd ed. Philadelphia: WB Saunders, 2000v–vi.
- 18.Drazner M H, Rame J E, Stevenson L W.et al Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. N Engl J Med 2001345574–581. [DOI] [PubMed] [Google Scholar]
- 19.Yock P G, Popp R L. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation 198470657–662. [DOI] [PubMed] [Google Scholar]
- 20.Katz J, Milliken M C, Stray‐Gundersen J.et al Estimation of human myocardial mass with MR imaging. Radiology 1988169495–498. [DOI] [PubMed] [Google Scholar]
- 21.Rominger M B, Bachmann G F, Pabst W.et al Right ventricular volumes and ejection fraction with fast cine MR imaging in breath‐hold technique: applicability, normal values from 52 volunteers, and evaluation of 325 adult cardiac patients. J Magn Reson Imaging 199910908–918. [DOI] [PubMed] [Google Scholar]
- 22.Drexler M, Erbel R, Muller U.et al Measurement of intracardiac dimensions and structures in normal young adult subjects by transesophageal echocardiography. Am J Cardiol 1990651491–1496. [DOI] [PubMed] [Google Scholar]
- 23.Alfakih K, Plein S, Thiele H.et al Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady‐state free precession imaging sequences. J Magn Reson Imaging 200317323–329. [DOI] [PubMed] [Google Scholar]
- 24.Scharhag J, Schneider G, Urhausen A.et al Athlete's heart: right and left ventricular mass and function in male endurance athletes and untrained individuals determined by magnetic resonance imaging. J Am Coll Cardiol 2002401856–1863. [DOI] [PubMed] [Google Scholar]
- 25.Thanopoulos B D, Laskari C V, Tsaousis G S.et al Closure of atrial septal defects with the Amplatzer occlusion device: preliminary results. J Am Coll Cardiol . 1998;311110–1116. [DOI] [PubMed]
- 26.Krumsdorf U, Keppeler P, Horvath K.et al Catheter closure of atrial septal defects and patent foramen ovale in patients with an atrial septal aneurysm using different devices. J Interv Cardiol 20011449–55. [DOI] [PubMed] [Google Scholar]
- 27.Helber U, Baumann R, Seboldt H.et al Atrial septal defect in adults: cardiopulmonary exercise capacity before and 4 months and 10 years after defect closure. J Am Coll Cardiol 1997291345–1350. [DOI] [PubMed] [Google Scholar]
- 28.Kort H W, Balzer D T, Johnson M C. Resolution of right heart enlargement after closure of secundum atrial septal defect with transcatheter technique. J Am Coll Cardiol 2001381528–1532. [DOI] [PubMed] [Google Scholar]
- 29.Ascione R, Lloyd C T, Underwood M J.et al Inflammatory response after coronary revascularization with or without cardiopulmonary bypass. Ann Thorac Surg 2000691198–1204. [DOI] [PubMed] [Google Scholar]
- 30.Tarnok A, Hambsch J, Emmrich F.et al Complement activation, cytokines, and adhesion molecules in children undergoing cardiac surgery with or without cardiopulmonary bypass. Pediatr Cardiol 199920113–125. [DOI] [PubMed] [Google Scholar]
- 31.Zahler S, Massoudy P, Hartl H.et al Acute cardiac inflammatory responses to postischemic reperfusion during cardiopulmonary bypass. Cardiovasc Res 199941722–730. [DOI] [PubMed] [Google Scholar]
- 32.Breisblatt W M, Stein K L, Wolfe C J.et al Acute myocardial dysfunction and recovery: a common occurrence after coronary bypass surgery. J Am Coll Cardiol 1990151261–1269. [DOI] [PubMed] [Google Scholar]
- 33.Chaturvedi R R, Lincoln C, Gothard J W.et al Left ventricular dysfunction after open repair of simple congenital heart defects in infants and children: quantitation with the use of a conductance catheter immediately after bypass. J Thorac Cardiovasc Surg 199811577–83. [DOI] [PubMed] [Google Scholar]
- 34.Papavassiliou D P, Parks W J, Hopkins K L.et al Three‐dimensional echocardiographic measurement of right ventricular volume in children with congenital heart disease validated by magnetic resonance imaging. J Am Soc Echocardiogr 199811770–777. [DOI] [PubMed] [Google Scholar]
- 35.Marzullo P, L 'Abbate A, Marcus M L. Patterns of global and regional systolic and diastolic function in the normal right ventricle assessed by ultrafast computed tomography. J Am Coll Cardiol 1991171318–1325. [DOI] [PubMed] [Google Scholar]
- 36.European Society of Cardiology, Association of European Paediatric Cardiologists. The clinical role of magnetic resonance in cardiovascular disease. Task Force of the European Society of Cardiology, in collaboration with the Association of European Paediatric Cardiologists. Eur Heart J 19981919–39. [PubMed] [Google Scholar]
- 37.Rebergen S A, Niezen R A, Helbing W A.et al Cine gradient‐echo MR imaging and MR velocity mapping in the evaluation of congenital heart disease. Radiographics 199616467–481. [DOI] [PubMed] [Google Scholar]
- 38.Helbing W A, Rebergen S A, Maliepaard C.et al Quantification of right ventricular function with magnetic resonance imaging in children with normal hearts and with congenital heart disease. Am Heart J 1995130828–837. [DOI] [PubMed] [Google Scholar]
- 39.Lorenz C H, Walker E S, Graham T P., Jret al Right ventricular performance and mass by use of cine MRI late after atrial repair of transposition of the great arteries. Circulation 199592II233–II239. [DOI] [PubMed] [Google Scholar]
- 40.Prasad S K, Pennell D J. Safety of cardiovascular magnetic resonance in patients with cardiovascular implants and devices. Heart 2004901241–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shellock F G. Magnetic resonance safety update 2002: implants and devices [review]. J Magn Reson Imaging 200216485–496. [DOI] [PubMed] [Google Scholar]
- 42.Giardini A, Donti A, Formigari R.et al Determinants of cardiopulmonary functional improvement after transcatheter atrial septal defect closure in asymptomatic adults. J Am Coll Cardiol 2004431886–1891. [DOI] [PubMed] [Google Scholar]
- 43.Alfakih K, Plein S, Bloomer T.et al Comparison of right ventricular volume measurements between axial and short axis orientation using steady‐state free precession magnetic resonance imaging. J Magn Reson Imaging 20031825–32. [DOI] [PubMed] [Google Scholar]
- 44.Jauhiainen T, Jarvinen V M, Hekali P E. Evaluation of methods for MR imaging of human right ventricular heart volumes and mass. Acta Radiol 200243587–592. [DOI] [PubMed] [Google Scholar]