Abstract
Objective
To determine the safety and effectiveness of cutting balloon angioplasty for pulmonary vein stenosis (PVS).
Design and setting
Retrospective review of case notes and cardiac catheterisation data at the Royal Brompton Hospital.
Main outcome measures
Diameter of pulmonary vein, tricuspid regurgitant jet velocity on echocardiogram, and percutaneous oxygen saturation before and after cutting balloon angioplasty.
Results
Three patients had congenital PVS and three had PVS associated with total anomalous pulmonary venous drainage. A total of 27 PVSs were treated during 12 catheterisation procedures. Median patient age at the time of procedure was 12.5 months (range 1.5–36 months) and weight was 7.1 kg (range 2.8–11.1 kg). Minimum pulmonary vein diameter increased significantly on angiography after cutting balloon angioplasty, from mean (SD) 2.3 (0.7) mm to 4.2 (1.9) mm, mean of differences 1.9 mm (95% confidence interval (CI) 0.9 to 2.9 mm, p = 0.0013). Mean (SD) oxygen saturation rose from 79.6 (12.9)% to 83.9 (9.0)%, mean of differences 4.3% (95% CI 0.7% to 8.0%, p = 0.0238). All children's symptoms improved subjectively. Tricuspid regurgitant jet velocity did not change significantly. The longest time interval before repeat intervention was six months. There were no acute deaths; one patient had a small pulmonary haemorrhage and developed a small aneurysm adjacent to the site of angioplasty.
Conclusion
Cutting balloon angioplasty is safe in the palliation of PVS in children. It gives some acute relief but often needs to be repeated, as improvement is rarely sustained.
Keywords: pulmonary vein stenosis, cutting balloon
Pulmonary vein stenosis (PVS) can be congenital or acquired after surgical repair of a total or partial anomalous pulmonary venous connection.1 Congenital forms may be isolated or associated with other forms of congenital heart disease.2,3 In both the congenital and acquired forms of PVS, histological findings show variable manifestation of neointimal proliferation leading to occlusion of the lumen of one or more of the pulmonary veins.3,4,5,6 Three patterns of PVS have been described: bilateral tubular hypoplasia extending from the venoatrial junction for a variable length, discrete hourglass constriction at the venoatrial junctions, and bilateral multiple short pulmonary veins that are hypoplastic for their entire extrapulmonary course.7 The prognosis is often poor with the development of progressive pulmonary venous congestion followed by pulmonary arterial hypertension and eventual death. Even with early treatment the overall results remain disappointing. Conventional balloon angioplasty, stent implantation, and surgery have all been attempted with limited success.8,9,10,11 More recently a novel sutureless technique has been introduced for surgical management of postoperative PVS occurring after the repair of total anomalous pulmonary venous drainage (TAPVC).12,13 This has also been used for primary repair of pulmonary vein abnormalities; mid term results of this technique are only just becoming available.14 The optimal treatment for PVS has yet to be established.
Cutting balloons have previously been used to treat stenosis of renal dialysis arteriovenous fistulae, in‐stent stenosis of coronary arteries, and more recently peripheral pulmonary artery stenosis.15,16,17,18,19 Histological analysis of in‐stent stenosis of the coronary arteries shows some similarities to PVS with luminal encroachment by neointimal hyperplasia.20
The objective of this study was to determine the safety and effectiveness of cutting balloon angioplasty in the treatment of both congenital and acquired PVS.
PATIENTS AND METHODS
We reviewed all cases of PVS at the Royal Brompton Hospital where cutting balloons have been used for treatment. Clinical, echocardiographic, and cardiac catheterisation data were analysed before and after the procedure. The procedure was approved by the local research ethics advisors.
Patients
Six patients were treated with cutting balloon angioplasty between November 2001 and April 2005. Three of these patients had isolated congenital PVS and three had PVS, which became evident after repair of TAPVC. Table 1 shows details of the patients' presentations and lesions.
Table 1 Presentation symptoms and diagnosis.
| Patient no | Sex | Birth weight (kg) | Age at presentation with PVS (months) | Presenting symptoms | Associated cardiac defects | Interventions before cutting balloon angioplasty |
|---|---|---|---|---|---|---|
| 1 | M | 3.23 | 5 | Dyspnoea, cyanosis, failure to thrive | Right atrial isomerism, biventricular atrioventricular connection, DORV with hypoplastic left ventricle, TAPVC to coronary sinus, subpulmonary stenosis | 1. Surgical repair of TAPVC; 2. central shunt; 3. right modified Blalock‐Taussig shunt; 4. surgical relief of PVS; 5. conventional balloon dilatation of PVS (twice) |
| 2 | M | 1.88 | 24 | Dyspnoea | Isolated PVS | None |
| 3 | M | 3.2 | 1 | Dyspnoea, cyanosis, failure to thrive | Obstructed supracardiac TAPVC to innominate vein | 1. Surgical repair of TAPVC |
| 4 | M | 2.3 | 1.5 | Cyanosis | Isolated PVS | None |
| 5 | M | 0.656 | 12 | Dyspnoea | Isolated PVS | None |
| 6 | M | 3.15 | 2 | Dyspnoea, cyanosis | Obstructed supracardiac TAPVC to azygous vein | 1. Surgical repair of TAPVC; 2. conventional balloon dilatation of pulmonary veins |
DORV, double outlet right ventricle; PVS, pulmonary vein stenosis; TAPVC, total anomalous pulmonary venous drainage.
Catheterisation procedure
Cardiac catheterisation was performed under general anaesthesia with percutaneous access through the femoral vein. The left atrium was entered by crossing an atrial septal defect or persistent foramen ovale. When the interatrial septum was intact, the left atrium was entered after transseptal puncture (n = 1). Either a Judkins right coronary or a multipurpose catheter was used to cannulate individual pulmonary veins and manually inject contrast. Where possible the pressure difference across the stenosis was measured. A 4.0 mm × 10 mm cutting balloon (Boston Scientific) was introduced over a 0.014 inch Support Hi Torque floppy guidewire for stability and through a 6 French guiding catheter to protect both the balloon and the cardiac structures. The guiding catheter was placed across the pulmonary vein and the balloon was positioned across the area of stenosis. The guiding catheter was then withdrawn to expose the balloon and, after de‐airing, the balloon was inflated several times. A manometer was used to measure inflation pressure to ensure the recommended burst pressure of 8 atm was not exceeded. In the majority of cases (n = 20) the pulmonary vein was then further dilated with a conventional angioplasty balloon, as no cutting balloon with a diameter > 4 mm was available. A final angiogram was recorded to assess the response to treatment and any evidence of vessel injury.
Vessel diameter before and after angioplasty was measured off line, by using the catheter diameter to correct for magnification. Haemodynamic variables were also assessed before and after balloon dilatation.
Statistical analysis
Patient and procedural characteristics were described as mean (SD) or median (range) as appropriate. Paired Student's t test was used to assess differences in diameter before and after the intervention. Significance was defined as p < 0.05.
RESULTS
There were 12 procedures with a total of 27 episodes of pulmonary vein angioplasty with the cutting balloon. Immediately after 20 of the 27 cutting balloon episodes, the pulmonary vein was further dilated by conventional balloon angioplasty (5–8 mm diameter). In all veins undergoing angioplasty there was a discrete stenosis at the venoatrial junction.
One patient required a needle puncture of the atrial septum to allow access to the left atrium. In this patient the cutting balloon was also used to enlarge the atrial communication.
The median patient age at the time of the procedure was 12.5 months (range 1.5–36 months) and weight was 7.1 kg (range 2.8–11.1 kg). The procedures were prolonged with a median procedure time of 125.5 minutes (range 75–217 minutes) and a median screening time of 49.8 minutes (range 15.2–103.2 minutes). Procedures were performed with a high fractional inspired oxygen, as the patients had pulmonary hypertension and were considered an anaesthetic risk.
On 10 occasions it was possible to record the pressure gradient before and after angioplasty (table 2). The mean pressure difference across the stenosis fell on average from 19.5 (5.0) mm Hg to 8.5 (2.9) mm Hg, mean of the difference being 11 mm Hg (95% confidence interval 7.5 to 14.5 mm Hg, p < 0.0001).
Table 2 Details of interventions.
| Patient number | Procedure number | Patient age (months) | Weight (kg) | Pulmonary veins dilated (vein no in fig 1) | Increase in vein diameter (%) | Mean pressure gradient across stenosis (mm Hg) | Transcutaneous oxygen saturation* (%) | Procedure and screening times (min) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | |||||||
| 1 | 1 | 11 | 7.1 | RUPV (1) | 46 | NM | NM | 65 | 73 | 135; 52.9 |
| RLPV (2) | 19 | NM | NM | |||||||
| LUPV (3) | 120 | NM | NM | |||||||
| LLPV | NM | NM | NM | |||||||
| Anastomosis† | NM | NM | NM | |||||||
| 2 | 14 | 7.3 | RUPV | NM | 23 | 15 | 60 | 68 | 97; 37.6 | |
| RLPV | NM | NM | NM | |||||||
| LUPV (4) | 41 | NM | NM | |||||||
| 3 | 16 | 8.4 | RUPV | NM | NM | NM | 65 | 75 | 142; 58.8 | |
| RMPV | NM | NM | NM | |||||||
| RPV origin | NM | NM | NM | |||||||
| RLPV | NM | 20 | 11 | |||||||
| LUPV | NM | 21 | 9 | |||||||
| 2 | 4 | 36 | 11.1 | RUPV (5) | 267 | 25 | 5 | 90 | 85 | 217; 103.2 |
| RLPV | NM | NM | NM | |||||||
| 3 | 5 | 4 | 4.5 | LUPV (6) | 190 | 17 | 7 | 85 | 92 | 94; 29.5 |
| 6 | 22 | 9.2 | RUPV (7) | 63 | 8 | 5 | 95 | 95 | 75; 15.2 | |
| 4 | 7 | 1.5 | 2.8 | LLPV (8) | 100 | NM | NM | 70 | 75 | 165; 56.4 |
| 5 | 8 | 12 | 5.8 | RUPV (11) | 82 | NM | NM | 86 | 90 | 203; 71.7 |
| LUPV (10) | 138 | NM | NM | |||||||
| LLPV (9) | 121 | NM | NM | |||||||
| 9 | 13 | 6.3 | RUPV (13) | −3.1 | 19 | 8 | 87 | 90 | 124; 47.4 | |
| LUPV (12) | 28 | 22 | 8 | |||||||
| LLPV | NM | 24 | 8 | |||||||
| 12 | 20 | 7.4 | LLPV (14) | 45 | 16 | 9 | 88 | 85 | 127; 52.2 | |
| 6 | 10 | 3 | – | Anastomosis† | NM | NM | NM | 94 | 94 | 88; 35.0 |
| 11 | 4.5 | 4 | RLPV | NM | NM | NM | 70 | 85 | 98; 40.7 | |
*Oxygen saturations were recorded where possible in air but if these data were not available, pre‐ and post‐procedural oxygen saturation was taken with the patient receiving the same quantity of oxygen (like compared with like); †surgical anastomosis between left atrium and pulmonary venous confluence in TAPVC repair.
LLPV, left lower pulmonary vein; LUPV, left upper pulmonary vein; NM, not measured; RLPV, right lower pulmonary vein; RMPV, right middle pulmonary vein; RPV, right pulmonary vein; RUPV, right upper pulmonary vein.
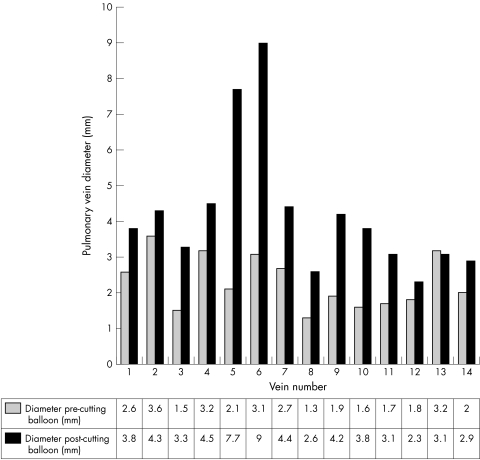
Suitable angiographic images to measure pulmonary vein diameter before and after angioplasty were available for only 14 pulmonary vein interventions, as there was an unacceptable degree of vessel overlap in the remainder. Where this was measured, minimum pulmonary vein diameter increased significantly after cutting balloon angioplasty, from 2.3 (0.7) mm to 4.2 (1.9) mm, mean of differences being 1.9 mm (95% confidence interval 0.9 to 2.9 mm, p = 0.0013). In 10 of these 14 interventions, further angioplasty with a conventional balloon was performed immediately after the cutting balloon procedure (figs 1 and 2).
Figure 1 Bar chart showing pulmonary vein diameter before and after cutting balloon angioplasty.
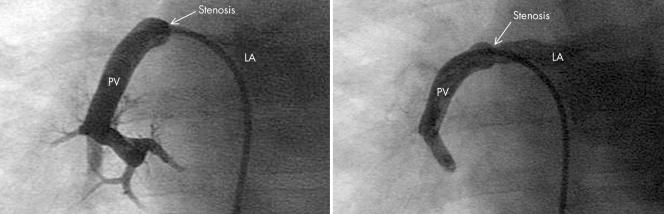
Figure 2 Angiogram of the right lower pulmonary vein (PV) in anteroposterior projection before (left) and after (right) cutting balloon angioplasty of a discrete stenosis at the junction between the left atrium (LA) and PV. Note the hypoplastic smaller peripheral vessels.
Other measurements of outcome
Echocardiography showed no significant difference in the velocity of the tricuspid valve regurgitation jet before and after the procedure.
Transcutaneous oxygen saturations differed significantly before and after intervention. Mean (SD) oxygen saturation rose from 79.6 (12.9)% to 83.9 (9.0)%, mean of differences being 4.3% (95% confidence interval 0.7% to 8.0%, p = 0.0238). Similarly, in virtually all the cases there appeared to be an important symptomatic improvement in the child's breathing pattern, which became more comfortable after the cutting balloon procedure.
Adverse events
One patient had a small self limiting pulmonary haemorrhage, which was diagnosed when the anaesthetist aspirated blood stained secretions from the endotracheal tube during the procedure. The same patient also developed a tiny aneurysm near the origin of the left pulmonary vein. Another patient had a severe bradycardia during injection of contrast into the pulmonary arteries but remained stable throughout the cutting balloon dilatation. There were no deaths related to the angioplasty.
Patient 4 developed focal seizures while on the intensive care unit 48 hours after angioplasty. This patient was known to have cutis marmorata telangiectasia congenita, which is associated with seizures. The baby did not have cranial imaging. He died of progressive pulmonary venous obstruction. No postmortem examination was performed making it impossible to determine whether the seizures were secondary to embolic phenomena during the procedure or due to his underlying syndrome.
Follow up
Table 3 shows the outcome after cutting balloon angioplasty. Three patients died of progressive PVS; three are still alive. Two of these patients have had significant improvement of symptoms now nine and three months from the last procedure. However, they both still have evidence of significant pulmonary hypertension. The third patient is deteriorating despite further surgical treatment (table 3).
Table 3 Outcome after cutting balloon angioplasty of PVS.
| Patient number | Procedure number | Details of next intervention | Final outcome |
|---|---|---|---|
| 1 | 1 | 1 month (Boston protocol chemotherapy) | Died aged 19 months |
| 2 | 2 months (cutting balloon angioplasty) | ||
| 3 | None | ||
| 2 | 4 | None | Died aged 38 months |
| 3 | 5 | 6 months (conventional balloon dilatation) | Alive aged 31 months |
| 6 | None | ||
| 4 | 7 | None | Died aged 3.5 months |
| 5 | 8 | 1 month (cutting balloon angioplasty) | Alive aged 23 months |
| 9 | 6 months (cutting balloon angioplasty) | ||
| 12 | 1 week (conventional balloon angioplasty and attempted stenting of pulmonary vein with drug eluting stent) | ||
| 6 | 10 | 6 weeks (cutting balloon angioplasty) | Alive aged 13 months |
| 11 | 1 month (surgical relief of pulmonary venous obstruction) |
DISCUSSION
To our knowledge, this is the first report of the use of cutting balloon angioplasty to treat patients with PVS. The technique appears to be most effective at reducing symptoms of pulmonary venous congestion; however, there seems to be little effect on the right ventricular pressure. The procedure can be performed safely with minimal complications and is effective where there is discrete PVS at the junction with the left atrium.
It has previously been shown that the results of conventional balloon angioplasty for PVS are poor.8,9 It is possible that conventional balloon dilatation fails because the area of stenosis is transiently stretched during the procedure but the intima is not ruptured—an effect that may be necessary for balloon dilatation to be successful.9 Cutting balloons are angioplasty balloons with three to four metal blades (0.1–0.4 mm thickness) mounted longitudinally on their surface. One would expect intimal rupture after balloon dilatation with such balloons.
Cutting balloons had previously been used with some success in adult patients to treat in‐stent stenosis of coronary arteries. Some studies have shown this technique to be more successful than conventional balloon angioplasty, although more recent studies have suggested there to be no difference between the two groups.16,21 In‐stent stenosis of coronary arteries is a disease that is also caused by neointimal hyperplasia. It was this relative success in the adult population as well as the theoretical benefits of using a cutting balloon that led us to consider using this technique on our paediatric patients with PVS.
The use of cutting balloons in the paediatric population is relatively rare. There have been isolated reports of successful use of cutting balloons to treat peripheral pulmonary artery stenosis and stenosed aortopulmonary collateral vessels and to allow enlargement of interatrial communications.17,18,19,22,23 Indeed we used a cutting balloon to enlarge the atrial communication made by needle septal puncture in patient 5 to gain access to the left atrium before cutting balloon angioplasty of the stenotic pulmonary veins.
This was a retrospective study and therefore the quantity of data available for analysis was limited. The emphasis during the procedure was to perform the intervention as quickly and effectively as possible. Patients were pulmonary hypertensive and inherently unstable under anaesthesia. As a result, little time was spent acquiring haemodynamic data and in most cases a right ventricular pressure to systemic pressure ratio was not determined before or after the cutting balloon intervention. Despite this, procedure times remained long, often exceeding two hours. Owing to the instability of the patients, the pressure measurements that were made should be treated with caution. In addition pulmonary venous pressures are unreliable when the stenosis is occluded by the catheter. An alternative way to measure effect would be with pulmonary venous wedge pressures. However, it is difficult to justify the time taken to acquire multiple pulmonary artery wedge pressures before and after balloon dilatation in such a group of unstable patients.
PVS is a progressive illness and, although there was evidence that our patients did have some reduction in the pulmonary venous obstruction, this effect was only temporary. With the exception of one patient (patient 3), who had his cutting balloon intervention nine months ago, the longest time to the next intervention was six months. Most of the patients had serial cardiac catheterisations; the progressive nature of the disease was shown by initial discrete stenoses becoming diffuse with venous hypoplasia over time. In all our patients, cutting balloon angioplasty was performed on veins with discrete hourglass constriction at the venoatrial junction. Although we have shown that this discrete narrowing was relieved, it is possible that the persistent pulmonary hypertension was secondary to remaining diffuse hypoplasia of the smaller intraparenchymal vessels. This was illustrated in some cases where retrograde injections of contrast into the pulmonary veins suggested a mesh of small vessels.
In patient 6, the temporary relief of the stenosis proved to be very helpful. The patient was due to have his pulmonary venous obstruction surgically relieved when he contracted respiratory syncytial virus positive bronchiolitis. The combination of pulmonary venous obstruction and bronchiolitis made him profoundly unwell, ventilator dependent, and requiring support with nitric oxide and inotropes. We performed cutting balloon angioplasty to give temporary relief to his pulmonary venous obstruction; he went on to recover from the bronchiolitis and have bypass surgery at a later date.
Although cutting balloon angioplasty can be used to palliate patients with PVS, it does not appear to halt the disease process. Indeed, no known current intervention can. Pathological studies have shown that the pulmonary venous obstruction is due to progressive neointimal proliferation. Previous studies have shown that cutting balloon angioplasty can minimise traumatic triggers of inflammatory cell and neointimal proliferation; however, the cutting balloon technique alone does not stop the neointimal proliferation from occurring.24,25
As discussed previously, neointimal proliferation is also responsible for in‐stent stenosis after coronary angioplasty. Exciting advances have recently been made in the treatment of adult patients with coronary heart disease. Studies have shown that suppression of neointimal proliferation is sustained by sirolimus eluting stents used in these adult patients.26,27 Sirolimus is a natural macrocyclic lactone with potent immunosuppressive action. It acts specifically on the late G1 phase of the cell cycle. Sirolimus may block cellular proliferation without inducing cell death and necrosis. In addition, sirolimus has been shown to stimulate apoptosis and reduce inflammation.
The future treatment of PVS possibly will also involve treatment with such sirolimus eluting stents. One can envisage a combination of cutting balloon angioplasty followed by stenting of the pulmonary vein with sirolimus eluting stents in an attempt not only to palliate but also to try to stop the progressive nature of this disease by preventing neointimal proliferation.
Our study has some limitations. It is retrospective and describes experience with only a small number of patients and procedures. In addition we were unable to obtain all measurements in all cases. PVS is a rare disease, thereby making it difficult to gain a large cohort of patients from a single institution.
Conclusion
We have described the use of cutting balloons to treat PVS. While the procedure appears to give acute relief to the stenosis and some clinical improvement, rarely is this sustained.
In summary, this technique can be used safely to palliate children with PVS. A combined approach of using cutting balloons followed by insertion of sirolimus eluting stents may, in the future, provide the best treatment for this devastating disease.
ACKNOWLEDGEMENTS
Dr Anna Seale is a research fellow at the Royal Brompton Hospital supported by grants from the Harrison Heart Foundation and the Joe Gandon Memorial Trust.
Abbreviations
PVS - pulmonary vein stenosis
TAPVC - total anomalous pulmonary venous drainage
Footnotes
Competing interests: none declared
References
- 1.Van Son J A M, Danielson G K, Puga F J.et al Repair of congenital and acquired pulmonary vein stenosis. Ann Thorac Surg 199560144–150. [DOI] [PubMed] [Google Scholar]
- 2.Breinholt J P, Hawkins J A, Minich L.et al Pulmonary vein stenosis with normal connection: associated cardiac abnormalities and variable outcome. Ann Thorac Surgery 199968164–168. [DOI] [PubMed] [Google Scholar]
- 3.Bini R M, Cleveland D C, Ceballos R.et al Congenital pulmonary vein stenosis. Am J Cardiol 198454369–375. [DOI] [PubMed] [Google Scholar]
- 4.Howarth S G. Total anomalous pulmonary venous return: prenatal damage to pulmonary vascular bed and extrapulmonary veins. Br Heart J 198248513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shone J D, Amplatz K, Anderson R C.et al Congenital stenosis of individual pulmonary veins. Circulation 196226574–581. [DOI] [PubMed] [Google Scholar]
- 6.Edwards J. Congenital stenosis of pulmonary veins. Lab Invest 1960946–66. [PubMed] [Google Scholar]
- 7.Fong L V, Anderson R H, Park S C.et al Morphologic features of stenosis of the pulmonary veins. Am J Cardiol 1988621136–1138. [DOI] [PubMed] [Google Scholar]
- 8.Driscoll D, Hesslein P, Mullins C. Congenital stenosis of individual pulmonary veins: clinical spectrum and unsuccessful treatment by transvenous balloon dilation. Am J Cardiol 1982491767–1772. [DOI] [PubMed] [Google Scholar]
- 9.Lock J E, Bass J L, Castaneda‐Zuniga W.et al Dilation angioplasty of congenital or operative narrowings of venous channels. Circulation 198470457–464. [DOI] [PubMed] [Google Scholar]
- 10.Coles J G, Yemets I, Najm H K, et a l. Experience with repair of congenital heart defects using endovascular devices. J Thorac Cardiovasc 19951101513–1520. [DOI] [PubMed] [Google Scholar]
- 11.Cullen S, Ho S Y, Shore D.et al Congenital stenosis of pulmonary veins: failure to modify natural history by intraoperative placements of stents. Cardiol Young 19944395–398. [Google Scholar]
- 12.Lacour‐Gayet F, Zoghbi J, Serraf A E.et al Surgical management of progressive pulmonary venous obstruction after repair of total anomalous pulmonary venous connection. J Thorac Cardiovasc Surg 1999117679–687. [DOI] [PubMed] [Google Scholar]
- 13.Najm H K, Caldarone C A, Smallhorn J.et al A sutureless technique for the relief of pulmonary vein stenosis with the use of in situ pericardium. J Thorac Cardiovasc Surg 1998115468–470. [DOI] [PubMed] [Google Scholar]
- 14.Yun T, Coles J G, Konstantinov I E.et al Conventional and sutureless techniques for management of the pulmonary veins: evolution of indications from postrepair pulmonary vein stenosis to primary pulmonary vein anomalies. J Thorac Cardiovasc Surg 2005129167–174. [DOI] [PubMed] [Google Scholar]
- 15.Vorwerk D, Gunther R W, Schurmann K.et al Use of a cutting balloon for resistant venous stenosis of a haemodynamic fistula. Cardiovasc Intervent Radiol 19951862–64. [DOI] [PubMed] [Google Scholar]
- 16.Muramatsu T, Tsukahara R, Ho M.et al Efficacy of cutting balloon angioplasty for in‐stent restenosis: an intravascular ultrasound evaluation. J Invasive Cardiol 200113439–444. [PubMed] [Google Scholar]
- 17.Schneider M B E, Zartner P A, Magee A G. Images in cardiology: cutting balloon for treatment of severe peripheral pulmonary stenoses in a child. Heart 199982108. [PMC free article] [PubMed] [Google Scholar]
- 18.Bergersen L J, Perry S B, Lock J E. Effect of cutting balloon angioplasty on resistant pulmonary artery stenosis. Am J Cardiol 200391185–189. [DOI] [PubMed] [Google Scholar]
- 19.Sugiyama H, Veldtman G R, Norgard G.et al Bladed balloon angioplasty for peripheral pulmonary artery stenosis. Catheter Cardiovasc Interv 20046271–77. [DOI] [PubMed] [Google Scholar]
- 20.Gordon P C, Gibson M, Cohen D J.et al Mechanisms of restenosis and redilation within coronary stents: quantitative angiographic assessment. J Am Coll Cardiol 1993211166–1174. [DOI] [PubMed] [Google Scholar]
- 21.Albiero R, Silber S, Di Mario C, et a l. Cutting balloon versus conventional balloon angioplasty for the treatment of in‐stent restenosis: results of the restenosis cutting balloon evaluation trial (RESCUT). J Am Coll Cardiol 200443943–949. [DOI] [PubMed] [Google Scholar]
- 22.Mertens L, Dens J, Gewillig M. Use of a cutting balloon catheter to dilate resistant stenoses in major aortic‐to‐pulmonary collateral arteries. Cardiol Young 200111574–577. [DOI] [PubMed] [Google Scholar]
- 23.Schneider M B E, Zartner P A, Magee A G. Transseptal approach in children after patch occlusion of atrial septal defect: first experience with the cutting balloon. Catheter Cardiovasc Interv 199948378–381. [DOI] [PubMed] [Google Scholar]
- 24.Inoue T, Sakai Y, Hoshi K.et al Lower expression of neutrophil adhesion molecule indicates less vessel wall injury and might explain lower restenosis rate after cutting balloon angioplasty. Circulation 1998972511–2518. [DOI] [PubMed] [Google Scholar]
- 25.Barath P, Fishbein M C, Vari S.et al Cutting balloon: a novel approach to percutaneous angioplasty. Am J Cardiol 1991681249–1252. [DOI] [PubMed] [Google Scholar]
- 26.Sousa J E, Costa M A, Abizaid A C.et al Sustained suppression of neointimal proliferation by sirolimus‐eluting stents: one‐year angiographic and intravascular ultrasound follow‐up. Circulation 20011042007–2011. [DOI] [PubMed] [Google Scholar]
- 27.Degertekin M, Serruys P W, Foley D P.et al Persistent inhibition of neointimal hyperplasia after sirolimus‐eluting stent implantation: long‐term (up to 2 years) clinical, angiographic, and intravascular ultrasound follow‐up. Circulation 20021061610–1613. [DOI] [PubMed] [Google Scholar]