Abstract
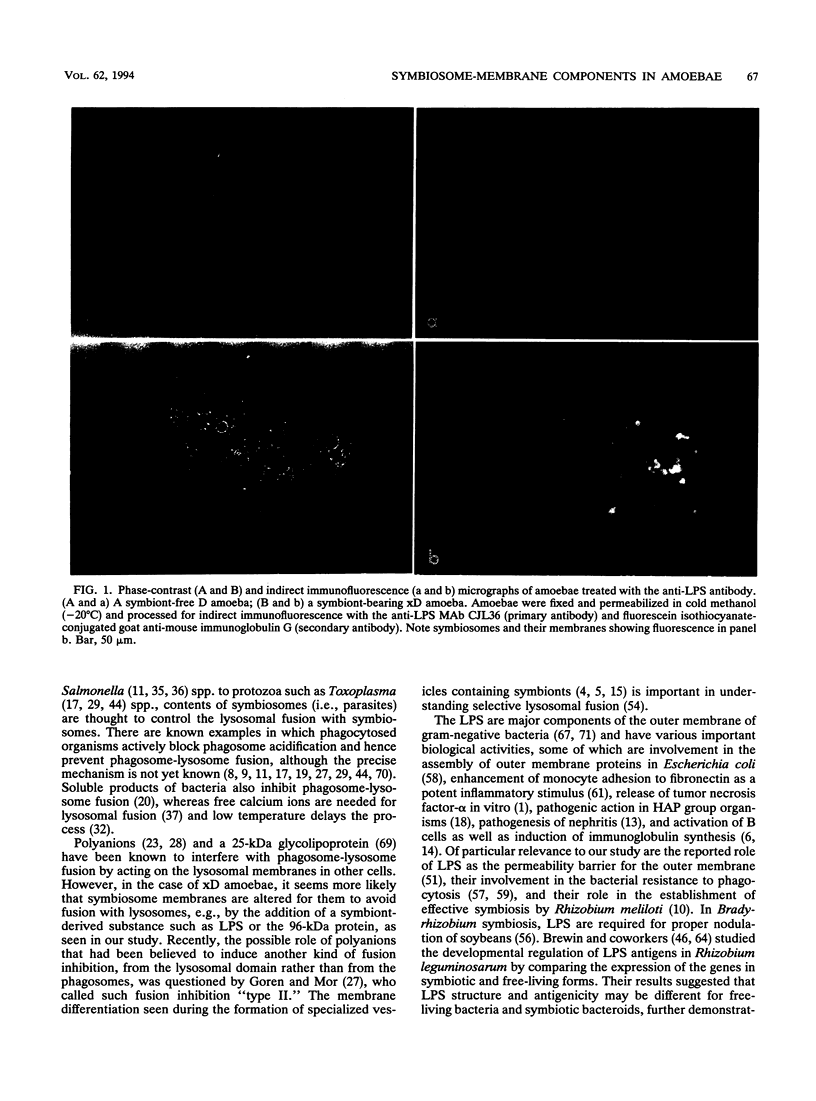
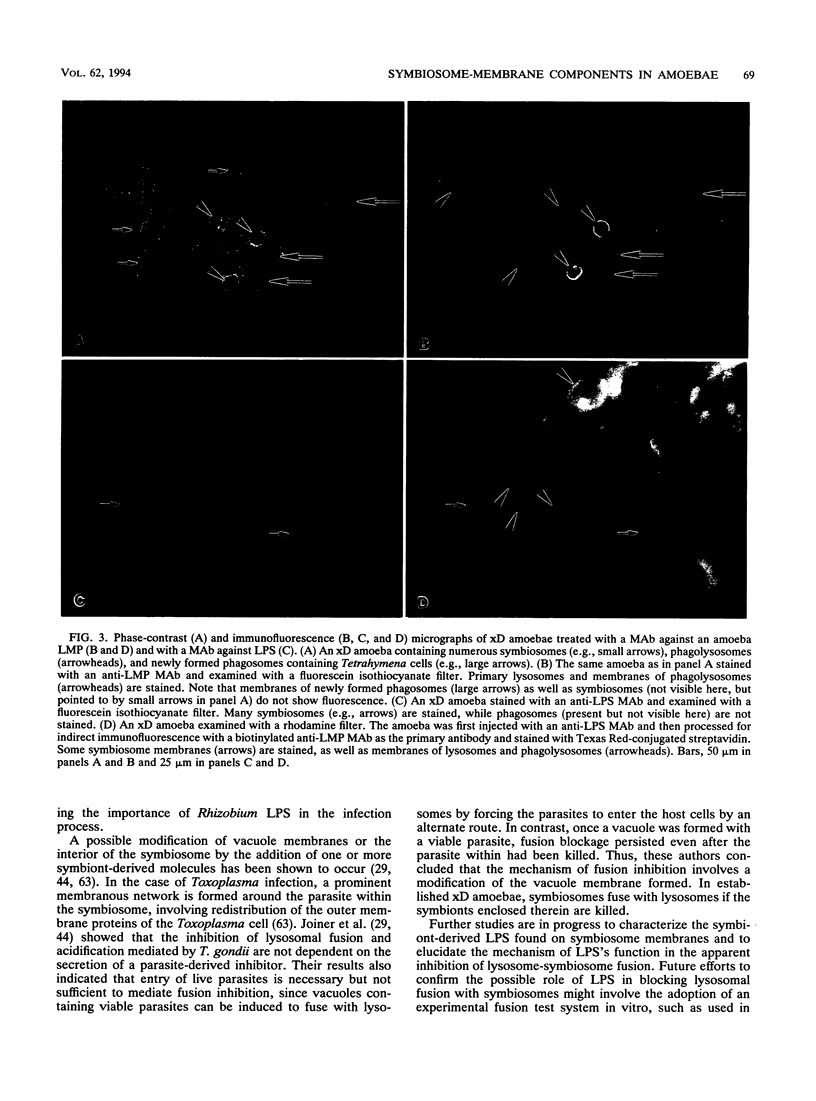
Experimental results are presented to support the view that symbiont-derived lipopolysaccharides are involved in the prevention of lysosome-symbiosome fusion in xD amoebae harboring bacterial endosymbionts. Monoclonal antibodies against lipopolysaccharides and a 96-kDa protein present on symbiosome membranes of amoebae were used to monitor the appearance of the membrane-specific components in newly infected amoebae with endosymbionts from xD amoebae. The lipopolysaccharides and protein appeared on the newly forming symbiosome membranes within 3 to 7 days, as detected by indirect immunofluorescence staining with monoclonal antibodies. The lysosome-symbiosome fusion was followed by double staining of two antigens with different monoclonal antibodies applied to the same amoeba. Antilipopolysaccharide monoclonal antibodies were detected by staining with a fluorescein isothiocyanate-conjugated secondary antibody, and a biotinylated anti-lysosomal protein monoclonal antibody was detected by staining with Texas Red-conjugated streptavidin. In xD amoebae injected with an antilipopolysaccharide antibody, lysosomes fused with some of the symbiosomes that did not fuse with lysosomes in noninjected cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. L., Czuprynski C. J. Bacterial lipopolysaccharide induces release of tumor necrosis factor-alpha from bovine peripheral blood monocytes and alveolar macrophages in vitro. J Leukoc Biol. 1990 Dec;48(6):549–556. doi: 10.1002/jlb.48.6.549. [DOI] [PubMed] [Google Scholar]
- Ahn T. I., Jeon K. W. Growth and electron microscopic studies on an experimentally established bacterial endosymbiosis in amoebae. J Cell Physiol. 1979 Jan;98(1):49–57. doi: 10.1002/jcp.1040980107. [DOI] [PubMed] [Google Scholar]
- Ahn T. I., Jeon K. W. Recycling of membrane proteins during endo- and exocytosis in amoebae. Exp Cell Res. 1985 Sep;160(1):54–62. doi: 10.1016/0014-4827(85)90235-6. [DOI] [PubMed] [Google Scholar]
- Ahn T. I., Jeon K. W. Structural and biochemical characteristics of the plasmalemma and vacuole membranes in Amoebae. Exp Cell Res. 1982 Feb;137(2):253–268. doi: 10.1016/0014-4827(82)90026-x. [DOI] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975 Jul 1;142(1):1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger K. H., Isberg R. R. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993 Jan;7(1):7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Black C. M., Bermudez L. E., Young L. S., Remington J. S. Co-infection of macrophages modulates interferon gamma and tumor necrosis factor-induced activation against intracellular pathogens. J Exp Med. 1990 Sep 1;172(3):977–980. doi: 10.1084/jem.172.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzoska P. M., Signer E. R. lpsZ, a lipopolysaccharide gene involved in symbiosis of Rhizobium meliloti. J Bacteriol. 1991 May;173(10):3235–3237. doi: 10.1128/jb.173.10.3235-3237.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Inhibition of macrophage phagosome-lysosome fusion by Salmonella typhimurium. Infect Immun. 1991 Jul;59(7):2232–2238. doi: 10.1128/iai.59.7.2232-2238.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W., Kalembasa S., Turowski D., Pachori P., Noel K. D. Characterization of the lipopolysaccharide from a Rhizobium phaseoli mutant that is defective in infection thread development. J Bacteriol. 1987 Nov;169(11):4923–4928. doi: 10.1128/jb.169.11.4923-4928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo T., Granholm N. A. Bacterial lipopolysaccharide transforms mesangial into proliferative lupus nephritis without interfering with processing of pathogenic immune complexes in NZB/W mice. Am J Pathol. 1990 Oct;137(4):971–978. [PMC free article] [PubMed] [Google Scholar]
- Fenwick B. W. Virulence attributes of the liposaccharides of the HAP group organisms. Can J Vet Res. 1990 Apr;54 (Suppl):S28–S32. [PubMed] [Google Scholar]
- Frenchick P. J., Markham R. J., Cochrane A. H. Inhibition of phagosome-lysosome fusion in macrophages by soluble extracts of virulent Brucella abortus. Am J Vet Res. 1985 Feb;46(2):332–335. [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fréhel C., Rastogi N. Phagosome-lysosome fusions in macrophages infected by Mycobacterium avium: role of mycosides-C and other cells surface components. Acta Leprol. 1989;7 (Suppl 1):173–174. [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Geisow M. J., Beaven G. H., Hart P. D., Young M. R. Site of action of a polyanion inhibitor of phagosome-lysosome fusion in cultured macrophages. Exp Cell Res. 1980 Mar;126(1):159–165. doi: 10.1016/0014-4827(80)90481-4. [DOI] [PubMed] [Google Scholar]
- Goldstein L., Ko C. A method for the mass culturing of large free-living amebas. Methods Cell Biol. 1976;13:239–246. doi: 10.1016/s0091-679x(08)61805-1. [DOI] [PubMed] [Google Scholar]
- Goosen-de Roo L., de Maagd R. A., Lugtenberg B. J. Antigenic changes in lipopolysaccharide I of Rhizobium leguminosarum bv. viciae in root nodules of Vicia sativa subsp. nigra occur during release from infection threads. J Bacteriol. 1991 May;173(10):3177–3183. doi: 10.1128/jb.173.10.3177-3183.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren M. B., Mor N. Influence of phagosomal contents on the apparent inhibition of phagosome-lysosome fusion mediated by polyanionic substances in mouse peritoneal macrophages. Biochem Cell Biol. 1990 Jan;68(1):24–32. doi: 10.1139/o90-004. [DOI] [PubMed] [Google Scholar]
- Goren M. B., Vatter A. E., Fiscus J. Polyanionic agents as inhibitors of phagosome-lysosome fusion in cultured macrophages: evolution of an alternative interpretation. J Leukoc Biol. 1987 Feb;41(2):111–121. doi: 10.1002/jlb.41.2.111. [DOI] [PubMed] [Google Scholar]
- Hall B. F., Joiner K. A. Strategies of obligate intracellular parasites for evading host defences. Immunol Today. 1991 Mar;12(3):A22–A27. doi: 10.1016/S0167-5699(05)80007-6. [DOI] [PubMed] [Google Scholar]
- Hammond S. M. Inhibitors of lipopolysaccharide biosynthesis impair the virulence potential of Escherichia coli. FEMS Microbiol Lett. 1992 Dec 15;100(1-3):293–297. doi: 10.1111/j.1574-6968.1992.tb14055.x. [DOI] [PubMed] [Google Scholar]
- Han J. H., Jeon K. W. Isolation and partial characterization of two plasmid deoxyribonucleic acids from endosymbiotic bacteria of Amoeba proteus. J Bacteriol. 1980 Mar;141(3):1466–1469. doi: 10.1128/jb.141.3.1466-1469.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haylett T., Thilo L. Endosome-lysosome fusion at low temperature. J Biol Chem. 1991 May 5;266(13):8322–8327. [PubMed] [Google Scholar]
- Hohman T. C., McNeil P. L., Muscatine L. Phagosome-lysosome fusion inhibited by algal symbionts of Hydra viridis. J Cell Biol. 1982 Jul;94(1):56–63. doi: 10.1083/jcb.94.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983 Dec 1;158(6):2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi Y., Arai T. Specific inhibition of phagosome-lysosome fusion in murine macrophages mediated by Salmonella typhimurium infection. FEMS Microbiol Immunol. 1990 May;2(1):35–43. doi: 10.1111/j.1574-6968.1990.tb03476.x. [DOI] [PubMed] [Google Scholar]
- Ishibashi Y., Nobuta K., Arai T. Mutant of Salmonella typhimurium lacking the inhibitory function for phagosome-lysosome fusion in murine macrophages. Microb Pathog. 1992 Oct;13(4):317–323. doi: 10.1016/0882-4010(92)90041-l. [DOI] [PubMed] [Google Scholar]
- Jaconi M. E., Lew D. P., Carpentier J. L., Magnusson K. E., Sjögren M., Stendahl O. Cytosolic free calcium elevation mediates the phagosome-lysosome fusion during phagocytosis in human neutrophils. J Cell Biol. 1990 May;110(5):1555–1564. doi: 10.1083/jcb.110.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon K. W., Jeon M. S. Cytoplasmic filaments and cellular wound healing in Amoeba proteus. J Cell Biol. 1975 Oct;67(1):243–249. doi: 10.1083/jcb.67.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon K. W., Jeon M. S. Endosymbiosis in amoebae: recently established endosymbionts have become required cytoplasmic components. J Cell Physiol. 1976 Oct;89(2):337–344. doi: 10.1002/jcp.1040890216. [DOI] [PubMed] [Google Scholar]
- Jeon K. W., Lorch I. J. Unusual intra-cellular bacterial infection in large, free-living amoebae. Exp Cell Res. 1967 Oct;48(1):236–240. doi: 10.1016/0014-4827(67)90313-8. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Fuhrman S. A., Miettinen H. M., Kasper L. H., Mellman I. Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc receptor-transfected fibroblasts. Science. 1990 Aug 10;249(4969):641–646. doi: 10.1126/science.2200126. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannenberg E. L., Rathbun E. A., Brewin N. J. Molecular dissection of structure and function in the lipopolysaccharide of Rhizobium leguminosarum strain 3841 using monoclonal antibodies and genetic analysis. Mol Microbiol. 1992 Sep;6(17):2477–2487. doi: 10.1111/j.1365-2958.1992.tb01424.x. [DOI] [PubMed] [Google Scholar]
- Kiehart D. P., Kaiser D. A., Pollard T. D. Monoclonal antibodies demonstrate limited structural homology between myosin isozymes from Acanthamoeba. J Cell Biol. 1984 Sep;99(3):1002–1014. doi: 10.1083/jcb.99.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana T., Mullock B. M., Luzio J. P. Identification of a protein capable of causing fusion of endosome and lysosome membranes. Biochem Soc Trans. 1993 May;21(2):299–300. doi: 10.1042/bst0210299. [DOI] [PubMed] [Google Scholar]
- Mayorga L. S., Bertini F., Stahl P. D. Fusion of newly formed phagosomes with endosomes in intact cells and in a cell-free system. J Biol Chem. 1991 Apr 5;266(10):6511–6517. [PubMed] [Google Scholar]
- Mullock B. M., Branch W. J., van Schaik M., Gilbert L. K., Luzio J. P. Reconstitution of an endosome-lysosome interaction in a cell-free system. J Cell Biol. 1989 Jun;108(6):2093–2099. doi: 10.1083/jcb.108.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Oates P. J., Touster O. In vitro fusion of Acanthamoeba phagolysosomes. III. Evidence that cyclic nucleotides and vacuole subpopulations respectively control the rate and the extent of vacuole fusion in Acanthamoeba homogenates. J Cell Biol. 1980 Jun;85(3):804–810. doi: 10.1083/jcb.85.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt J. L., Michael A. F. Retardation of fading and enhancement of intensity of immunofluorescence by p-phenylenediamine. J Histochem Cytochem. 1983 Jun;31(6):840–842. doi: 10.1177/31.6.6341464. [DOI] [PubMed] [Google Scholar]
- Puvanesarajah V., Schell F. M., Gerhold D., Stacey G. Cell surface polysaccharides from Bradyrhizobium japonicum and a nonnodulating mutant. J Bacteriol. 1987 Jan;169(1):137–141. doi: 10.1128/jb.169.1.137-141.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Cooney M. H., Spitznagel J. K. Susceptibility of lipopolysaccharide mutants to the bactericidal action of human neutrophil lysosomal fractions. Infect Immun. 1977 Apr;16(1):145–151. doi: 10.1128/iai.16.1.145-151.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried G., Hindennach I., Henning U. Role of lipopolysaccharide in assembly of Escherichia coli outer membrane proteins OmpA, OmpC, and OmpF. J Bacteriol. 1990 Oct;172(10):6048–6053. doi: 10.1128/jb.172.10.6048-6053.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth P., Polin R. A. Lipopolysaccharide enhances monocyte adherence to matrix-bound fibronectin. Clin Immunol Immunopathol. 1990 Dec;57(3):363–373. doi: 10.1016/0090-1229(90)90111-3. [DOI] [PubMed] [Google Scholar]
- Sanjuan J., Carlson R. W., Spaink H. P., Bhat U. R., Barbour W. M., Glushka J., Stacey G. A 2-O-methylfucose moiety is present in the lipo-oligosaccharide nodulation signal of Bradyrhizobium japonicum. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8789–8793. doi: 10.1073/pnas.89.18.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Krahenbuhl J. L. Modification of host cell phagosomes by Toxoplasma gondii involves redistribution of surface proteins and secretion of a 32 kDa protein. Eur J Cell Biol. 1988 Oct;47(1):81–87. [PubMed] [Google Scholar]
- Sindhu S. S., Brewin N. J., Kannenberg E. L. Immunochemical analysis of lipopolysaccharides from free-living and endosymbiotic forms of Rhizobium leguminosarum. J Bacteriol. 1990 Apr;172(4):1804–1813. doi: 10.1128/jb.172.4.1804-1813.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff U., Golecki J. R., Kazda J., Kaufmann S. H. Evidence for phagosome-lysosome fusion in Mycobacterium leprae-infected murine Schwann cells. Infect Immun. 1989 Mar;57(3):1008–1010. doi: 10.1128/iai.57.3.1008-1010.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheul A. F., Snippe H., Poolman J. T. Meningococcal lipopolysaccharides: virulence factor and potential vaccine component. Microbiol Rev. 1993 Mar;57(1):34–49. doi: 10.1128/mr.57.1.34-49.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadee A. A., Cohen J. D., Rabson A. R. Gamma interferon reverses inhibition of leukocyte bactericidal activity by a 25-kilodalton fraction from Mycobacterium tuberculosis. Infect Immun. 1987 Nov;55(11):2777–2782. doi: 10.1128/iai.55.11.2777-2782.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner E., Sibley L. D. Phagocytized intracellular microsporidian blocks phagosome acidification and phagosome-lysosome fusion. J Protozool. 1985 May;32(2):311–317. doi: 10.1111/j.1550-7408.1985.tb03056.x. [DOI] [PubMed] [Google Scholar]
- Westphal O., Jann K., Himmelspach K. Chemistry and immunochemistry of bacterial lipopolysaccharides as cell wall antigens and endotoxins. Prog Allergy. 1983;33:9–39. [PubMed] [Google Scholar]
- de Carvalho L., de Souza W. Internalization of surface anionic sites and phagosome-lysosome fusion during interaction of Toxoplasma gondii with macrophages. Eur J Cell Biol. 1990 Apr;51(2):211–219. [PubMed] [Google Scholar]





