Abstract
Objective
To evaluate prospectively the impact on left ventricular (LV) remodelling of an intracoronary aspiration thrombectomy device as adjunctive therapy in primary percutaneous coronary intervention (PCI) in patients with anterior ST elevation myocardial infarction (STEMI).
Methods
76 consecutive patients with anterior STEMI (65.3 (11.2) years, 48 men) were randomly assigned to intracoronary thrombectomy and stent placement (n = 38) or to conventional stenting (n = 38) of the infarct related artery. Each patient underwent transthoracic echocardiography immediately after PCI and at six months. At the time of echocardiographic control, major adverse cardiovascular events (MACE) in terms of death, new onset of myocardial infarction, and hospitalisation for heart failure were also evaluated.
Results
After a successful primary PCI, patients in the thrombectomy group achieved a higher rate of post‐procedure myocardial blush grade 3 (36.8% v 13.1%, p = 0.03) and effective ST segment resolution at 90 minutes (81.6% v 55.3%, p = 0.02). Six months after the index intervention, 19 patients (26.8%) developed LV dilatation, defined as an increase in end diastolic volume (EDV) ⩾ 20%: 15 in the conventional group and four in the thrombectomy group (p = 0.006). Accordingly, at six months patients treated conventionally had significantly higher end systolic volumes (82 (7.7) ml v 75.3 (4.9) ml, p < 0.0001) and EDV (152.5 (18.1) ml v 138.1 (10.7) ml, p < 0.0001) than patients treated with thrombectomy. No differences in cumulative MACE were observed (10.5% in the conventional group v 8.6% in the thrombectomy group, not significant).
Conclusion
Compared with conventional stenting, adjunctive aspiration thrombectomy in successful primary PCI seems to be associated with a significantly lower incidence of LV remodelling at six months in patients with anterior STEMI.
Keywords: primary angioplasty, ST elevation myocardial infarction, thrombectomy, Diver CE aspiration catheter, left ventricular remodelling, echocardiography
Left ventricular (LV) remodelling is a precursor of the development of congestive heart failure (CHF) and results in a significantly worse prognosis after an ST elevation myocardial infarction (STEMI).1,2 Recently, it has been shown that LV remodelling occurs even after successful primary percutaneous coronary intervention (PCI) and despite sustained patency of the infarct related artery (IRA).3
In patients with STEMI, primary PCI of the IRA produces excellent epicardial vessel patency and flow. However, primary PCI in lesions with a large thrombotic burden may cause thrombus dislodgment followed by reduced flow and microvascular dysfunction, which is a negative independent predictor of myocardial function recovery and long term survival.4,5
Adjunctive intracoronary thrombectomy used in conjunction with primary PCI has recently emerged as an attractive tool to prevent distal embolisation in the microvasculature.6,7,8,9,10 Recent studies showed that thrombectomy devices can preserve myocardial salvage and microvascular function.11,12,13 On the other hand, reliable data on the application of these mechanical devices in STEMI and their additional benefits in reducing long term complications and clinical events are limited.14
We sought to evaluate the impact on LV remodelling of an intracoronary aspiration thrombectomy device as adjunctive therapy in primary PCI compared with conventional PCI in patients with anterior STEMI.
METHODS
Study population
All patients with anterior STEMI undergoing primary PCI with stenting of a de novo coronary artery lesion were identified and screened for participation in this study.
Anterior STEMI was defined as chest pain lasting more than 30 minutes in conjunction with new persistent ST segment elevation of ⩾ 1 mm in at least two contiguous anterior leads.
To be eligible for the study, patients with anterior STEMI needed to be > 18 years old and to have an identifiable thrombus on IRA at coronary angiography. Angiographically identifiable thrombus was defined as the presence of a filling defect within the coronary lumen, surrounded by contrast material, seen in multiple projections and in the absence of calcium within the filling defect, or as the persistence of contrast material within the coronary lumen.15 Exclusion criteria were previous myocardial infarction (MI) or coronary artery bypass grafting, three vessel coronary artery disease, severe valvar heart disease, TIMI (thrombolysis in myocardial infarction) grade 2 or 3 flow at the time of initial angiography, or unsuccessful PCI defined as no antegrade flow or > 50% residual stenosis in the IRA.
Study protocol
Primary PCI was performed within 12 hours after the onset of symptoms through a 7 French sheath in the femoral artery, according to standard clinical practice.
All patients were randomly assigned in a 1:1 ratio to intracoronary aspiration thrombectomy and stent placement or to conventional stenting of the IRA.
The TIMI flow and myocardial blush grade (MBG) were analysed off line.16,17 The sum of ST segment elevations was measured manually 80 ms after the end of the QRS complex (J point) in anterior leads at baseline and 90 minutes after the index procedure. Resolution of ST segment elevation was expressed as a percentage of the initial ST segment elevation. Resolution of > 70% was defined as indicative of good myocardial reperfusion.18
All patients were treated with aspirin 300 mg orally and heparin 8000 IU intravenously before the procedure and with abciximab as a 0.25 mg/kg bolus and 0.125 μg/kg/min intravenous infusion immediately before the revascularisation and continued for 12 hours.
Patients subsequently received heparin for 48 hours, aspirin 100 mg daily, and ticlopidine (250 mg orally twice a day for at least four weeks) or clopidogrel (loading dose of 300 mg followed by 75 mg/day for at least four weeks). Other adjunctive pharmacotherapy was administered at the discretion of the operator.
The protocol was accepted by the institutional ethical board and was performed in accordance with institutional guidelines and the Declaration of Helsinki. All patients gave written informed consent before entering the study.
Diver aspiration thrombectomy catheter
The Diver CE aspiration thrombectomy catheter (Invatec, Brescia, Italy) has a central aspiration lumen running through its full length, a soft atraumatic tip with multiple side holes communicating with the central lumen, and a radio‐opaque marker band located 1 mm from its distal end. A luer lock‐type hub in the proximal end allows the connection of a stopcock and a syringe for blood aspiration and clot removal. The catheter has a rapid exchange design, with the guidewire lumen running from the distal tip to 22.7 cm proximally in the catheter body, where the exit port is located.
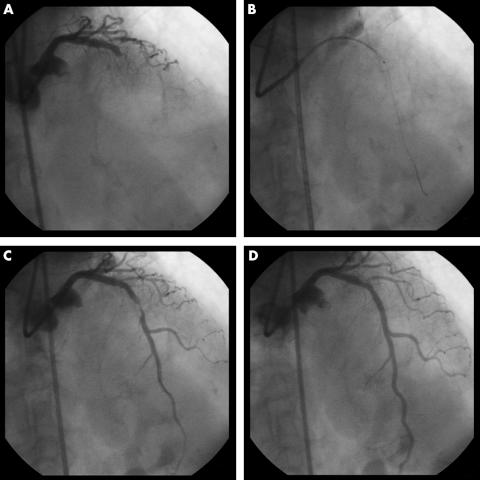
The Diver catheter was inserted through a 7 French guiding catheter over a 0.014 inch guidewire and was gently advanced to the culprit lesion (fig 1).
Figure 1 (A) Left coronary angiography showing a total occlusion (TIMI (thrombolysis in myocardial infarction) flow grade 0) in the middle tract of the left anterior descending (LAD) coronary artery. (B) The Diver CE aspiration thrombectomy catheter was advanced to the distal LAD. (C) Angiography after intracoronary aspiration showing TIMI 2 coronary blood flow and a significant stenosis in the middle tract of the LAD. (D) After successful direct stenting, a final TIMI flow grade of 3 was achieved.
Echocardiographic examination
Each patient was examined by transthoracic echocardiography immediately after PCI and six months after the index infarction with a commercially available machine (Vivid 7; GE Medical Systems) equipped with 2.5 and 3.5 MHz transducers and stored on videotape for later analysis.
LV end systolic volume (ESV), end diastolic volume (EDV), and ejection fraction (EF) were determined from apical two and four chamber views by Simpson's biplane formula. Volumes were normalised for body surface area and expressed as indices. LV dilatation was defined as an increase in EDV ⩾ 20%, based on repeated measurements in individual patients.3,19
LV wall motion score index (WMSi) was calculated by using a 16 segment model. Wall motion for each segment was graded semiquantitatively as normokinesia = 1, hypokinesia = 2, akinesia = 3, and dyskinesia = 4. WMSi was calculated by summing the scores for each segment and dividing by the number of analysed segments.
Interobserver and intraobserver variability
To test the reproducibility of the echocardiographic data, ESV, EDV, EF, and WMSi were measured by two experienced observers and twice by each observer in 20 randomly selected patients.
Interobserver coefficients of variation for measuring ESV, EDV, EF, and WMSi were 8%, 5%, 8%, and 3%, respectively. Intraobserver coefficients of variation for repeated measurements were 5%, 3%, 5%, and 2%, respectively.
Clinical follow up
Patients were also followed up clinically at the time of echocardiographic control. The end point of interest was major adverse cardiovascular events (MACE) (death, new onset of MI, and hospitalisation for CHF) at six months. All events were confirmed by review of the hospital chart or the physician's record.
Statistical analysis
All continuous variables are expressed as mean (SD) and are analysed by Student's t test. Categorical variables are expressed as number of patients and percentages and are analysed by the χ2 or Fisher's exact test, as appropriate. Differences were considered significant at p ⩽ 0.05. Analysis of variance with the Scheffé post hoc test was used to analyse repeated measures of LV volumes, LV EF, and WMSi.
A backwards stepwise multivariable logistic regression analysis of clinical and angiographic variables was also used to assess further the independent predictive value of aspiration thrombectomy on LV remodelling.
Data were statistically analysed with StatView version 5.0 (SAS Institute, Cary, North Carolina, USA).
RESULTS
Baseline characteristics
Seventy six consecutive patients (65.3 (11.2) years, 48 men) with anterior STEMI underwent a primary PCI at 6.8 (2.3) hours from symptoms onset. Thrombectomy followed by stent implantation was performed in 38 (thrombectomy group) patients and conventional stenting in 38 (conventional group).
Patients who underwent aspiration thrombectomy were less likely to have a family history of coronary artery disease (36.8% in the conventional group v 13.1% in the thrombectomy group, p = 0.03) and had significantly lower cholesterol concentrations (4.17 (0.28) v 4.32 (0.39) mmol/l, p = 0.04) (table 1). Other clinical characteristics were similar between the two groups.
Table 1 Clinical characteristics of the study patients.
| Total population (n = 76) | Conventional group (n = 38) | Thrombectomy group (n = 38) | |
|---|---|---|---|
| Age (years) | 65.3 (11.2) | 64.6 (12.5) | 66.7 (14.1) |
| Men | 48 (63.1%) | 21 (55.3%) | 27 (71%) |
| Risk factors | |||
| Hypertension | 34 (44.7%) | 19 (50%) | 15 (39.5%) |
| Diabetes | 16 (21%) | 7 (18.4%) | 9 (23.7%) |
| Smoking | 17 (22.4%) | 10 (26.3%) | 7 (18.4%) |
| Obesity | 4 (5.3) | 1 (2.6) | 3 (7.9) |
| Family history of CAD | 19 (25%) | 14 (36.8%) | 5 (13.1%)* |
| Cholesterol (mmol/l) | 4.22 (0.70) | 4.32 (0.39) | 4.17 (0.28)* |
| Triglycerides (mmol/l) | 1.38 (0.42) | 1.41 (0.29) | 1.37 (0.44) |
| Renal failure | 8 (10.5%) | 3 (7.9%) | 5 (13.1%) |
| Killip class III | 19 (25%) | 11 (28.9%) | 8 (21%) |
| Previous PCI | 11 (14.5%) | 4 (10.5%) | 7 (18.4%) |
| Symptoms to balloon (hours) | 7.3 (1.3) | 7.6 (1.8) | 7.2 (1.9) |
| Medications at follow up | |||
| ACE‐I/ARBs | 67 (88.1%) | 31 (81.6%) | 36 (94.7%) |
| Aldosterone blockers | 41 (53.9%) | 22 (57.9%) | 19 (50%) |
| β Blockers | 58 (76.3%) | 27 (71%) | 31 (81.6%) |
| Statins | 68 (89.5%) | 36 (94.7%) | 32 (84.2%) |
| Aspirin | 74 (97.4%) | 37 (97.4%) | 37 (97.4%) |
Data are mean (SD) or number (%).
*p<0.05 between conventional and thrombectomy groups.
ACE‐I, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CAD, coronary artery disease; PCI, percutaneous coronary angioplasty.
Procedural characteristics
Among the 38 patients undergoing an intracoronary thrombectomy procedure, one had a coronary dissection immediately proximal to the culprit lesion. This complication was successfully treated with a stent implantation covering both the dissection and the culprit lesion.
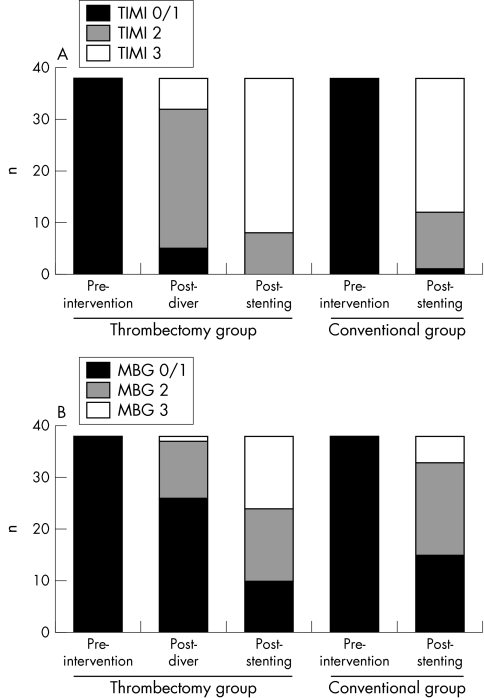
Compared with the conventional group, patients treated with aspiration thrombectomy had a significantly higher incidence of direct stenting (92.1% v 5.3%, p < 0.0001) (table 2). Patients in the thrombectomy group also had a higher rate of post‐procedural MBG 3 (36.8% v 13.1%, p = 0.03) (fig 2). No statistical differences were observed in final TIMI flow grade between the two groups (fig 2). Other procedural characteristics and angiographic measurements were similar between groups (table 2).
Table 2 Procedural characteristics of the study population.
| Total population (n = 76) | Conventional group (n = 38) | Thrombectomy group (n = 38) | |
|---|---|---|---|
| Location of infarct related artery | |||
| Left main | 1 (1.3%) | 0 | 1 (2.6%) |
| LAD/diagonal branch | 75 (98.7%) | 38 (100%) | 37 (97.4%) |
| Two vessel CAD | 16 (21%) | 7 (18.4%) | 9 (23.7%) |
| Intra‐aortic balloon pump | 7 (9.2%) | 5 (13.1%) | 3 (7.9%) |
| Bifurcation | 14 (18.4%) | 5 (13.1%) | 9 (23.7%) |
| Lesion length (mm) | 14.0 (5.3) | 14.4 (6.6) | 13.9 (7.5) |
| Vessel size (mm) | 2.9 (0.6) | 2.9 (0.5) | 2.9 (0.6) |
| MLD before intervention (mm) | 0.9 (0.3) | 0.9 (0.4) | 0.9 (0.3) |
| Procedure | |||
| Direct stenting | 37 (48.7%) | 2 (5.3%) | 35 (92.1%)** |
| Postdilatation | 5 (6.6%) | 3 (7.9%) | 2 (5.3%) |
| Stent type | |||
| Bare metal | 33 (43.4%) | 17 (44.7%) | 16 (42.1%) |
| Drug eluting | 43 (56.6%) | 21 (55.3%) | 22 (57.9%) |
| MLD after intervention (mm) | 2.9 (0.8) | 2.9 (0.7) | 2.9 (0.1) |
| Diameter stenosis after intervention (%) | 3.7 (4.8) | 3.6 (5.4) | 3.7 (5.1) |
| Peak creatine kinase‐MB (U/l) | 161 (134) | 167 (129) | 146 (122) |
| 90° ST segment resolution | 52 (68.4%) | 21 (55.3%) | 31 (81.6)* |
Data are mean (SD) or number (%).
*p<0.05; **p<0.0001 between conventional and thrombectomy groups.
LAD, left anterior descending; MLD, minimum lumen diameter.
Figure 2 (A) TIMI flow and (B) myocardial blush grade (MBG) before and after intervention in both groups.
Mean summed ST segment elevation was 25.3 (11.1) mV before PCI and 7.8 (6.4) mV after the procedure resulting in 17.5 (12.2) mV absolute ST segment resolution. The relative resolution was 70.3 (22.6)%. Mean time between reperfusion and the second ECG was 105 (28) minutes. Compared with patients in the conventional group, patients treated with thrombectomy achieved a significantly higher effective ST segment resolution 90 minutes after the PCI procedure (81.6% v 55.3%, p = 0.02) (table 2).
Echocardiographic data
Six months (187 (23) days) after the index intervention, two patients died (both in the conventional group) and three patients of the thrombectomy group declined the follow up echocardiogram. Therefore, the echocardiographic follow up was completed in 71 of 76 (93.4%) patients.
Nineteen patients (26.8%) developed LV dilatation at six months, 15 in the conventional group and four in the thrombectomy group (p = 0.006).
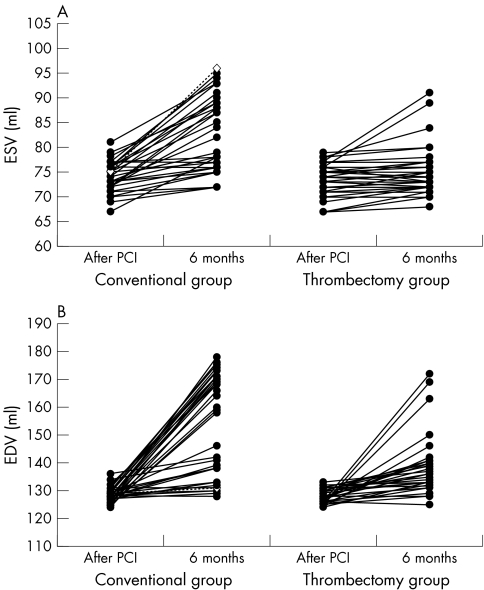
Baseline ESV (74 (3) ml v 73.7 (3.3) ml, p = 0.69) and EDV (128.5 (2.5) ml v 127.9 (2.4) ml, p = 0.3) were similar between patients who received a conventional PCI and those who underwent thrombectomy, respectively (fig 3). At six months, patients treated conventionally had significantly higher ESV (82 (7.7) ml v 75.3 (4.9) ml, p < 0.0001) and EDV (152.5 (18.1) ml v 138.1 (10.7) ml, p < 0.0001) than the patients treated with aspiration thrombectomy (fig 3).
Figure 3 Changes in (A) left ventricular end systolic volume (ESV) and (B) end diastolic volume (EDV) from the index percutaneous coronary intervention (PCI) to the six month follow up in patients treated with thrombectomy and conventionally.
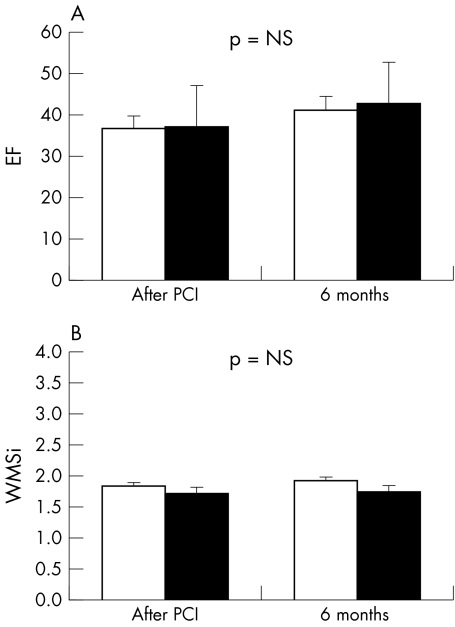
No differences were found in either EF or WMSi between the two groups at baseline and during the follow up period (fig 4).
Figure 4 Changes in (A) left ventricular ejection fraction (EF) and (B) wall motion score index (WMSi) from the index PCI to the six month follow up in the thrombectomy (black bars) and conventional groups (white bars). NS, not significant.
In the multivariate analysis, including the baseline clinical and angiographic parameters, the only independent predictors of LV remodelling were random assignment to conventional PCI (versus thrombectomy, p = 0.04) and a postprocedural MBG grade of 0 (p = 0.07).
Clinical outcomes
Incidences of death, recurrent MI, hospitalisation for CHF, and cumulative MACE at six months after the index intervention were similar in all patients with anterior STEMI, whether they were in the thrombectomy or the conventional group (table 3).
Table 3 Major adverse cardiovascular events (MACE) at six months.
| Conventional group (n = 38) | Thrombectomy group (n = 35) | |
|---|---|---|
| Death | 2 (5.3%) | 0 |
| New onset MI | 0 | 1 (2.8%) |
| Hospitalisation for CHF | 3 (7.9%) | 2 (5.7%) |
| MACE | 4 (10.5%) | 3 (8.6%) |
CHF, congestive heart failure; MI, myocardial infarction.
DISCUSSION
The major findings of the present study are, firstly, that compared with conventional PCI, adjunctive intracoronary aspiration thrombectomy during primary angioplasty in patients with anterior STEMI can safely improve myocardial tissue perfusion; and secondly, that this is associated with a significant decrease in the incidence of LV remodelling at six months; however, overall MACE rates are similar.
LV remodelling after successful primary PCI
LV dilatation carries a grim prognosis after a STEMI.1 The most favourable interventions to limit or prevent post‐MI LV remodelling are those addressed to obtain the earliest and most sustained IRA patency.20,21,22 Although mechanical reperfusion for STEMI significantly improves coronary epicardial flow, it does not necessarily mean that microvascular flow and myocardial perfusion are normalised. In fact, especially after conventional primary PCI, it is possible that distal microemboli cause microvascular obstruction and limit distal tissue perfusion.
Bolognese et al3 reported that LV dilatation is significant in about 30% of patients with STEMI successfully treated with primary PCI, comparable with the rate of 34% observed in patients receiving intravenous thrombolysis.23 In addition, as in patients receiving thrombolysis, patients with STEMI treated with primary PCI apparently have subsequent LV remodelling despite spontaneous recovery of regional and global LV function, suggesting that the incidence of post‐MI LV dilatation is independent of which reperfusion strategy has been used.3,23 In our series, LV dilatation occurred in 27% of patients after MI treated with mechanical reperfusion. Since we enrolled only patients with anterior STEMI and the extent of LV dilatation depends not only on the infarct size but also on the type of infarct healing and coexistent LV wall stress, this incidence can be considered to be relatively low.24 However, our observation of a significant reduction in the occurrence of LV remodelling after an adjunctive thrombectomy in successful primary PCI highlights that optimal reperfusion entails both early and sustained epicardial patency and optimal tissue reperfusion.
Myocardial tissue perfusion and LV remodelling
The clinical relevance of distal tissue perfusion has been shown in several studies that have used surrogate markers such as ST segment resolution or other non‐invasive diagnostic modalities.4,5,25,26,27,28,29,30,31 Patients with impaired microperfusion have increased early and late mortality, larger irreversible myocardial injury, and consequently a higher incidence of adverse LV remodelling leading to CHF.4,5,32,33,34
In our study, both indices of microvascular flow restoration after PCI were significantly more favourable in the thrombectomy group than in the conventional group. ST segment resolution and MBG are both highly specific indicators of myocardial tissue perfusion.35,36 ST segment recovery in patients with STEMI represents both reversal of ischaemia and interruption of infarction and it provides important prognostic information.26,27,28,37 In addition, the MBG may reflect the patency of the microvasculature and has been found to have an additive predictive value for long term mortality beyond Killip class, TIMI grade flow, and LV EF and to relate to the enzymatic infarct size after primary PCI.33,38 However, LV remodelling seems to be induced by factors other than initial infarct size, as also suggested by the non‐significant difference in CK‐MB concentrations between the two groups in the present study.3
Effects of thrombectomy during primary PCI
Because of the limited ability of coronary stenting to attenuate the no reflow phenomenon, mechanical or pharmacological approaches have been added to conventional PCI. There are several systems for intracoronary thrombectomy that differ considerably in construction, principles of operation, and management. Most of these systems have reported efficacies for epicardial flow restoration similar to that seen with the aspiration device used in this study.39,40,41 Aspiration thrombectomy devices are characterised by a simple manual aspiration that can safely extract atherothrombotic material from the target lesion, thus restoring flow and increasing the patency rate of the IRA before stenting and myocardial perfusion, as recently shown by the randomised evaluation of the effect of mechanical reduction of distal embolisation by thrombus aspiration in primary and rescue angioplasty (REMEDIA) study.42
Although thrombectomy during primary PCI initially resulted in high procedural success rates and improved outcomes in patients with STEMI with angiographically large intracoronary thrombus, results of recent clinical trials are discouraging.39
The enhanced myocardial efficacy and recovery by aspiration of liberated debris (EMERALD) trial failed to show any improvement in microvascular flow, reperfusion success, infarct size, and event‐free survival in patients with STEMI undergoing primary PCI with a balloon occlusion and aspiration distal microcirculatory protection system.43 In the large AngioJet rheolytic thrombectomy in patients undergoing primary angioplasty for acute myocardial infarction (AiMI) trial, 480 patients with STEMI were randomly assigned to either AngioJet plus direct PCI or to direct PCI alone(Ali A, Annual international meeting TCT, Washington, 2004). This multicentre, prospective clinical trial did not find a significant reduction in MI size with the use of thrombectomy. Moreover, while this study was not powered for clinical end points, group mortality differed significantly, with a remarkably low mortality in the control arm. Differences in both thrombectomy systems and patient selection can explain such divergences with our findings. In the AiMI trial, TIMI flow grade 0 was found in baseline angiography of only 55% of patients in the thrombectomy group and in 53% of controls, and an absent or minimal thrombus was reported in 23% and in 27%, respectively. Conversely, the present study enrolled only patients with angiographically identifiable thrombus, where a thrombectomy device should be more useful. The present study was not intended or powered to evaluate the potential clinical benefits of aspiration thrombectomy in patients with STEMI at six months.
Impact of thrombectomy on LV remodelling
Kondo et al44 described the effects of intracoronary thrombectomy on LV remodelling in 109 patients with STEMI who underwent PCI plus thrombus aspiration with the Rescue catheter and 86 controls treated with conventional PCI. The incidence of LV remodelling, evaluated by cine angiography, was significantly lower in the thrombectomy group at six months. No differences were observed in cardiac events, including death, reinfarction, and target vessel revascularisation or changes in EF during follow up. Even in our series, only a slight but not significant improvement in EF and WMSi was observed among patients without progressive dilatation throughout follow up. These findings may be related to the presence and extension of viable myocardium, the severity of LV dilatation, and the accuracy of echocardiography in assessing EF.45,46 Kondo et al44 reported the outcomes of a retrospective cohort of unselected types of STEMI, compared with our study; importantly, only 66% of their patients had an initial TIMI 0 flow and the authors did not specify whether the Rescue system was used in the presence of visible thrombus only. In addition, a coronary stent was implanted in only 34% of patients randomly selected for thrombectomy and in 67% of controls, and none of the patients received glycoprotein IIb/IIIa inhibitors.
Conclusion
Compared with conventional stenting, in patients with intracoronary visible thrombus, pretreatment with aspiration thrombectomy during primary PCI improves the parameters of myocardial tissue perfusion. These findings appear to be associated with a significantly lower incidence of LV remodelling at six months in patients presenting with anterior STEMI.
Abbreviations
AiMI - AngioJet rheolytic thrombectomy in patients undergoing primary angioplasty for acute myocardial infarction
CHF - congestive heart failure
EDV - end diastolic volume
EF - ejection fraction
ESV - end systolic volume
EMERALD - enhanced myocardial efficacy and recovery by aspiration of liberated debris
IRA - infarct related artery
LV - left ventricular
MACE - major adverse cardiovascular events
MBG - myocardial blush grade
MI - myocardial infarction
PCI - percutaneous coronary intervention
REMEDIA - randomised evaluation of the effect of mechanical reduction of distal embolisation by thrombus aspiration in primary and rescue angioplasty
STEMI - ST elevation myocardial infarction
TIMI - thrombolysis in myocardial infarction
WMSi - wall motion score index
Footnotes
Competing interests: None declared
Part of this paper was accepted for the Annual Meeting of the European Society of Cardiology, Stockholm, 3–7 September 2005.
References
- 1.Pfeffer M A, Braunwald E. Ventricular remodeling after myocardial infarction: experimental observations and clinical implications. Circulation 1990811161–1172. [DOI] [PubMed] [Google Scholar]
- 2.Gaudron P, Eilles C, Kugler I.et al Progressive left ventricular dysfunction and remodeling after myocardial infarction: potential mechanisms and early predictors. Circulation 199387755–763. [DOI] [PubMed] [Google Scholar]
- 3.Bolognese L, Neskovic A N, Parodi G.et al Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long‐term prognostic implications. Circulation 20021062351–2357. [DOI] [PubMed] [Google Scholar]
- 4.Morishima I, Sone T, Okumura K.et al Angiographic no‐reflow phenomenon as a predictor of adverse long‐term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol 2000361202–1209. [DOI] [PubMed] [Google Scholar]
- 5.Ito H, Maruyama A, Iwakura K.et al Clinical implications of the “no reflow” phenomenon: a predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation 199693223–228. [DOI] [PubMed] [Google Scholar]
- 6.Belli G, Pezzano A, De Biase A M.et al Adjunctive thrombus aspiration and mechanical protection from distal embolization in primary percutaneous intervention for acute myocardial infarction. Catheter Cardiovasc Interv 200050362–370. [DOI] [PubMed] [Google Scholar]
- 7.Kawaguchi R, Hoshizaki H, Oshima S.et al Effectiveness of thrombectomy before stent implantation in acute myocardial infarction. Circ J 200367951–954. [DOI] [PubMed] [Google Scholar]
- 8.Antoniucci D, Valenti R, Migliorini A.et al Comparison of rheolytic thrombectomy before direct infarct artery stenting versus direct stenting alone in patients undergoing percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol 2004931033–1035. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa Y, Matsuo S, Kimura T.et al Thrombectomy with AngioJet catheter in native coronary arteries for patients with acute or recent myocardial infarction. Am J Cardiol 199983994–999. [DOI] [PubMed] [Google Scholar]
- 10.Silva J A, Ramee S R, Cohen D J.et al Rheolytic thrombectomy during percutaneous revascularization for acute myocardial infarction: experience with the AngioJet catheter. Am Heart J 2001141353–359. [DOI] [PubMed] [Google Scholar]
- 11.Yip H K, Wu C J, Chang H W.et al Effect of the PercuSurge GuardWire device on the integrity of microvasculature and clinical outcomes during primary transradial coronary intervention in acute myocardial infarction. Am J Cardiol 2003921331–1335. [DOI] [PubMed] [Google Scholar]
- 12.Napodano M, Pasquetto G, Sacca S.et al Intracoronary thrombectomy improves myocardial reperfusion in patients undergoing direct angioplasty for acute myocardial infarction. J Am Coll Cardiol 2003421395–1402. [DOI] [PubMed] [Google Scholar]
- 13.Beran G, Lang I, Schreiber W.et al Intracoronary thrombectomy with the X‐sizer catheter system improves epicardial flow and accelerates ST‐segment resolution in patients with acute coronary syndrome: a prospective, randomized, controlled study. Circulation 20021052355–2360. [DOI] [PubMed] [Google Scholar]
- 14.Moses J, Moussa I. Do new devices add to the results of PTCA in acute myocardial infarction? Am Heart J 1999138S158–S163. [DOI] [PubMed] [Google Scholar]
- 15.Violaris A G, Melkert R, Herrman J P R.et al Role of angiographically identifiable thrombus on long‐term luminal renarrowing after coronary angioplasty: a quantitative angiographic analysis. Circulation 199693889–897. [DOI] [PubMed] [Google Scholar]
- 16.The TIMI Study Group The thrombolysis in myocardial infarction (TIMI) trial. The TIMI study group. N Engl J Med 1985312932–936. [DOI] [PubMed] [Google Scholar]
- 17.Van't Hof A W J, Liem A, Suryapranata H.et al Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction. Circulation 1998972302–2306. [DOI] [PubMed] [Google Scholar]
- 18.Van 't Hof A W, Liem A, de Boer M J.et al Clinical value of 12‐lead electrocardiogram after successful reperfusion therapy for acute myocardial infarction. Zwolle Myocardial infarction Study Group. Lancet 1997350615–619. [DOI] [PubMed] [Google Scholar]
- 19.Bolognese L, Cerisano G, Buonamici P.et al Influence of infarct‐zone viability on left ventricular remodeling following acute myocardial infarction. Circulation 1997963353–3359. [DOI] [PubMed] [Google Scholar]
- 20.Kern M J. Patterns of left ventricular dilatation with an opened artery after acute myocardial infarction: missing links to long‐term prognosis. Circulation 20021062294–2295. [DOI] [PubMed] [Google Scholar]
- 21.GUSTO Angiographic Investigators The effects of tissue plasminogen activator, streptokinase or both on coronary artery patency, ventricular function, and survival after acute myocardial infarction. GUSTO angiographic investigators. N Engl J Med 19933291615–1622. [DOI] [PubMed] [Google Scholar]
- 22.Waldecker B, Waas W, Habersbosh W.et al Long‐term follow‐up after direct percutaneous transluminal coronary angioplasty for acute myocardial infarction. J Am Coll Cardiol 1998321320–1325. [DOI] [PubMed] [Google Scholar]
- 23.Giannuzzi P, Temporelli P L, Bosimini E.et al Heterogeneity of left ventricular remodeling after acute myocardial infarction: results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardio‐3 echo substudy. Am Heart J 2001141131–138. [DOI] [PubMed] [Google Scholar]
- 24.Healy B, Aweisman H F. Myocardial infarct expansion, infarct extension, and reinfarction: pathophysiologic concepts. Prog Cardiovasc Dis 19873073–109. [DOI] [PubMed] [Google Scholar]
- 25.Ito H, Tomooka T, Sakai N.et al Lack of myocardial perfusion immediately after successful thrombolysis: a predictor of poor recovery of left ventricular function in anterior myocardial infarction. Circulation 1992851699–1705. [DOI] [PubMed] [Google Scholar]
- 26.Prasad A, Stone G W, Aymong E.et al Impact of ST‐segment resolution after primary angioplasty on outcomes after myocardial infarction in elderly patients: an analysis from the CADILLAC trial. Am Heart J 2004147669–675. [DOI] [PubMed] [Google Scholar]
- 27.De Lemos J A, Antman E M, Giugliano R P.et al ST‐segment resolution and infarct‐related artery patency and flow after thrombolytic therapy. Thrombolysis in Myocardial Infarction (TIMI) 14 investigators. Am J Cardiol 200085299–304. [DOI] [PubMed] [Google Scholar]
- 28.De Luca G, van 't Hof A W J, Ottervanger J P.et al Ageing, impaired myocardial perfusion and mortality in patients with ST‐segment elevation myocardial infarction treated with primary angioplasty. Eur Heart J 200526662–666. [DOI] [PubMed] [Google Scholar]
- 29.Corbalan R, Larrain G, Nazzal C.et al Association of noninvasive markers of coronary artery reperfusion to assess microvascular obstruction in patients with acute myocardial infarction treated with primary angioplasty. Am J Cardiol 200188342–346. [DOI] [PubMed] [Google Scholar]
- 30.Agati L. Microvascular integrity after reperfusion therapy. Am Heart J 1999138S76–S78. [DOI] [PubMed] [Google Scholar]
- 31.Agati L, Voci P, Hickle P.et al Tissue‐type plasminogen activator therapy versus primary coronary angioplasty: impact on myocardial tissue perfusion and regional function 1 month after uncomplicated myocardial infarction. J Am Coll Cardiol 199831338–343. [DOI] [PubMed] [Google Scholar]
- 32.Garot P, Pascal O, Simon M.et al Impact of microvascular integrity and local viability on left ventricular remodelling after reperfused acute myocardial infarction. Heart 200389393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone G W, Peterson M A, Lansky A J.et al Impact of normalized myocardial perfusion after successful angioplasty in acute myocardial infarction. J Am Coll Cardiol 200239591–597. [DOI] [PubMed] [Google Scholar]
- 34.Agati L, Voci P, Bilotta F.et al Influence of residual perfusion within the infarct zone on the natural history of left ventricular dysfunction after acute myocardial infarction: a myocardial contrast echocardiographic study. J Am Coll Cardiol 199424336–342. [DOI] [PubMed] [Google Scholar]
- 35.Agewall S. How should we evaluate an open artery in STEMI patients? Eur Heart J 200526634–636. [DOI] [PubMed] [Google Scholar]
- 36.Sorajja P, Gersh B J, Costantini C.et al Combined prognostic utility of ST‐segment recovery and myocardial blush after primary percutaneous coronary intervention in acute myocardial infarction. Eur Heart J 200526667–674. [DOI] [PubMed] [Google Scholar]
- 37.De Luca G, van 't Hof A W, de Boer M J.et al Time‐to‐treatment significantly affects the extent of ST‐segment resolution and myocardial blush in patients with acute myocardial infarction treated by primary angioplasty. Eur Heart J 2004251009–1013. [DOI] [PubMed] [Google Scholar]
- 38.Gibson C M, Schömig A. Coronary and myocardial angiography: angiographic assessment of both epicardial and myocardial perfusion. Circulation 20041093096–3105. [DOI] [PubMed] [Google Scholar]
- 39.Ali A, Schreiber T L. The role of percutaneous thrombectomy in the contemporary treatment of acute myocardial infarction. J Invasive Cardiol 200416546–548. [PubMed] [Google Scholar]
- 40.Murakami T, Mizuno S, Takahashi Y.et al Intracoronary aspiration thrombectomy for acute myocardial infarction. Am J Cardiol 199882839–844. [DOI] [PubMed] [Google Scholar]
- 41.Wang H J, Kao H L, Liau C S.et al Export aspiration catheter thrombosuction before actual angioplasty in primary coronary intervention for acute myocardial infarction. Catheter Cardiovasc Interv 200257332–339. [DOI] [PubMed] [Google Scholar]
- 42.Burzotta F, Trani C, Romagnoli E.et al Manual thrombus‐aspiration improves myocardial reperfusion the randomized evaluation of the effect of mechanical reduction of distal embolization by thrombus‐aspiration in primary and rescue angioplasty (REMEDIA) trial. J Am Coll Cardiol 200546371–376. [DOI] [PubMed] [Google Scholar]
- 43.Stone G W, Webb J, Cox D A.et al Distal microcirculatory protection during percutaneous coronary intervention in acute ST‐segment elevation myocardial infarction: a randomised controlled trial. JAMA 20052931063–1072. [DOI] [PubMed] [Google Scholar]
- 44.Kondo H, Suzuki T, Fukutomi T.et al Effects of percutaneous coronary arterial thrombectomy during acute myocardial infarction on left ventricular remodeling. Am J Cardiol 200493527–531. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi A, Ino T, Adachi H.et al Left ventricular volume predicts postoperative course in patients with ischemic cardiomyopathy. Ann Thorac Surg 199865434–438. [DOI] [PubMed] [Google Scholar]
- 46.Bax J J, Schinkel A F L, Boersma E.et al Extensive left ventricular remodeling does not allow viable myocardium to improve in left ventricular ejection fraction after revascularization and is associated with worse long‐term prognosis. Circulation 2004110(suppl II)II18–II22. [DOI] [PubMed] [Google Scholar]