Abstract
Objectives
To estimate the frequency and nature of complications in patients undergoing diagnostic cardiac catheterisation and to assess time trends in complications since the introduction of a voluntary cooperative audit.
Methods
Cardiac centres undertaking diagnostic cardiac catheterisation in England and Wales during the 10 years 1990–9 were invited to join the study. Each participating centre reported numbers of patients catheterised each month and details of complications and deaths as they occurred. Complication rates were calculated for the main diagnostic procedures and for each participating hospital and time trends in complications were examined.
Results
41 cardiac centres contributed. 211 645 diagnostic procedures in adults and 7582 paediatric procedures were registered. The majority (87%) of diagnostic catheter studies in adults were left heart studies with coronary arteriography. The overall complication rate for adult procedures was 7.4/1000, with mortality at 0.7/1000; for paediatric procedures the complication rate was similar but mortality rather higher. Complication rates varied between centres but there was little association with caseload. Time trends across the decade showed both complication and mortality decreasing; from 9.5 to 5.8/1000 and from 1.4 to 0.4/1000, respectively.
Conclusion
Complication rates of diagnostic catheterisation are low but neither negligible nor irreducible. While voluntary audit of cardiac catheter complications is useful and inexpensive, there is a clear need to establish a formal reporting system in all cardiac catheter laboratories, with clear definitions of reportable complications.
Keywords: complications, cardiac catheterisation, medical audit
Diagnostic cardiac catheterisation is an integral part of cardiological practice. Coronary angiography remains invaluable in the assessment of patients with ischaemic heart disease and is essential in mapping anatomy preparatory to revascularisation. Cardiac catheterisation, which involves inserting catheters into the heart or great vessels, is not entirely free from risk of complications and, in some patients, death. Risk associated with technique or operator can be reduced by audit, the continuing identification and analysis of untoward events with feedback to practitioners.1,2 In some countries, there is a statutory requirement for catheter laboratory audit.3 In the UK audit of cardiac surgery and coronary angioplasty is established on a voluntary basis and, following formation of the Joint Audit Committee of the British Cardiac Society and the Royal College of Physicians in 1989, one of its first projects was a voluntary audit of diagnostic cardiac catheterisation.4,5,6 This project, the confidential inquiry into cardiac catheter complications, was led by the late David de Bono.7 The present report summarises the findings over the 10 years 1990–9 inclusive.
METHODS
An open invitation to participate was issued through the British Cardiac Society newsletter and by writing to centres known to be performing diagnostic angiography. Centres were asked to identify a link person, usually a catheter laboratory nurse or manager, to be responsible for local data collection. Each month the link person sent a single page form to the coordinating centre, listing the number of procedures performed, categorised by type of procedure, and reported complications when they occurred, also on a single page form. The complication report included a description of the complication, date, type of procedure, and status of the operator. The identity of the patient was coded to enable centres to identify their own patients and prevent double entry. Follow up reports were sent if a late complication or death occurred.
A complication was defined as any untoward event that jeopardised the patient's life or prolonged the planned hospital stay. There was no specific time limit but there had to be a definite or probable link between the catheter procedure itself and the complication. Thus, the death of a patient while awaiting surgery for an acute post‐infarct ventricular septal defect would not be regarded as a catheter complication, even though it occurred shortly after the catheter study, whereas late death from renal failure resulting from cholesterol embolism at the time of catheterisation would be counted, even if separated from the study by some days or weeks. Several examples were provided in the initial information pack sent to centres.
All reports were scrutinised by one observer (David de Bono until 1998, NB from 1998) to ensure compliance with the guidelines before being entered on the database. Complications were classified into groups according to their primary manifestation: arrhythmias (ventricular fibrillation, ventricular tachycardia, asystole, and vasovagal), vascular access complication (including haematoma that required transfusion), ischaemia (where there was evidence based on symptoms or ECG changes of severe and persisting myocardial ischaemia as a complication of the catheter procedure), coronary dissection, haemodynamic collapse, cerebrovascular accident (including transient ischaemic attack), inter‐ or transmyocardial injection, and allergy. A few patients had complications that could fall into two or more groups, but in the present analysis all were ascribed to one principal complication. The voluntary reporting scheme closed at the end of 1999. Procedures, complications, and deaths were reviewed again (by GE) to confirm inclusion and classification under original guidelines. Returns from centres that registered fewer than 1000 patients have been pooled. Confidence intervals have been calculated, assuming Poisson distributions for complications and deaths.
RESULTS
Forty one centres participated (see appendix). Three further centres registered but logged no data. Monthly returns and notification of complications were not continuous in all participating hospitals when, for example, a nominated link person changed jobs. In this analysis sporadic reports have been omitted and only runs of three consecutive months or more included.
Between 1 August 1990 and 31 December 1999, the coordinating centre logged 219 227 procedures. Of these 211 645 were diagnostic catheterisations in adults and 7582 were paediatric procedures. Procedures involving coronary angioplasty or balloon dilatation of a valve are not included in the present report, as the British Cardiac Intervention Society analyses interventional complications.5 One thousand six hundred and fourteen complications and 164 procedure related deaths were registered.
Table 1 shows numbers of procedures in the 30 centres that registered over 1000 adult procedure, and in the pooled group of 10 centres that registered fewer than 1000 procedures each. Some monthly returns gave total procedure numbers, without distinguishing type of procedure: these were distributed pro rata according to the completed returns from the hospital. Left heart catheterisation with coronary angiography accounted for 87% of adult diagnostic procedures, and 95% of paediatric procedures were right and left heart without coronary angiography.
Table 1 Types of diagnostic catheter procedures in adults and complications and death rates by centre.
| Centre | Total no | LCA (%) | L no C (%) | RLCA (%) | RL no C (%) | R (%) | Complications rate/1000 (95% CI) | Mortality/1000 (95% CI) |
|---|---|---|---|---|---|---|---|---|
| 1 | 14081 | 86.2 | 3.1 | 9.5 | 0.6 | 0.7 | 8.4 (7.0 to 18.2) | 1.6 (1.0 to 2.4) |
| 2 | 21975 | 84.4 | 2.9 | 8.9 | 0.9 | 2.9 | 9.0 (7.8 to 10.4) | 0.7 (0.4 to 1.2) |
| 3 | 1174 | 82.7 | 1.9 | 10.7 | 0.6 | 4.2 | 8.5 (4.1 to 15.7) | 1.7 (0.2 to 6.1) |
| 4 | 3936 | 93.3 | 0.2 | 6.1 | 0.0 | 0.4 | 6.4 (4.1 to 9.4) | 0.3 (0.0 to 1.4) |
| 5 | 17474 | 92.4 | 0.2 | 6.0 | 0.2 | 1.3 | 6.2 (5.2 to 7.6) | 0.1 (0.0 to 0.4) |
| 6 | 2194 | 91.3 | 0.6 | 7.7 | 0.1 | 0.4 | 4.1 (1.9 to 7.8) | 0.0 (0.0 to 1.7) |
| 7 | 2381 | 84.2 | 1.6 | 14.2 | 0.0 | 0.0 | 11.8 (7.8 to 17.0) | 2.1 (0.7 to 4.9) |
| 10 | 5049 | 90.3 | 0.7 | 7.8 | 0.5 | 0.7 | 8.9 (6.5 to 11.9) | 1.8 (0.8 to 3.4) |
| 11 | 3840 | 90.2 | 0.7 | 8.2 | 0.1 | 0.9 | 6.8 (4.4 to 9.9) | 1.8 (0.7 to 3.8) |
| 12 | 21030 | 80.9 | 0.0 | 18.3 | 0.1 | 0.7 | 1.9 (1.3 to 2.5) | 0.4 (0.2 to 0.8) |
| 13 | 8523 | 89.5 | 1.4 | 6.8 | 1.6 | 0.7 | 3.3 (2.2 to 4.8) | 0.9 (0.4 to 1.9) |
| 15 | 5517 | 85.6 | 0.6 | 11.6 | 0.9 | 1.3 | 6.2 (4.4 to 8.7) | 0.9 (0.3 to 2.1) |
| 17 | 11461 | 78.1 | 0.3 | 19.8 | 0.3 | 1.5 | 5.4 (4.2 to 6.9) | 0.2 (0.0 to 0.6) |
| 18 | 22694 | 87.6 | 0.5 | 11.0 | 0.3 | 0.6 | 10.7 (9.4 to 12.2) | 0.7 (0.4 to 1.2) |
| 19 | 10473 | 85.8 | 0.9 | 10.3 | 0.1 | 2.9 | 7.5 (6.0 to 9.4) | 0.3 (0.1 to 0.8) |
| 20 | 1578 | 85.9 | 1.8 | 8.2 | 0.0 | 4.2 | 5.1 (2.2 to 10.0) | 1.3 (0.1 to 4.6) |
| 23 | 1555 | 83.2 | 1.4 | 12.4 | 1.9 | 1.2 | 3.9 (1.4 to 8.4) | 1.9 (0.4 to 5.7) |
| 25 | 2214 | 94.3 | 0.5 | 3.6 | 0.8 | 0.7 | 8.6 (5.2 to 13.4) | 0.0 (0.0 to 1.7) |
| 27 | 2274 | 91.0 | 0.2 | 7.8 | 0.2 | 0.7 | 13.6 (9.2 to 19.4) | 1.8 (0.5 to 4.5) |
| 28 | 1387 | 98.6 | 0.6 | 0.6 | 0.0 | 0.3 | 0.0 (0.0 to 2.7) | 0.0 (0.0 to 2.7) |
| 29 | 8412 | 87.1 | 0.7 | 10.8 | 0.2 | 1.3 | 7.6 (5.8 to 9.8) | 1.0 (0.4 to 1.9) |
| 30 | 3531 | 88.7 | 0.1 | 9.9 | 0.3 | 1.0 | 7.1 (4.5 to 10.5) | 0.6 (0.1 to 2.0) |
| 31 | 13618 | 92.3 | 0.1 | 7.1 | 0.0 | 0.5 | 9.3 (7.8 to 11.1) | 0.6 (0.3 to 1.2) |
| 33 | 5123 | 89.4 | 0.8 | 5.9 | 3.6 | 0.4 | 3.5 (2.1 to 5.5) | 0.6 (0.1 to 1.7) |
| 34 | 3287 | 92.2 | 0.5 | 7.3 | 0.0 | 0.0 | 0.0 (0.0 to 1.1) | 0.0 (0.0 to 1.1) |
| 36 | 3589 | 93.3 | 0.1 | 6.0 | 0.1 | 0.5 | 3.6 (1.9 to 6.2) | 0.6 (0.1 to 2.0) |
| 38 | 2350 | 91.8 | 0.0 | 7.1 | 0.1 | 1.0 | 13.2 (8.9 to 18.7) | 0.4 (0.0 to 2.4) |
| 39 | 2699 | 93.2 | 0.0 | 6.7 | 0.0 | 0.2 | 11.9 (8.2 to 16.7) | 0.4 (0.0 to 2.1) |
| 40 | 2300 | 92.9 | 0.0 | 4.9 | 0.6 | 1.7 | 15.7 (10.9 to 21.7) | 1.7 (0.5 to 4.4) |
| 42 | 1508 | 88.7 | 0.5 | 6.4 | 1.9 | 2.6 | 13.9 (8.6 to 21.2) | 0.0 (0.0 to 2.5) |
| Pooled* | 4418 | 88.8 | 0.5 | 6.4 | 1.8 | 2.6 | 15.4 (12.0 to 19.5) | 1.8 (0.8 to 3.6) |
| Total | 211645 | 87.4 | 0.9 | 10.0 | 0.5 | 1.2 | 7.4 (7.0 to 7.7) | 0.7 (0.6 to 0.9) |
*Pooled results in 10 centres registering fewer than 1000 patients each.
CI, confidence interval; L no C, left heart no coronary arteries; LCA, left heart and coronary arteries; R, right heart only; RLCA, right and left heart and coronaries; RL no C, right and left heart no coronaries.
Table 1 also lists complication rates and mortality by centre for all centres registering over 1000 cases and pooled for those centres that registered fewer than 1000 cases. Nine centres recorded high complication rates (lower 95% confidence limit greater than the overall mean of 7.4) and the 10 small centres combined also recorded a high complication rate, double the overall mean. Two centres plus the 10 small centres combined recorded high death rates (lower 95% confidence limit greater than the overall mean of 0.7).
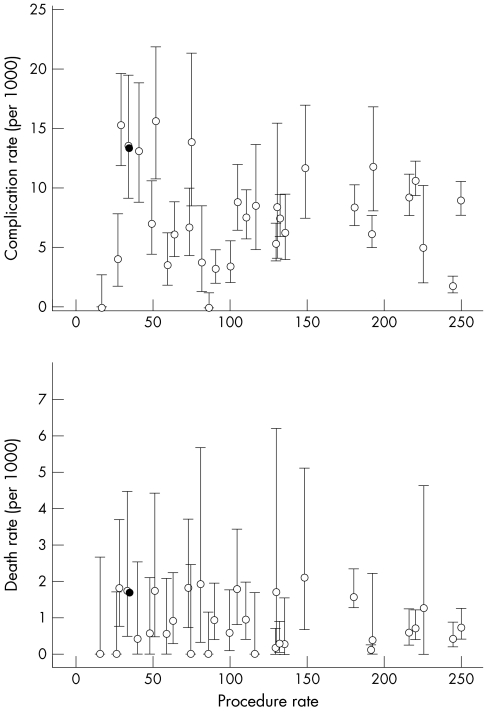
Figure 1 shows the relation between complication and death rates and monthly caseload for 30 centres and 10 small centres pooled. Overall, there was no significant relation between complication rate and caseload (for 31 “hospitals”). However, the highest complication rates were recorded in four hospitals performing fewer than 100 procedures a month and in the 10 small hospitals combined, which together averaged fewer than 30 procedures a month.
Figure 1 Complications and death rates with 95% confidence intervals by hospital according to monthly procedure rate.
Hospitals with high complication rates also recorded high death rates (p < 0.05). The association between death rate and caseload was significant, suggesting higher mortality in hospitals that undertake fewer procedures a month (p < 0.05). The pooled group of 10 small hospitals recorded one of the highest death rates.
Table 2 shows numbers of complication and deaths for different types of diagnostic procedure. The overall complication rate for adult diagnostic catheterisation was 7.4/1000 (95% confidence interval (CI) 7.0 to 7.7) and overall mortality was 0.72/1000 (95% CI 0.61 to 0.84). The corresponding rates for paediatric procedures were 7.1 (95% CI 5.4 to 9.2) and 1.45 (95% CI 0.73 to 2.60), respectively (7582 procedures, 4404 in one hospital and 3012 in five others, 54 complications, and 11 deaths). Paediatric procedures are not strictly comparable with adult, since most involve intervention and general anaesthesia.
Table 2 Complications and deaths by type of procedure and classification of complications in all centres combined.
| Procedures | Complications | Deaths | |
|---|---|---|---|
| Procedure type | |||
| LCAs | 184 909 (87%) | 1 368 (88%) | 146 (94%) |
| L no C | 1 855 (1%) | 3 (0%) | 1 (1%) |
| RCLA | 21 234 (10%) | 107 (7%) | 7 (5%) |
| RL no C | 1 055 (0%) | 4 (0%) | 0 (0%) |
| R | 2 592 (1%) | 13 (1%) | 1 (1%) |
| Not known | 0 (0%) | 65 (4%) | 0 (0%) |
| All adult diagnostic | 211 645 | 1 560 | 155 |
| Classification | |||
| Allergy | 53 (3%) | 0 (0%) | |
| Arrhythmias | 553 (35%) | 18 (12%) | |
| Cerebrovascular accident | 137 (8%) | 8 (5%) | |
| Coronary dissection | 71 (5%) | 6 (4%) | |
| Haemodynamic collapse | 94 (6%) | 29 (19%) | |
| Intramyocardial injection | 16 (1%) | 0 (0%) | |
| Ischaemia | 178 (11%) | 74 (48%) | |
| Myocardial infarction | 12 (1%) | 5 (3%) | |
| Vascular | 335 (22%) | 7 (5%) | |
| Miscellaneous | 25 (2%) | 5 (3%) | |
| Unknown | 86 (6%) | 3 (2%) |
Table 2 also gives numbers of complications and deaths by types of complication. The most common were arrhythmias (35%), with low case fatality (3%), and second were vascular complications (22%), with low case fatality (2%). Third most common were ischaemias (11%), which accounted for a large proportion of the deaths (case fatality 42%).
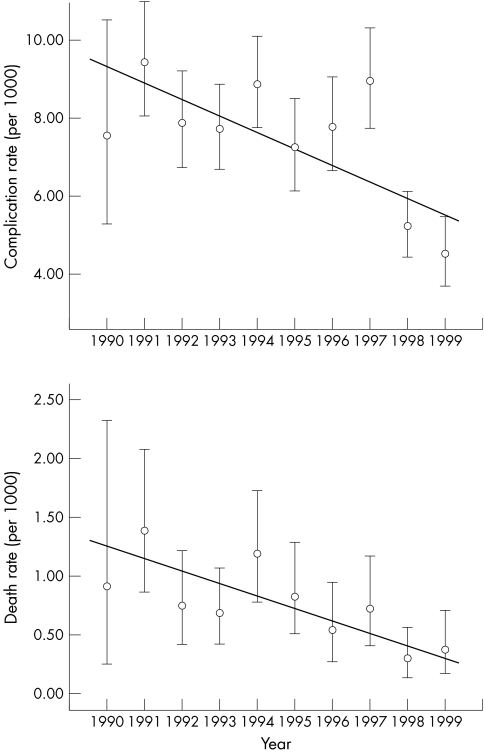
Analysis of the time trend in complication rates shows higher rates in the early years and lower rates later, an average decline of 0.42/1000 per annum (p < 0.05; fig 2). The time trend in death rate shows a significant decline of 0.09/1000 per annum (p < 0.02; fig 2).
Figure 2 Complications and death rates by year.
DISCUSSION
We report the frequency and nature of complications in patients undergoing diagnostic cardiac catheterisation in 41 cardiac centres in England and Wales during the period from 1990 to 1999. The study is based on a voluntary reporting system established by the Joint Audit Committee of the British Cardiac Society and Royal College of Physicians of London. The study follows the same methods and analysis, including grouping of procedures and reported complications, as were used for the first two years.7 There are weaknesses in voluntary registration of complications: relatively few hospitals participated for the whole period and several contributed for only a year. There were some inconsistencies in data capture—for example, numbers of complications on a monthly return not matching exactly the number of complication forms registered; and classification in denominators—for example, types of procedures in monthly returns. Despite feedback in the early years of the study, centres were likely to vary somewhat in the threshold deemed notifiable. Nevertheless, we consider that this voluntary audit is representative of diagnostic cardiac catheterisation undertaken in England and Wales during the audit and is comparable with other published studies.8,9,10
The majority of cases, 87% of diagnostic catheter studies in adults, were left heart studies with coronary arteriography, as reported in other similar series. It was not possible to distinguish the number of procedures involving coronary arteriography only. Historically, the majority of left heart studies have involved both coronary arteriography and left ventriculography. However, in recent years, with the advent of echocardiography, the requirement for left ventriculography has diminished and that procedure is practised less often. It is known that retrograde crossing of the aortic valve in patients with aortic stenosis is accompanied by substantial risk of complications, especially neurological.11 The option of coding procedures that involve coronary arteriography alone should be included in future audits.
The overall complication rate of 7.4/1000 compares favourably with those reported previously by multicentre registries—for example, 8.8/1000 in the USA.8,9,10,12 The complication rate seemed to fall over the course of the decade. Artefact cannot be ruled out—for example, a change in threshold of complication deemed sufficiently serious to report, or different hospitals contributing in later years. Since mortality showed a clear trend and recording of mortality is less subjective, we conclude that the complication rate probably did fall over the decade. Reasons underpinning a real fall are likely to be multifactorial, attributable to improvements in equipment and contrast material, changes in arterial access site with reduced use of brachial arteriotomy, changes in technique with reduced use of transseptal puncture, and improved periprocedural preparation. However, such trends may be offset, to some extent, by the use of cardiac catheterisation in older patients with more complex co‐morbidity.
Arrhythmias continue to be the leading type of complication (35%). However, few deaths (12%) were attributable to arrhythmias, which may reflect continued improvements in arrhythmia management with increased advanced cardiac life support uptake. Vascular complications made up the second most common type (22%) and these also had a low associated mortality. The preferred site of arterial access has altered over the years. Brachial arteriotomy has been overtaken by femoral puncture and radial puncture has become the preferred access site in many centres. Brachial arteriotomy is associated with an increased risk of vascular complications compared with use of the femoral route.13 Recent comparisons between the use of the femoral and radial routes have suggested that the radial route is associated with a reduced risk of complication, but smaller catheter sizes and recently developed closure devices seem to have comparable complication rates.14 Future audits should clearly document access site.
This study found some association between centre caseload and mortality. American guidelines in 1991 suggested a minimum annual caseload of 300 per centre.15 Although complication rates in low volume centres have consistently been shown to be higher than in high volume centres, more recent guidelines have recognised that setting a minimum caseload is fraught with problems.16 Findings based on voluntary audit and without patient specific data do not provide sufficiently robust evidence to make recommendations over minimum caseloads. However, the trend with caseload lends support for the need for formal audit of all patients. It should be noted that the majority of centres reporting in this study had caseloads far exceeding 25 a month.
Practice in cardiac catheterisation changes with time. The tendency is increasing to undertake coronary arteriography and proceed immediately to percutaneous coronary intervention, provided the anatomy and clinical presentation are appropriate. The British Society for Interventional Cardiology is committed to use of the central cardiac audit database and regular audit. We suggest that the recording of complications in diagnostic procedures should follow the lead of interventional cardiology. The increasing number of cardiac catheterisation facilities, the development of newer and safer invasive techniques, and the evolution of non‐invasive investigations present a strong case for a robust means of monitoring diagnostic cardiac catheterisation studies throughout the UK. This process should be compulsory, be funded appropriately, use coding based on current practice, and be transparent. Centres that perform studies with unduly high complication rates need to be identified to maintain high standards of cardiac practice.
ACKNOWLEDGEMENTS
The authors thank David de Bono for initiating and directing the study for many years, Lynne Walker for managing the hospital returns, Janet Hill for converting the database to SPSS and cleaning data, and Carell Alden for typing the manuscript.
Appendix: Participating hospitals
Airedale General Hospital, Keighley; Birch Hill Hospital, Rochdale; Bristol Royal Infirmary; Broadgreen Hospital, Liverpool; BUPA Hospital, Leicester; Conquest Hospital, St Leonards‐on‐Sea; Derriford Hospital, Plymouth; Dudley Road Hospital, Birmingham; Glasgow Royal Infirmary; Glenfield Hospital, Leicester; Great Ormond Street Hospital, London WC1; John Radcliffe Hospital, Oxford; King's College Hospital, London SE5; Leeds General Infirmary; London Chest Hospital, London E2; Maidstone Hospital; Mayday Hospital, Croydon; Norfolk & Norwich Hospital, Norwich; North Staffordshire Royal Infirmary, Stoke‐on‐Trent; Nottingham City Hospital; Papworth Hospital, Cambridge; Queen Elizabeth Hospital, Birmingham; Queen Mary's Hospital, Sidcup; Queen's Medical Centre, Nottingham; Royal Cornwall Hospital, Truro; Royal Infirmary of Edinburgh; Royal London Hospital, London E1; Royal Postgraduate Medical School, London W12; Royal United Hospital, Bath; South Cleveland Hospital, Middlesbrough; St Bartholomew's Hospital, London EC1; St Georges Hospital, London SW17; St Mary's Hospital & Medical School, London W2; Torbay Hospital, Torquay; University Hospital of Wales, Cardiff; Victoria Hospital, Blackpool; Wythenshawe Hospital, Manchester; York District Hospital
Footnotes
Competing interests: none declared
References
- 1.Ward Kennedy J, for the Registry Committee of the Society for Cardiac Angiography and Interventions Symposium on catheterisation complications: complications associated with cardiac catheterisation and angiography. Cathet Cardiovasc Diagn 198285–11. [DOI] [PubMed] [Google Scholar]
- 2.Noto T J, Johnson L W, Krone R.et al Cardiac catheterisation 1990: a report of the Registry of the Society for Cardiac Angiography and Interventions (SCA&I). Cathet Cardiovasc Diagn 19912475–83. [DOI] [PubMed] [Google Scholar]
- 3.Silber S, Albrecht A, Gohring S.et al First annual report of practitioners of interventional cardiology in private practice in Germany: results of procedures of left heart catheterisation and coronary interventions in the year 1996. Herz 19982347–57. [DOI] [PubMed] [Google Scholar]
- 4.English T A H, Bailey A R, Dark J F.et al The UK cardiac surgical register, 1977–82. BMJ 19842891205–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hubner P J B. Cardiac interventional procedures in the United Kingdom during 1990. Br Heart J 199248434–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Belder M A. Cardiac intervention procedures in the United Kingdom 1997: developments in data collection. Heart 199982(suppl 2)112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Bono D, on behalf of the Joint Audit Committee of the British Cardiac Society and Royal College of Physicians of London Complications of diagnostic cardiac catheterisation: results from 34,041 patients in the United Kingdom confidential enquiry into cardiac catheter complications. Br Heart J 199370297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson L W, Krone R. Cardiac catheterisation 1991: a report of the Registry of the Society for Cardiac Angiography and Interventions. Cathet Cardiovasc Diagn 199328219–220. [DOI] [PubMed] [Google Scholar]
- 9.Krone R J, Johnson L, Noto T. Five year trends in cardiac catheterisation: a report from the Registry of the Society for Cardiac Angiography and Interventions. Cathet Cardiovasc Design 19963931–35. [DOI] [PubMed] [Google Scholar]
- 10.Chandrasekar B, Doucet S, Bilodeau L.et al Complications of cardiac catheterization in the current era: a single‐center experience. Catheter Cardiovasc Interv 200152289–295. [DOI] [PubMed] [Google Scholar]
- 11.Omran H, Schmidt H, Hackenbroch M.et al Silent and apparent cerebral embolism after retrograde catheterisation of the aortic valve in valvular stenosis: a prospective, randomised study. Lancet 20033611241–1246. [DOI] [PubMed] [Google Scholar]
- 12.Bashore T, Bates E R, Berger P B.et al American College of Cardiology/Society for Cardiac Angiography and Interventions Clinical Expert Consensus Document on cardiac catheterization laboratory standards. A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2001372170–2214. [DOI] [PubMed] [Google Scholar]
- 13.Scanlon P J, Faxon D P, Audet A M.et al ACC/AHA guidelines for coronary angiography: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol 1999331756–1824. [DOI] [PubMed] [Google Scholar]
- 14.Reddy B K, Brewster P S, Walsh T.et al Randomized comparison of rapid ambulation using radial, 4 French femoral access, or femoral access with Angioseal closure. Catheter Cardiovasc Interv 200462143–149. [DOI] [PubMed] [Google Scholar]
- 15.American College of Cardiology American Heart Association. ACC/AHA guidelines for cardiac catheterization and cardiac catheterization laboratories. American College of Cardiology/American Heart Association ad hoc task force on cardiac catheterization. J Am Coll Cardiol 1991181149–1182. [PubMed] [Google Scholar]
- 16.Hirshfield J W, Ellis S G, Faxon D P. Recommendations for the assessment and maintenance of proficiency in coronary interventional procedures: statement of the American College of Cardiology. J Am Coll Cardiol 199831722–743. [DOI] [PubMed] [Google Scholar]