Abstract
Objectives
To evaluate the prevalence of atrial thrombi in patients with atrial fibrillation undergoing different anticoagulation regimens before cardioversion; to evaluate the usefulness of transoesophageal echocardiography (TOE) guided cardioversion to prevent thromboembolic complications; and to correlate the presence of atrial thrombi with clinical and echocardiographic data.
Methods
757 consecutive patients admitted as candidates for cardioversion of atrial fibrillation were enrolled in the study. They were divided into four groups: effective conventional oral anticoagulation, short term anticoagulation, ineffective oral anticoagulation or subtherapeutic anticoagulation, and effective oral anticoagulation with a duration of < 3 weeks for various clinical reasons. All patients underwent TOE before cardioversion; in the presence of atrial thrombi or extreme left atrial echo contrast, cardioversion was postponed. The incidence of thromboembolic events was evaluated after cardioversion.
Results
Atrial thrombi were detected in 48 of the 757 (6.3%) patients. No significant differences in the percentage of atrial thrombosis were found in the four study groups. Patients with atrial thrombosis were older and had a higher percentage of mitral prosthetic valves, lower left ventricular ejection fraction, more severe atrial spontaneous echo contrast, and lower Doppler left atrial appendage velocities. 648 patients were scheduled for cardioversion. Cardioversion was successful in 89% of patients without any major thromboembolic event.
Conclusions
The prevalence of atrial thrombosis before cardioversion despite different treatments with anticoagulants is about 7% and a TOE guided approach may prevent the risk of embolic events.
Keywords: atrial thrombosis, atrial fibrillation, transoesophageal echocardiography
Atrial fibrillation is the most common sustained arrhythmia in clinical practice.1,2 Patients with atrial fibrillation have a decreased functional capacity and a substantial risk of thromboembolism.2,3 Therefore, cardioversion is performed to relieve symptoms, improve functional capacity, and reduce embolic risk. However, despite different protocols and anticoagulant regimens, cardioversion related thromboembolism may occur due both to migration of thrombi present at the time of the procedure and to formation (and migration) of thrombi in the post‐cardioversion period.4,5 Transoesophageal echocardiography (TOE) is the method of choice for the study of the anatomy and function of atrial cavities and, in particular, of the left atrial appendage (LAA), allowing atrial thrombosis to be detected with a high degree of accuracy.6 Clinical data are emerging in support of the usefulness of the TOE guided approach to cardioversion to prevent thromboembolic complications or to perform early cardioversion.7,8 Moreover, nowadays several patients with paroxysmal or chronic atrial fibrillation or flutter may be candidates for radiofrequency ablation and in these cases TOE may be very useful. Despite all these observations, few data are available concerning the prevalence of atrial thrombi in patients with atrial fibrillation and different regimens of anticoagulation before cardioversion.9
This is a single centre, observational study of a consecutive series of patients undergoing cardioversion with different anticoagulant regimens.
The objectives of the study were, firstly, to evaluate the prevalence of atrial thrombi in patients with atrial fibrillation receiving various anticoagulant regimens before cardioversion; secondly, to evaluate the usefulness of TOE guided cardioversion to prevent thromboembolic complications; and thirdly, to correlate the presence of atrial thrombi with clinical and echocardiographic data in an unselected anticoagulated population admitted to the hospital for cardioversion.
PATIENTS AND METHODS
Patients admitted to our hospital as candidates for cardioversion of atrial fibrillation or atrial flutter were enrolled in this study. Patients who had haemodynamically unstable atrial fibrillation and had to be rapidly cardioverted were excluded. Seven hundred and fifty nine consecutive patients were therefore enrolled in this observational study.
Study design
Patients were divided into four groups according to their anticoagulant regimens: group 1, effective conventional oral anticoagulation (warfarin with an international normalised ratio (INR) ⩾ 2 for three or four weeks); group 2, short term anticoagulation with intravenous unfractionated heparin (target activated thromboplastin time 1.5–2.5 times the control value) or with intravenous unfractionated heparin associated with warfarin for ⩽ 4 days; group 3, ineffective oral anticoagulation or subtherapeutic anticoagulation (patients with prolonged treatment for > 3 weeks with warfarin with INR < 2 on at least one measurement in the preceding three weeks—in this group heparin was given for 24 hours before cardioversion if the INR was < 2 at hospital admission); and group 4, effective oral anticoagulation with warfarin for < 3 weeks for various clinical reasons (high haemorrhagic risk, decision of the treating physician).
Patients who were free from atrial thrombosis were scheduled for cardioversion. In the presence of atrial thrombi cardioversion was postponed and in cases of severe spontaneous echo contrast in the left atrial cavity the decision to convert was left to the treating physician. A six month clinical and anticoagulant follow up was performed. TOE was repeated in patients with detected thrombus. Determination of thromboembolic events was based on clinical findings and supported by computed tomography.
The local ethics committee approved the study. Informed consent was obtained from all patients.
Transthoracic and transoesophageal studies
All patients underwent chest radiography and transthoracic echocardiography (Sonos 5500 model 21364A; Philips, Andover, Massachusetts, USA, or Acuson Sequoia C256; Siemens Medical Solutions). Two dimensional measurements were obtained according to the standards of the American Society of Echocardiography.10 Left ventricular volumes and ejection fraction were measured by the area–length method.10
Multiplane TOE was performed within 24 hours of the scheduled cardioversion. Patients received pharyngeal anaesthesia with lidocaine spray. The majority of patients were sedated intravenously with midazolam (2–5 mg intravenously); the probe was then inserted into the oesophagus and connected to the ultrasound unit (model 5500, Philips). Investigations were carried out with the patients in the supine left lateral position.
The probe was initially advanced to a depth of 25–35 cm and then manipulated to optimise imaging of the atria and of the LAA. Left atrial and LAA images were obtained in several planes from 0–180°. All images were recorded while zooming the LAA and optimising gain settings and post‐processing to minimise grey noise artefacts.
According to methods previously described4,11,12 we analysed the following: LAA maximum area in short axis, long axis, and at 120° views (measured by tracing a line starting from the top of the limbus of the left upper pulmonary vein along the entire endocardial border, immediately preceding the beginning of the QRS complex); the number of LAA lobes (a lobe was defined as a visible outpouching from the main tubular body of the LAA); LAA peak filling and peak emptying velocities, obtained by placing the pulsed Doppler sample volume into the outlet of the appendage cavity (average of five cardiac cycles); the presence or absence of left atrial or LAA thrombosis, described as echo dense masses, mobile or immobile, connected to the left atrial or LAA wall; and the presence of spontaneous echo contrast, graded as 0 (none, with no echogenicity), 1+ (mild, with minimal echogenicity located in the LAA or sparsely distributed in the main cavity of the left atrium, which may be detectable only transiently during the cardiac cycle and is imperceptible at operating gain settings for two dimensional echocardiographic analysis), 2+ (mild to moderate, with a denser swirling pattern than in 1+ but with similar distribution, detectable without increased gain settings), 3+ (moderate, with a dense swirling pattern in the LAA, generally associated with a somewhat lower intensity in the main cavity, which may fluctuate in intensity but is detectable constantly throughout the cardiac cycle), and 4+ (severe, with intense echo density and very slow swirling patterns in the LAA, usually with similar density in the main cavity).
Artefact images were considered to be present when location and echogenicity suggestive of reverberations were obtained at different TOE rotational angles. In particular artefacts were supposed to be found in the LAA twice as far from the transducer as an anatomical interface (in particular the partition bend between the left upper pulmonary vein and the LAA).
All images were recorded on videotape and re‐evaluated in the echocardiographic laboratory by two independent observers. Interobserver discrepancies were resolved by two other well trained observers not involved in the study. This consensus was requested in only two cases.
Statistical analysis
All data are expressed as the mean (SD). Data from different groups were compared by Student's unpaired t test. We compared categorical variables by using the χ2 test (with continuity correction for small numbers). A value of p < 0.05 was considered to be significant.
RESULTS
Multiplane TOE was performed in all patients without complications. Two patients (0.3%) who did not tolerate the procedure were excluded from the study.
Table 1 lists clinical characteristics of the study population and duration of their arrhythmia. The main pathological conditions associated with atrial fibrillation were systemic hypertension (21%), mitral valve disease (11%), dilated cardiomyopathy (10%), prosthetic valve (9%), and coronary artery disease (7%). One hundred and thirty six patients (18%) had lone fibrillation and 4% had previous thromboembolic events.
Table 1 Clinical features of the study population (n = 757).
| Men | 508 (67%) |
| Women | 249 (33%) |
| Age (years) | 66 (10) |
| <60 years | 188 (25%) |
| >61 years | 569 (75%) |
| Duration of arrhythmia (634 patients) | 82 (132) (range 3–1000) |
| Unknown | 123 (16.2%) |
| <30 days | 291 (38%) |
| 31–90 days | 190 (25.1%) |
| >91 days | 153 (20.2%) |
| Atrial fibrillation | 639 (84.5%) |
| Atrial flutter | 118 (15.5%) |
Data are number (%) or mean (SD).
According to anatomical data the majority of patients had a multilobed LAA (table 2, fig 1).
Table 2 Transoesophageal echocardiography findings in the study population (n = 757).
| No of LAA lobes | |
| 1 | 198 (26.2%) |
| 2 | 366 (48.3%) |
| 3 | 155 (20.5%) |
| ⩾4 | 38 (5%) |
| Spontaneous echo contrast grade | |
| 0 | 184 (24.3%) |
| 1 | 237 (31.3%) |
| 2 | 172 (22.7%) |
| 3 | 87 (11.5%) |
| 4 | 77 (10.2%) |
| Patients with thrombi | 48 (6.3%) |
| LA thrombi | 2 (4.2%) |
| LAA thrombi | 42 (87.5%) |
| RAA thrombi | 4 (8.3%) |
| Patients with LAA artefacts | 115 (15%) |
LA, left atrium; LAA, left atrial appendage; RAA, right atrial appendage.
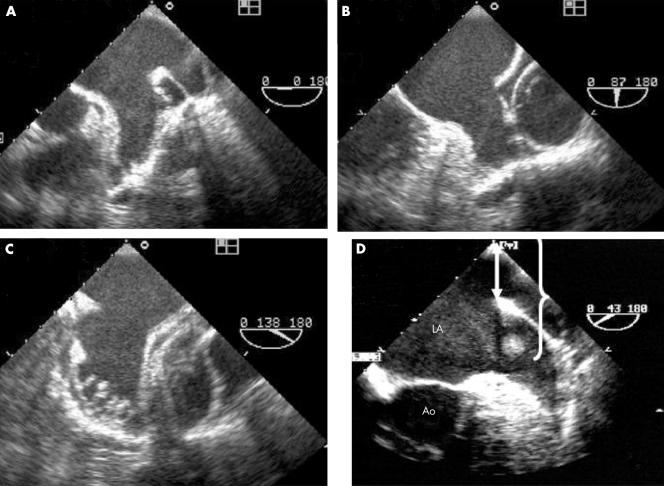
Figure 1 Multiplane transoesophageal echocardiography (TOE) example of (A, B, C) a complete and systematic examination of a left atrial appendage (LAA) without thrombosis and (D) of an LAA artefact. Panels A, B, and C show a multilobed appendage displayed at 0°, 87°, and 138°, respectively. Panel D shows an image inside the LAA that is exactly twice as far from the transducer as from the anatomical interface produced by the bend between the LAA and left pulmonary vein (arrow and brackets). Ao, aorta; LA, left atrium.
Atrial thrombi were detected in 48 patients (6.3%) (table 2); in the majority of patients they were located in the LAA (fig 2) and in a minority in the right atrial appendage (four patients) or the left atrium (two patients). Size (all thrombi were small) and mobility (34 were immobile and 14 mobile) were similar in the four study groups.
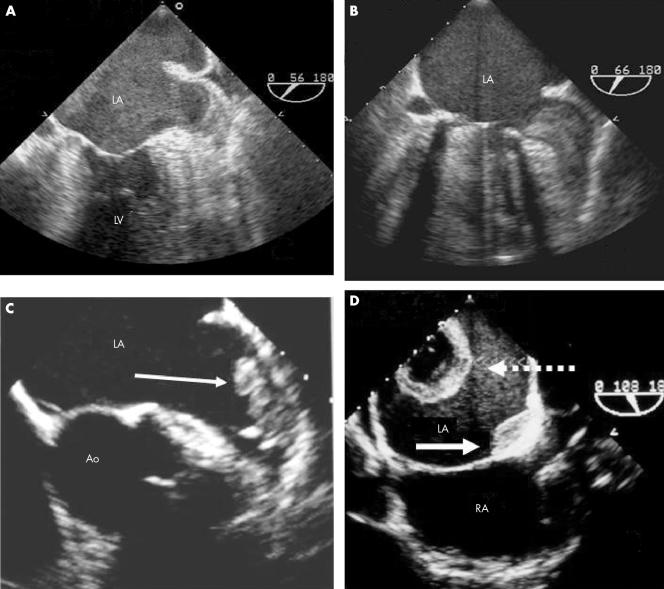
Figure 2 Four transoesophageal examples of LA thrombosis: (A) extreme smoke‐like effect associated with sludge in the LAA; (B) soft LAA thrombus; (C) organised LAA thrombus (arrow); (D) thrombus located in the posterior wall of the LA with an atypical configuration (cavity inside the thrombus) (dashed arrow) associated with a second thrombus (arrow) located at the atrial septum level. LV, left ventricle; RA, right atrium.
Severe spontaneous echo contrast was detected in 77 patients and 53 presented with a distinct intermediate stage of thrombosis termed “sludge” (extreme echo contrast without clot formation) (fig 2).
Artefacts were observed in 115 patients (table 2); by definition their position was precisely twice as far from the transducer (4.8 (1.1) cm) as from the partition bend between the left upper pulmonary vein and left appendage (2.26 (0.59) cm), suggesting a reverberation (fig 1).
By selection and inclusion criteria 486 (64.2%) patients composed group 1, 142 (18.8%) group 2, 51 (6.7%) group 3, and 78 (10.3%) group 4. Table 3 shows the percentages of thrombi detected in each group of the study. No significant differences in the prevalence of atrial thrombosis and in the severity of spontaneous echo contrast were found between the four study groups. On the basis of the presence (group T) of absence (group NT) of left atrial thrombi the study population was divided into two groups (table 4). Patients in group T were older and had a higher percentage of mitral prosthetic valves, lower left ventricular ejection fraction, more severe atrial spontaneous echo contrast, and lower Doppler LAA velocities.
Table 3 Clinical and TOE findings in the four groups of patients.
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| Number of patients | 486 | 142 | 51 | 78 |
| Age (years) | 65.9 (9.1) | 66.4 (11.3) | 65.4 (10.4) | 68.1 (10) |
| Arrhythmia duration (days) | 104.8 (137)* | 36.3 (108) | 42.6 (85.2) | 37.1 (127) |
| Atrial thrombosis | 32 (6.6%) | 8 (5.6%) | 1 (2%) | 7 (9%) |
| SEC grade | ||||
| 0 | 109 (22.4%) | 36 (25.4%) | 14 (27.5%) | 25 (32.1%) |
| 1 | 146 (30%) | 47 (33.1%) | 22 (43.1%) | 22 (28.2%) |
| 2 | 120 (24.7%) | 33 (23.2%) | 5 (9.8%) | 14 (17.9%) |
| 3 | 64 (13.2%) | 7 (4.9%) | 5 (9.8%) | 11 (14.1%) |
| 4 | 47 (9.7%) | 19 (13.4%) | 5 (9.8%) | 6 (7.7%) |
| ECV not performed because of severe SEC | 34 (7%) | 11 (7.7%) | 2 (4%) | 6 (7.7%) |
Data are number (%) or mean (SD).
*p<0.05 versus groups 2, 3, and 4.
ECV, electrical cardioversion; SEC, spontaneous echo contrast.
Table 4 Clinical and echocardiographic features of patients with (group T) and without (group NT) atrial thrombi.
| Group T | Group NT | |
|---|---|---|
| Age | ||
| <60 years | 4 (8.3%) | 184 (26%) |
| >60 years | 44 (91.7%)* | 525 (74%) |
| Atrial flutter | 5 (10.4%) | 113 (15.9%) |
| Atrial fibrillation | 43 (89.6%) | 596 (84.1%) |
| Mitral prosthetic valve | 7 (14.6%)* | 32 (4.5%) |
| Left atrial dimension (mm) | 48 (7.2) | 45.7 (7.4) |
| LVEF (%) | 49.7 (13)* | 54.7 (11.3) |
| SEC grade | ||
| 0 | 0 (0%)* | 184 (26%) |
| 1 | 5 (10.4%)* | 232 (32.7%) |
| 2 | 9 (18.8%)* | 163 (23%) |
| 3 | 13 (27.1%)* | 74 (10.4%) |
| 4 | 21 (43.8%)* | 56 (7.9%) |
| LAA short axis(cm2) | 6 (2.5) | 5.7 (2.3) |
| LAA long axis (cm2) | 5.9 (2.6) | 5.3 (2.2) |
| LAA 120° (cm2) | 7.3 (2.4) | 6.3 (2.6) |
| LAA inflow velocity (cm/s) | 26 (15.3)* | 40.1 (17.7) |
| LAA outflow velocity (cm/s) | 23.1 (12.4)* | 35.6 (16.7) |
Data are number (%) or mean (SD).
*p<0.05 versus group NT.
LVEF, left ventricular ejection fraction.
In 109 patients (48 with atrial thrombosis, 53 with sludge, and 8 for clinical reasons) cardioversion was postponed. Six hundred forty eight patients were scheduled for cardioversion: 16 (2.1%) had spontaneous cardioversion before any procedure, 76 (11.7%) had pharmacological cardioversion, 506 (78%) had electrical cardioversion, and 50 (7.7%) had electrical cardioversion during a transcatheter ablation procedure.
Cardioversion was successful in 582 (89.8%) patients. No major thromboembolic events occurred; one patient had a brief episode (12 hours) of hemianopsia that resolved completely with a normal head computed tomogram.
Twenty three patients who had not been cardioverted because of atrial thrombosis or sludge underwent a second TOE after accurate anticoagulation (warfarin with INR between 2.5–3.5 eventually associated with antiplatelet agents) for two months; four of these patients had a third TOE because of thrombus persistence. Atrial thrombosis or sludge resolved in 20 of them, who were then scheduled for cardioversion. Cardioversion was obtained without thromboembolic complications.
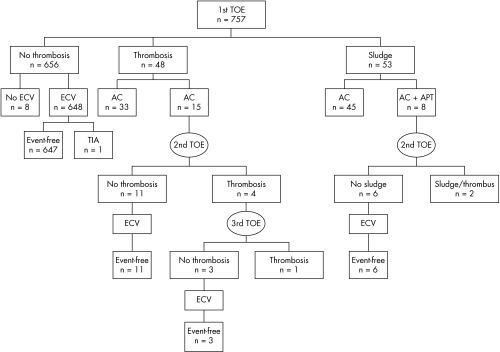
No bleeding event occurred in the study population. Figure 3 summarises the decision making flow chart for the study population, results of TOE, and outcomes.
Figure 3 Decision making flow chart according to results of TOE and clinical outcomes. AC, anticoagulation treatment; APT, antiplatelet treatment; ECV, electrical cardioversion; TIA, transient ischaemic attack.
DISCUSSION
This is the first observational study of the prevalence of left atrial thrombosis in a consecutive series of a patients undergoing cardioversion with different anticoagulant regimens. The main findings of this study are: firstly, that the prevalence of left atrial thrombi is about 7% and is independent of the anticoagulant regimen; secondly, that TOE guided cardioversion in this heterogeneous anticoagulated population may prevent the risk of embolic events; and thirdly, that clinical and echocardiographic data may facilitate the recognition of patients with a higher incidence of left atrial thrombi.
TOE accurately detects atrial thrombosis and is more sensitive than transthoracic echocardiography.6 Several studies with different anticoagulant regimens examined the incidence of left atrial thrombi before cardioversion. Manning et al13 reported that in patients with short term anticoagulation the incidence of atrial thrombi was 13%. In their study TOE showed 14 atrial thrombi in 12 of 94 patients with atrial fibrillation (> 48 hours' duration). Seidl et al14 reported a 7.7% incidence of left atrial thrombi in a consecutive series of 1076 patients scheduled for electrical cardioversion with effective anticoagulation (> 3 weeks' duration). In the ACUTE (assessment of cardioversion using transoesophageal echocardiography) pilot study,15 cardioversion was postponed in seven (13%) patients undergoing a TOE guided approach with short term anticoagulation. More recently Corrado et al16 showed that before cardioversion subtherapeutic levels of anticoagulation, even with a conventional anticoagulant approach, may expose patients to systemic thromboembolism.17 In their study left atrial thrombi were identified in 9.8% of patients with subtherapeutic INR, and these data are very consistent with another larger retrospective study of patient with subtherapeutic INR.18
Despite all these studies, ours is the first report concerning routine TOE screening for the detection of left atrial thrombi in a consecutive series of patients admitted to hospital with different anticoagulant schemes. In our study, as previously described,19 we used a very accurate protocol for multiplane TOE detection, trying to investigate the LAA as accurately as possible. No significant difference in the prevalence of atrial thrombosis was found in patients with four different anticoagulant schemes: effective conventional oral anticoagulation (6.6%), short term anticoagulation (5.6%), ineffective or subtherapeutic anticoagulation (1.9%), and effective oral anticoagulation for < 3 weeks (9%). These results are very similar to those of the previously cited studies confirming that despite treatment with various anticoagulants the prevalence of left atrial thrombi is about 7%.
Even though in anticoagulated patients (particularly with long term conventional treatment), thromboembolism may more often arise as a consequence of the effects of cardioversion than from the dislodgement of a pre‐existing thrombi (this explains the very low incidence of thromboembolic events after conventional cardioversion despite a 7% prevalence of thrombi), our data suggest caution in the selection of candidates for cardioversion and underline the role of TOE in these cases.5 In general the combination of three weeks of warfarin followed by TOE is considered unnecessary and relatively cost ineffective.17 Manning,9 however, suggested that an expanded exploration of the role of TOE guided cardioversion may be reconsidered after the publication of the high prevalence of thrombi by Seidl et al14 in patients receiving a conventional three week anticoagulation before cardioversion. Our data not only confirm the data of Seidl et al14 but also show that the incidence of thromboembolism is almost cancelled with a TOE guided approach in an unselected series of patients undergoing cardioversion with different anticoagulant schemes. Moreover, even if we do not suggest extending the TOE guided approach to all patients undergoing cardioversion, our data clearly show that several clinical and echo Doppler variables may facilitate the presence of atrial thrombosis. Age, poor left ventricular function, and mitral valve prosthesis are correlated with a higher incidence of atrial thrombi.20 Therefore, in these cases even in patients with conventional long term anticoagulation TOE guided cardioversion may be suggested; this may be particularly applicable for mitral valve prosthesis, in which TOE is also useful for prosthetic valve function evaluation. Another reason for more extended use of TOE guided cardioversion is the high prevalence of patients who may have subtherapeutic anticoagulation.21,22 Lastly, at least in centres that perform complex invasive procedures in association with cardioversion, routine use of TOE may facilitate the selection of these candidates and the decision to immediately convert one procedure to another.
Conventional anticoagulation and a TOE guided strategy with short term anticoagulation have been shown to have similar low rates of embolisation (approximately 0.8% in the majority of studies).8,23 In our study no major embolic event occurred (0%) and only one patient (0.15%) had a minor symptom related to cardioversion (hemianopsia that resolved completely). Our favourable results may be explained by two main reasons. Firstly, atrial thrombi were very accurately researched by the multiplane TOE approach, which is particularly useful in patients with multilobed LAA. Secondly, even though there is no consensus on the role of a very pronounced smoke‐like effect with sludge in the images, we arbitrarily considered these entities to indicate a pre‐thrombotic status and the majority of these patients (53 of 757 equally distributed in the four study groups) were not cardioverted.
In conclusion, despite different anticoagulant schemes before cardioversion the prevalence of left atrial thrombi is about 7% and a TOE guided approach may prevent the risk of embolic events. Even though several studies have documented that the TOE guided protocol is not necessary and relatively cost ineffective after conventional (three weeks) anticoagulation, our data suggest that the TOE guided approach may be more fully explored and used. This is particularly true in centres that receive a very heterogeneous population with different anticoagulant schemes, in patients with suspected subtherapeutic anticoagulation, and in patients scheduled for complex electrophysiological procedures associated with cardioversion.
Abbreviations
ACUTE - assessment of cardioversion using transoesophageal echocardiography
INR - international normalised ratio
LAA - left atrial appendage
TOE - transoesophageal echocardiography
References
- 1.Ryder K M, Benjamin E J. Epidemiology and significance of atrial fibrillation. Am J Cardiol 199984131R–8R. [DOI] [PubMed] [Google Scholar]
- 2.Krahn A D, Manfreda J, Tate R B.et al The natural history of atrial fibrillation: incidence, risk factors and prognosis in the Manitoba follow‐up study. Am J Med 199598476–484. [DOI] [PubMed] [Google Scholar]
- 3.Daoud E G, Weiss R, Bahu M.et al Effect of an irregular ventricular rhythm on cardiac output. Am J Cardiol 1996781433–1436. [DOI] [PubMed] [Google Scholar]
- 4.Fatkin D, Kuchar D L, Thorburn C W.et al Transesophageal echocardiography before and during direct current cardioversion of atrial fibrillation: evidence of “atrial stunning” as a mechanism of thromboembolic complications. J Am Coll Cardiol 199423307–316. [DOI] [PubMed] [Google Scholar]
- 5.Black I V, Fatkin D, Sagar K B.et al Exclusion of atrial thrombus by transesophageal echocardiography does not preclude embolism after cardioversion of atrial fibrillation: a multicenter study. Circulation 1994892509–2513. [DOI] [PubMed] [Google Scholar]
- 6.Manning W J, Weintraub R M, Wakmonski C A.et al Accuracy of transesophageal echocardiography for identifying left atrial thrombi: a prospective, intraoperative study. Ann Intern Med 1995123817–822. [DOI] [PubMed] [Google Scholar]
- 7.Silverman D I, Manning W J. Role of echocardiography in patients undergoing elective cardioversion of atrial fibrillation. Circulation 199898479–486. [DOI] [PubMed] [Google Scholar]
- 8.Klein A L, Grimm R A, Murray R D.et al Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med 20043441411–1420. [DOI] [PubMed] [Google Scholar]
- 9.Manning W J, Silverman D I, Seto T B.et al Value of precardioversion transesophageal echocardiography in managing cardioversion of atrial fibrillation [letter]. J Am Coll Cardiol 2002401889. [DOI] [PubMed] [Google Scholar]
- 10.Schiller N B, Shah P M, Crawford M.et al Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. American Society of Echocardiography, committee on standards, subcommittee on quantitation of two‐dimensional echocardiograms. J Am Soc Echocardiogr 19892358–367. [DOI] [PubMed] [Google Scholar]
- 11.Chan S K, Kannam J P, Douglas P S.et al Multiplane transesophageal echocardiographic assessment of left atrial appendage anatomy and function. Am J Cardiol 199576528–530. [DOI] [PubMed] [Google Scholar]
- 12.Rubin D N, Katz S E, Riley M F.et al Evaluation of left atrial appendage anatomy and function in recent‐onset atrial fibrillation by transesophageal echocardiography. Am J Cardiol 199678774–778. [DOI] [PubMed] [Google Scholar]
- 13.Manning W J, Silverman D I, Keighley C S.et al Transesophageal echocardiography facilitated early cardioversion from atrial fibrillation using short‐term anticoagulation: final results of a prospective 4.5‐year study. J Am Coll Cardiol 1995251354–1361. [DOI] [PubMed] [Google Scholar]
- 14.Seidl K, Rameken M, Drögemüller A.et al Embolic events in patients with atrial fibrillation and effective anticoagulation value of transesophageal echocardiography to guide direct‐current cardioversion. J Am Coll Cardiol 2002391436–1442. [DOI] [PubMed] [Google Scholar]
- 15.Klein A L, Grimm R A, Black I W.et al Cardioversion guided by transesophageal echocardiography: the ACUTE pilot study. Ann Intern Med 1997126200–209. [DOI] [PubMed] [Google Scholar]
- 16.Corrado G, Beretta S, Sormani L.et al Prevalence of atrial thrombi in patients with atrial fibrillation/flutter and subtherapeutic anticoagulation prior to cardioversion. Eur J Echocardiogr 20045257–261. [DOI] [PubMed] [Google Scholar]
- 17.Laupacis A, Albers G, Dunn M.et al Antithrombotic therapy in atrial fibrillation. Chest 1992102(4 suppl)426S–33S. [DOI] [PubMed] [Google Scholar]
- 18.Shen X, Li H, Rovang K.et al Prevalence of intra‐atrial thrombi in atrial fibrillation patients with subtherapeutic international normalized ratios while taking conventional anticoagulation. Am J Cardiol 200290660–662. [DOI] [PubMed] [Google Scholar]
- 19.Maltagliati A, Pepi M, Tamborini G.et al Usefulness of multiplane transesophageal echocardiography in the recognition of artifacts and normal anatomical variants that may mimic left atrial thrombi in patients with atrial fibrillation. Ital Heart J 20034797–802. [PubMed] [Google Scholar]
- 20.The Stroke Prevention in Atrial Fibrillation Investigators Predictors of thromboembolism in atrial fibrillation. I. Clinical features of patients at risk. The stroke prevention in atrial fibrillation investigators. Ann Intern Med 199211789–90. [DOI] [PubMed] [Google Scholar]
- 21.Schlicht J R, Davis R C, Naqi K.et al Physician practices regarding anticoagulation and cardioversion of atrial fibrillation. Arch Intern Med 1996156290–294. [PubMed] [Google Scholar]
- 22.Carlsson J, Tebbe U, Rox J.et al Cardioversion of atrial fibrillation in the elderly. ALKK_study Group. Arbeitsgemeinschaft Leitender Kardiologischer Krankenhausaerzte. Am J Cardiol 1996781380–1384. [DOI] [PubMed] [Google Scholar]
- 23.Berkelund C, Orning O. The efficacy of anticoagulant therapy in preventing embolism related to DC electrical cardioversion of atrial fibrillation. Am J Cardiol 196923208–216. [DOI] [PubMed] [Google Scholar]