Abstract
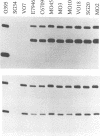
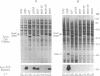
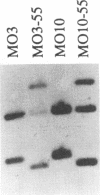
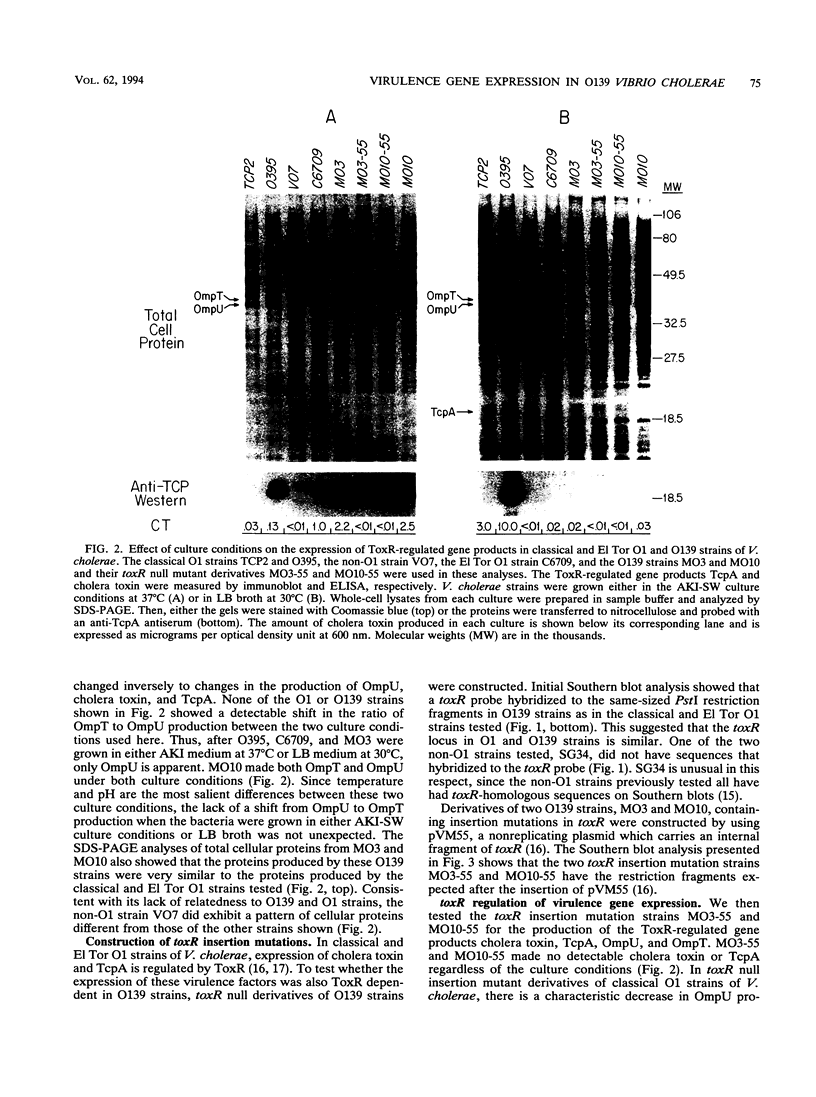
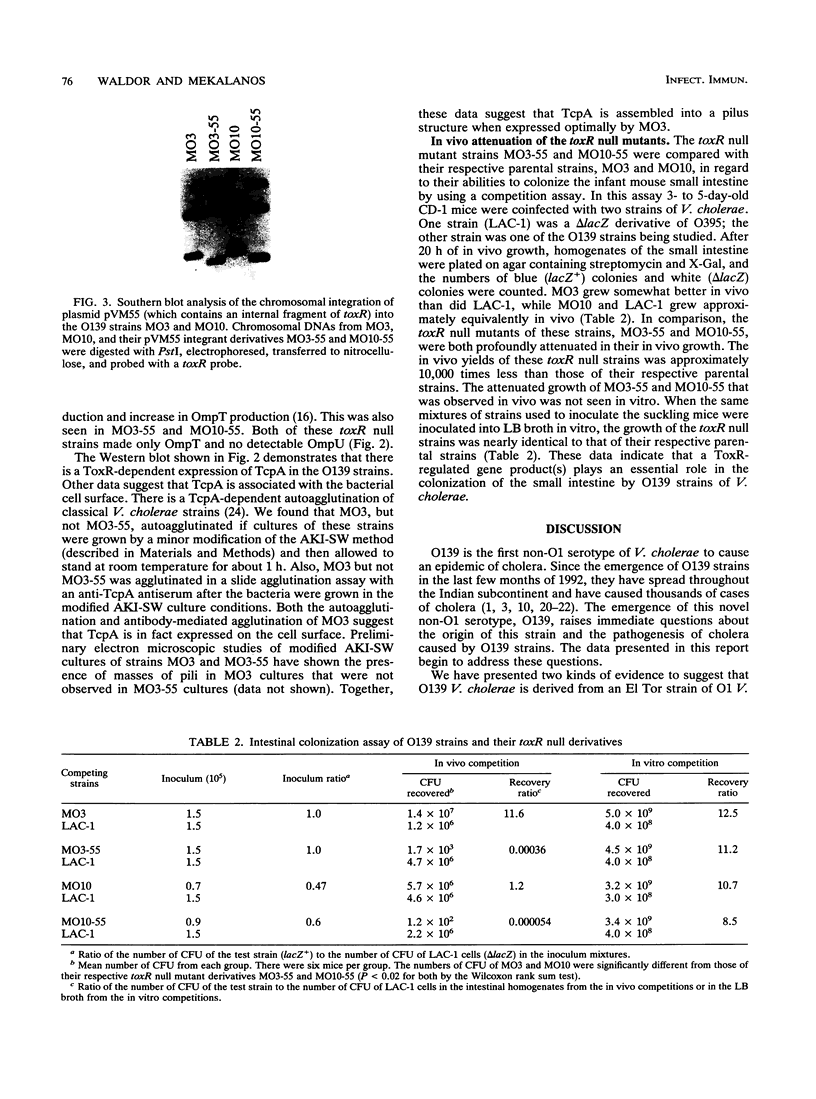
Vibrio cholerae serogroup O1 has historically been thought to be the exclusive cause of epidemic cholera. O139 is a novel serogroup of V. cholerae which emerged on the Indian subcontinent in the last few months of 1992 and is the first non-O1 serogroup of V. cholerae to cause epidemic cholera. We have investigated the expression of some of the known virulence factors of classical and El Tor O1 strains of V. cholerae in clinical isolates of O139 strains. We show that, in contrast to other non-O1 strains, O139 strains express TcpA, the major subunit of the toxin-coregulated pilus found in O1 strains. As in O1 strains, the expression of cholera toxin and TcpA is coordinately regulated by environmental parameters in O139 strains. Derivatives of O139 strains that contain a toxR null mutation were constructed and used to demonstrate that the expression of cholera toxin, TcpA, and the outer membrane protein OmpU in O139 strains, as in O1 strains, is dependent on ToxR. Two kinds of evidence suggest that O139 strains are closely related to El Tor strains of V. cholerae. First, both O139 and El Tor strains share a restriction fragment length polymorphism for tcpA, which distinguishes El Tor from classical strains of V. cholerae. Second, cholera toxin production in O139 strains is greatly enhanced by culture conditions that have been previously shown to promote production of cholera toxin in El Tor strains and not in classical strains of V. cholerae. Although O139 is a novel serotype of V. cholerae, O139 strains conform to a fundamental theme that has evolved from the study of O1 strains: ToxR mediates coordinate regulation of virulence gene expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert M. J., Siddique A. K., Islam M. S., Faruque A. S., Ansaruzzaman M., Faruque S. M., Sack R. B. Large outbreak of clinical cholera due to Vibrio cholerae non-O1 in Bangladesh. Lancet. 1993 Mar 13;341(8846):704–704. doi: 10.1016/0140-6736(93)90481-u. [DOI] [PubMed] [Google Scholar]
- Bhattacharya M. K., Bhattacharya S. K., Garg S., Saha P. K., Dutta D., Nair G. B., Deb B. C., Das K. P. Outbreak of Vibrio cholerae non-O1 in India and Bangladesh. Lancet. 1993 May 22;341(8856):1346–1347. doi: 10.1016/0140-6736(93)90855-b. [DOI] [PubMed] [Google Scholar]
- Bhattacharya M. K., Bhattacharya S. K., Garg S., Saha P. K., Dutta D., Nair G. B., Deb B. C., Das K. P. Outbreak of Vibrio cholerae non-O1 in India and Bangladesh. Lancet. 1993 May 22;341(8856):1346–1347. doi: 10.1016/0140-6736(93)90855-b. [DOI] [PubMed] [Google Scholar]
- DiRita V. J. Co-ordinate expression of virulence genes by ToxR in Vibrio cholerae. Mol Microbiol. 1992 Feb;6(4):451–458. doi: 10.1111/j.1365-2958.1992.tb01489.x. [DOI] [PubMed] [Google Scholar]
- Hanchalay S., Seriwatana J., Echeverria P., Holmgren J., Tirapat C., Moseley S. L., Taylor D. N. Non-O1 Vibrio cholerae in Thailand: homology with cloned cholera toxin genes. J Clin Microbiol. 1985 Feb;21(2):288–289. doi: 10.1128/jcm.21.2.288-289.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington D. A., Hall R. H., Losonsky G., Mekalanos J. J., Taylor R. K., Levine M. M. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988 Oct 1;168(4):1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J. Comparison of the tissue receptors for Vibrio cholerae and Escherichia coli enterotoxins by means of gangliosides and natural cholera toxoid. Infect Immun. 1973 Dec;8(6):851–859. doi: 10.1128/iai.8.6.851-859.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga M., Yamamoto K., Higa N., Ichinose Y., Nakasone N., Tanabe M. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol Immunol. 1986;30(11):1075–1083. doi: 10.1111/j.1348-0421.1986.tb03037.x. [DOI] [PubMed] [Google Scholar]
- Jesudason M. V., John T. J. Major shift in prevalence of non-O1 and El Tor Vibrio cholerae. Lancet. 1993 Apr 24;341(8852):1090–1091. doi: 10.1016/0140-6736(93)92447-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983 Nov;35(1):253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988 Jun;170(6):2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. Synthesis of cholera toxin is positively regulated at the transcriptional level by toxR. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3471–3475. doi: 10.1073/pnas.81.11.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Taylor R. K., Mekalanos J. J. Cholera toxin transcriptional activator toxR is a transmembrane DNA binding protein. Cell. 1987 Jan 30;48(2):271–279. doi: 10.1016/0092-8674(87)90430-2. [DOI] [PubMed] [Google Scholar]
- Morris J. G., Jr Non-O group 1 Vibrio cholerae: a look at the epidemiology of an occasional pathogen. Epidemiol Rev. 1990;12:179–191. doi: 10.1093/oxfordjournals.epirev.a036052. [DOI] [PubMed] [Google Scholar]
- Peterson K. M., Mekalanos J. J. Characterization of the Vibrio cholerae ToxR regulon: identification of novel genes involved in intestinal colonization. Infect Immun. 1988 Nov;56(11):2822–2829. doi: 10.1128/iai.56.11.2822-2829.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthy T., Garg S., Sharma R., Bhattacharya S. K., Nair G. B., Shimada T., Takeda T., Karasawa T., Kurazano H., Pal A. Emergence of novel strain of Vibrio cholerae with epidemic potential in southern and eastern India. Lancet. 1993 Mar 13;341(8846):703–704. doi: 10.1016/0140-6736(93)90480-5. [DOI] [PubMed] [Google Scholar]
- Sarkar B. L., De S. P., Sircar B. K., Garg S., Nair G. B., Deb B. C. Polymyxin B sensitive strains of Vibrio cholerae non-O1 from recent epidemic in India. Lancet. 1993 Apr 24;341(8852):1090–1090. doi: 10.1016/0140-6736(93)92446-z. [DOI] [PubMed] [Google Scholar]
- Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987 May;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R., Shaw C., Peterson K., Spears P., Mekalanos J. Safe, live Vibrio cholerae vaccines? Vaccine. 1988 Apr;6(2):151–154. doi: 10.1016/s0264-410x(88)80019-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsmuth I. K., Evins G. M., Fields P. I., Olsvik O., Popovic T., Bopp C. A., Wells J. G., Carrillo C., Blake P. A. The molecular epidemiology of cholera in Latin America. J Infect Dis. 1993 Mar;167(3):621–626. doi: 10.1093/infdis/167.3.621. [DOI] [PubMed] [Google Scholar]