Abstract
Objective
To find out whether B‐type natriuretic peptide (BNP) detects silent myocardial ischaemia in patients with type 2 diabetes, since many of these patients have silent ischaemia leading to unexpected cardiac deaths.
Design
Prospective cross‐sectional study with consecutive recruitment of patients.
Setting
Outpatient, single centre.
Patients
219 patients with type 2 diabetes. Patients were excluded if they had a history or evidence of cardiac failure.
Outcome measures
BNP, echocardiography and exercise tolerance test (ETT). BNP was compared with the ETT result in all patients and specifically in those who had no apparent ischaemic heart disease (IHD).
Results
121 patients had no history of IHD or cardiac failure and of these patients 85 had a clearly abnormal or normal ETT result. BNP was higher in patients with an abnormal than with a normal ETT (mean 58.2 (SD 46.3) v 24.4 (SD 15.7) pg/ml, p < 0.001). In univariate analysis BNP was an independent predictor of an abnormal ETT (p < 0.001). In multivariate analysis BNP remained an independent predictor of the ETT result. BNP concentration over 20 pg/ml predicted an abnormal ETT result with a sensitivity of 87% and specificity of 37%, and BNP over 40 pg/ml had a sensitivity of 63% and a specificity of 81%.
Conclusion
BNP is of value in predicting silent ischaemia on exercise testing in asymptomatic patients with type 2 diabetes.
Keywords: natriuretic peptides, myocardial ischaemia, exercise tolerance testing
Fifty per cent of all sudden cardiac deaths occur in people with no history of cardiac disease.1 In fact, sudden cardiac death in people without prior cardiac disease accounts for nearly 10% of all adult deaths. This may be particularly pertinent to patients with diabetes, who have a high risk of coronary artery disease that is often silent. To prevent such events the first necessary step would be to develop a simple test (for example, a blood test) that can identify those patients with diabetes with silent coronary artery disease. Recent studies have led us to hypothesise that B‐type natriuretic peptide (BNP) may be able to do this. This is in addition to the well known ability of BNP to identify left ventricular (LV) systolic dysfunction.2,3
Our hypothesis is based on three main observations. Firstly, Goetze et al4 recently showed that ischaemic myocardial tissue expresses more BNP than non‐ischaemic tissue. Secondly, in vitro cardiomyocytes degranulate and release BNP when they are made to be hypoxic.5 Thirdly, in symptomatic angina BNP can identify the presence of coronary artery disease and myocardial ischaemia.6,7,8,9,10 No study has yet addressed the key population of asymptomatic patients who are nevertheless at high risk of cardiac events, such as patients with type 2 diabetes.
Our objective was to see whether BNP can identify silent myocardial ischaemia identified by an exercise tolerance test (ETT) in asymptomatic patients with type 2 diabetes.
METHODS
Design and setting
Between 1999 and 2001, 219 patients with type 2 diabetes diagnosed between 3–6 years earlier were consecutively recruited into this pilot study from the Diabetes Centre outpatient department at Ninewells Hospital. All patients successfully recruited underwent a single three hour assessment in the Department of Clinical Pharmacology, Ninewells Hospital, Dundee. The Tayside committee on medical research ethics gave ethical approval, and written consent was obtained from each patient. The study was conducted in accordance with the Declaration of Helsinki.
A comprehensive cardiac history and examination were performed, followed by a 12 lead ECG, transthoracic echocardiography and treadmill exercise testing. Patients were excluded if a history or clinical examination found evidence of heart failure or significant valvular disease. They were also excluded if they were unable to exercise or had intercurrent illness, rest pain, severe hypertension and significant bradycardia or tachyarrhythmias.
BNP sampling
Venous blood samples were taken to measure BNP concentrations before exercise and after the patient had been supine for 30 minutes. The samples were measured in a single batch by an experienced technician. BNP was measured by a standard commercially available radioimmunoassay kit (Peninsula Laboratories, Merseyside, UK). A BNP concentration of < 100 pg/ml was used to exclude cardiac failure.
Resting ECG
Resting ECGs were classified as abnormal in the presence of pathological Q waves, LV hypertrophy on voltage criteria, or ST/T wave abnormalities. As the purpose of this study was to determine the usefulness of BNP as a screening test in patients with no known ischaemic heart disease (IHD), those with significant ECG abnormalities were excluded.
Exercise testing
A graded symptom limited maximal exercise test was performed according to the Bruce protocol.11 ECG monitoring and heart rate and blood pressure responses were assessed before and during exercise and for at least five minutes into the recovery period. The duration of the test was noted. The ECG tracings were analysed for the presence of ⩾ 1 mm horizontal or downsloping ST segment depression measured at 0.08 seconds after the J point. The ETT was classified as normal or abnormal based on these criteria.
Cardiac symptoms experienced during and after the test were carefully recorded. Reasons for termination of the test were limiting symptoms experienced by the patient (for example, fatigue, dyspnoea, chest pain, dizziness and palpitations), maximum predicted heart rate (220 − age) or stage 4 of the protocol reached, significant ST depression (⩾ 3 mm), appearance of ventricular arrhythmias or conduction abnormalities and a fall in heart rate or systolic blood pressure.
We also calculated the Duke treadmill score,12 a composite index designed to provide survival estimates based on results from the exercise test. It has been shown to provide diagnostic and prognostic predictive accuracy of coronary artery disease in symptomatic patients.13 The Duke score is calculated as follows: [exercise time (minutes)]−[5× maximum ST segment deviation (mm)]−[4× treadmill angina index] (the angina index is 0 for no angina, 1 for no limiting angina and 2 for exercise limiting angina). A treadmill score of 5 or more is considered to indicate low risk, −10 to 4 intermediate risk, and less than −10 high risk of significant coronary artery disease. A score of less than 5 was used to define a test as abnormal where necessary in the calculations.
LV function assessment
A single experienced physician blinded to the rest of the test results assessed transthoracic echocardiograms recorded with a Hewlett Packard Sonos 2000 scanner equipped with a 2.5 MHz transducer. Measurements were taken in accordance with the recommendations of the American Society of Echocardiography. Intraobserver variability was 9%. The operator was blinded to the clinical details and results of the other investigations of each patient.
Quantitative assessment of LV function was possible in 155 patients. The remainder of patients had LV function subjectively assessed. LV ejection fraction (LVEF) was calculated with the modified Simpson's rule. LV systolic dysfunction was defined as LVEF < 45%, which complies with the local guidelines for defining LV dysfunction. We chose a deliberately conservative cut off to ensure that only patients with no LV dysfunction were included in the analysis.
LV mass index (LVMI) was calculated with the formula of Devereux.14 LV hypertrophy was defined as LVMI greater than 110 g/m2 for women and greater than 135 g/m2 for men.
Statistical analysis
Data were statistically analysed with SPSS for Windows (V.10.1; SPSS, Chicago, Illinois, USA). BNP values were log transformed before analysis to achieve normal distribution. To determine difference between variables the independent samples t test was used for continuous data and χ2 for discrete data. Forward, stepwise logistic regression analyses were performed to assess whether variables were independent predictors of outcome (ETT or Duke score). Receiver operating characteristic curves were constructed to assess the clinical usefulness of predicting outcome. Sensitivities and specificities were also calculated.
BNP values are presented as median and interquartile range (IQR).
RESULTS
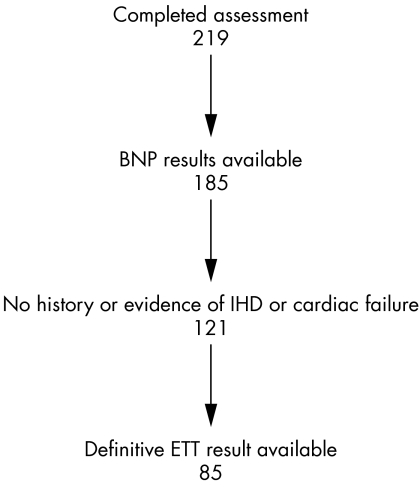
Of the 219 patients who were screened (fig 1), BNP results were unavailable for 34. Ten samples were mislaid, 15 samples were of insufficient volume and for nine samples the results were not reproducible even when the test was done in triplicate. Therefore, 185 patients (85%) had BNP samples that were suitable for analysis. Of the 185 patients who had BNP samples suitable for analysis, 121 (65%) had no history or evidence of IHD or congestive cardiac failure. Of these 121 patients 85 (70%) had an ETT result that could be classed definitively as abnormal or normal, rather than equivocal (20 patients were unable to complete the ETT). Table 1 presents characteristics of these 85 patients who had no evidence of IHD or congestive cardiac failure and had results for both BNP and ETT.
Figure 1 Selection and exclusion of study participants showing number remaining after each analysis. BNP, B‐type natriuretic peptide; ETT, exercise tolerance test; IHD, ischaemic heart disease.
Table 1 Characteristics of the 85 patients with definitive exercise tolerance test (ETT) result who had both B‐type natriuretic peptide (BNP) sample results and no evidence or history of ischaemic heart disease or cardiac failure.
| ETT normal | ETT abnormal | |
|---|---|---|
| Age (years) | 54 (11) | 60(7)* |
| Sex (male/female) | 53/17 | 10/5 |
| Smoking (no/yes) | 48/22 | 14/1* |
| Hypertension (no/yes) | 41/29 | 6/9 |
| Hyperlipidaemia (no/yes) | 56/14 | 9/6 |
| Haemoglobin A1c (%) | 7.6 (1.4) | 7.8 (1.9) |
| Cholesterol (mmol/l) | 5.3 (1.6) | 5.5 (1.3) |
| SBP (mm Hg) | 142 (17) | 151 (21) |
| DBP (mm Hg) | 82 (12) | 83 (11) |
| BMI (kg/m2) | 30(5.7) | 30 (6.3) |
| BNP (pg/ml) | 24.4 (15.7) | 58.2 (46.3)*** |
Data are described as patient number (%), median (interquartile range) for BNP or mean (SD).
*p⩽0.05; ***p<0.001 between normal and abnormal test.
BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Table 2 summarises baseline characteristics of the 185 patients: 19% of patients had evidence of microalbuminuria, 49% were taking metformin, 41% were taking a sulfonylurea, 12% were taking insulin and 9% were controlled by diet alone. With respect to cardiovascular drugs, 27% were taking an angiotensin converting enzyme inhibitor, 25% were taking a calcium channel blocker, 14% were taking a β blocker, 25% were taking aspirin and 12% were taking diuretics.
Table 2 Characteristics of the 185 patients with B‐type natriuretic (BNP) samples.
| All patients (n = 185) | Patients with no IHD or cardiac failure (n = 121) | Patients with IHD or cardiac failure (n = 64) | |
|---|---|---|---|
| Age (years) | 58.6 (10) | 57 (10) | 65 (7)*** |
| Sex (male/female) | 109/76 | 74/47 | 35/29 |
| Smoking (no/yes) | 147/38 | 93/28 | 54/10 |
| Hypertension (no/yes) | 100/85 | 58/63/ | 42/22/ |
| Hyperlipidaemia (no/yes) | 132/53 | 90/31 | 42/22 |
| Haemoglobin A1c (%) | 7.79 (1.5) | 7.6 (1.5) | 7.8 (1.3) |
| Cholesterol (mmol/l) | 5.3 (1.3) | 5.3 (1.5) | 5.3 (0.9) |
| SBP (mm Hg) | 142 (21) | 142 (21) | 145 (15) |
| DBP (mm Hg) | 82 (11) | 81 (10) | 79 (10) |
| BMI (kg/m2) | 30.1 (5.98) | 31 (5.9) | 30 (5.1) |
| BNP (pg/ml) | 32.0 (30.6) | 29.8 (25.7) | 44.7 (38.0) |
Data are described as patient number (%), median (interquartile range) for BNP or mean (SD).
***p<0.001 between the ischaemic heart disease (IHD) or cardiac failure and the no IHD or cardiac failure groups.
BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Median BNP (IQR) concentration for the group as a whole was 32.0 (30.6) pg/ml. This is comparable with previous studies of patients with diabetes without known cardiac disease.15,16
Is BNP raised in patients with an abnormal exercise test?
Table 3 shows the median (IQR) of BNP defined by a normal or abnormal test result.
Table 3 B‐type natriuretic peptide (BNP) concentrations defined by normal and abnormal test results for all patients.
| Test | Number with valid test result | BNP (pg/ml) | |
|---|---|---|---|
| Normal test result | Abnormal test result | ||
| ETT | 104 | 26.1 (18.7) | 59.2 (44.9)*** |
| Duke score | 181 | 25.9 (19.1) | 33.9 (31.3)* |
BNP concentration is median (interquartile range) for each normal or abnormal test result.
*p<0.05, ***p<0.001 between normal and abnormal result.
ETT, exercise tolerance test.
BNP was significantly raised in patients with an abnormal compared with a normal exercise test (p < 0.001).
When only those patients with no evidence of IHD or cardiac failure were considered, BNP concentration remained significantly greater in those with an abnormal ETT than in those whose ETT was normal; median (IQR) BNP values of patients with a normal and abnormal ETT were 24.4 (15.7) pg/ml and 58.2 (46.3) pg/ml, respectively (p for difference < 0.001). When known cardiovascular risk factors and confounders of BNP concentration (history of hypertension, sex, age and smoking history as dichotomous variables, plus microalbuminuria, cholesterol, body mass index, haemoglobin A1c, LVMI and LVEF as continuous variables) were included in a multivariate logistic regression analysis along with BNP, BNP remained an independent predictor of an abnormal ETT (table 4). Indeed, it was the strongest independent predictor.
Table 4 Independent predictors of an abnormal exercise tolerance test in multivariate analysis.
| Variable | B value | 95% CI of B value | p Value | SE |
|---|---|---|---|---|
| Age | 0.23 | 0.04 to 0.42 | 0.01 | 0.97 |
| Haemoglobin A1c | 1.54 | 0.23 to 2.85 | 0.02 | 0.67 |
| Cholesterol | 1.07 | 0.13 to 2.01 | 0.03 | 0.48 |
| lnBNP | 11.15 | 3.67 to 18.6 | 0.003 | 3.81 |
BNP, B‐type natriuretic peptide; B value, β coefficient.
Is BNP raised in patients with a negative Duke score?
The Duke score was used as a second index of myocardial ischaemia. The Duke score was calculated for 181 patients, of whom 99 had no history of IHD or cardiac failure. More patients had a Duke score calculated than had an ETT result, as it is possible to ascribe a Duke score even when the ETT result is clinically equivocal.
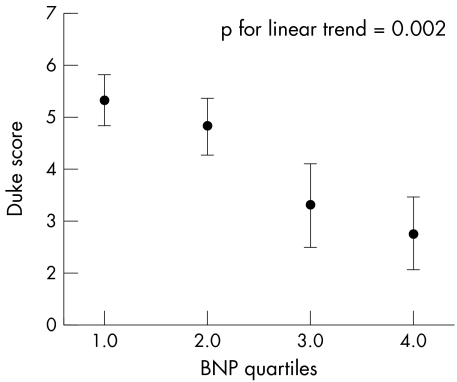
In the whole group BNP was significantly greater in those with a Duke score that indicated ischaemia than in those whose Duke score did not indicate ischaemia (p = 0.003). BNP concentrations correlated inversely with Duke score on exercise testing—that is, the more negative the Duke score the higher the BNP concentration (correlation coefficient –0.184, p = 0.013). When patients with high BNP values (> 100 pg/ml) were excluded from analysis BNP quartiles still inversely correlated with the Duke score (r = −0.26, p = 0.002) (fig 2).
Figure 2 B‐type natriuretic peptide (BNP) quartiles within the normal range compared with Duke score (mean and 95% confidence interval).
When patients with no evidence of IHD or cardiac failure were analysed alone BNP was still higher in those with a positive than in those with a negative Duke score; median (IQR) BNP values for those with a positive and negative Duke score were 33.4 (31.2) pg/ml and 24.7 (16.9) pg/ml, respectively (p for difference = 0.05). There was also a significant inverse correlation between BNP and Duke score (as a continuous variable) (r = −0.23, p = 0.01). When known cardiovascular risk factors and confounders of BNP concentration (history of hypertension, sex, age and smoking history as dichotomous variables, plus microalbuminuria, cholesterol, body mass index, haemoglobin A1c, LVMI and LVEF as continuous variables) were included in a multivariate analysis along with BNP, BNP was no longer a significant predictor of the Duke score.
Would BNP be a useful screening test to identify those likely to have an abnormal exercise test?
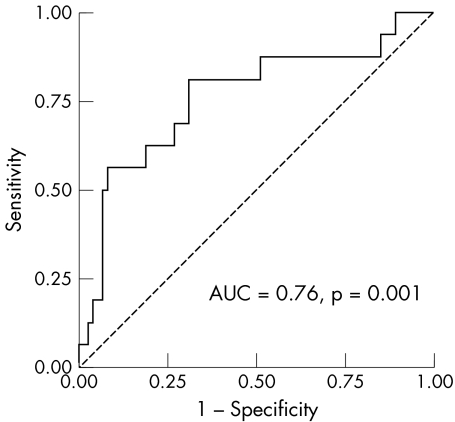
To determine BNP's usefulness as a screening test for prediction of an abnormal exercise test in patients with no history of cardiovascular disease, we constructed a receiver operating characteristic curve (fig 3). BNP was sensitive in predicting who would have an abnormal exercise test but it lacked specificity. For a BNP value above 20 pg/ml the sensitivity and specificity for prediction of an abnormal ETT are 87% and 37%, respectively (table 5).
Figure 3 Receiver operative characteristic curve describing the ability of B‐type natriuretic peptide to predict an abnormal exercise tolerance test in patients with no history of ischaemic heart disease. AUC, area under the curve.
Table 5 Sensitivity and specificity of B‐type natriuretic peptide (BNP) in predicting an abnormal exercise tolerance test in patients with no history of ischaemic heart disease.
| BNP (pg/ml) | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|
| >20 | 87 | 37 | 21 | 90 |
| >35 | 69 | 72 | 34 | 91 |
| >40 | 63 | 81 | 42 | 90 |
DISCUSSION
The present study suggests that BNP can identify which asymptomatic patients with type 2 diabetes will have an abnormal exercise test when patients with apparent IHD have been excluded. The area under the curve in this study (0.76) was similar to that seen by Weber et al6 in symptomatic angina patients.
These results may partly explain recent data showing that BNP predicts cardiovascular events, even in patients who do not have heart failure.17,18,19,20 More pertinent to this current study are recent publications showing that BNP predicts mortality among diabetic clinic attendees.15,21,22
Although a BNP concentration of 20 pg/ml is sensitive, it lacks specificity to identify patients who will have an abnormal ETT. This means that a normal BNP is good at ruling out ischaemia, which would mean that a high‐risk patient would probably not be missed.
The question that naturally arises is what treatment options may be useful for patients identified to have silent ischaemia. Firstly, younger patients at higher risk may be eligible for invasive intervention (for example, angioplasty). There have not been any large scale trials of intervention in silent ischaemia, but small studies have found angioplasty to be safe and to give a better outcome than either medical treatment or no treatment.23,24,25 The ACIP (Asymptomatic Cardiac Ischemia Pilot) trial also found that coronary artery bypass grafting was of value in patients with silent ischaemia.25 A second possible outcome arises from the EUROASPIRE (European Action on Secondary Prevention through Intervention to Reduce Events) data showing how poor risk factor control is in practice.26 This can be exemplified here by the average systolic blood pressure being 142 mm Hg despite the target systolic blood pressure for a patient with diabetes being < 130 mm Hg. It is possible that a high BNP concentration would spur the consulting doctor on to achieve better risk factor control than the EUROASPIRE average: such an approach has in general been shown to work.27 A third potential outcome is that patients with silent ischaemia will be targeted to receive more aggressive treatment of blood pressure, cholesterol or glucose to lower than current target concentrations. Such an intensive risk reduction strategy when applied generally in diabetes has recently been shown to reduce cardiovascular and microvascular events by almost 50%.28
Potential study weaknesses
We are assuming that an abnormal ETT indicates silent myocardial ischaemia. Although the ETT has been shown to be useful in patients with diabetes29 the ideal test for coronary disease remains angiography. As our patients were all asymptomatic, however, at the time of the study it was deemed unethical for most of them to proceed to angiography. In addition, exercise testing is by far the most common investigation used routinely in the UK to investigate for a possible diagnosis of coronary disease. Also, whether or not further imaging shows coronary artery lesions, the ETT result is independently predictive of outcome in patients with diabetes.30 In the current study, coronary artery disease was indeed seen in all five of the patients who were selected for coronary angiography because their exercise test was abnormal at exceptionally low workloads.
Another limitation is that we did not formally assess diastolic function in this study. This was because, firstly, at the time of the study there was no consensus on parameters to diagnose diastolic dysfunction and, secondly, there are no treatments that significantly reduce cardiovascular events even in symptomatic diastolic dysfunction.
Patients attended for assessment throughout the day and there is some evidence to suggest that BNP concentrations fluctuate throughout the day.31 It is highly unlikely, however, that all patients with an abnormal (or normal) ETT result were randomly studied at a particular time of day.
In conclusion, these results suggest that BNP may be useful as a screening test for silent myocardial ischaemia in asymptomatic patients with type 2 diabetes. Thus, BNP prescreening followed by an ETT in selected patients may become a useful strategy in asymptomatic patients with diabetes with the aim ultimately of reducing cardiovascular events by better targeting of cardioprotective treatments. Future research in larger populations is now required to give more accurate figures on the precise diagnostic accuracy of BNP and to see whether different cut offs for BNP can perform better in different subgroups of patients.
ACKNOWLEDGEMENTS
Dr Simon Ogston (medical statistician) reviewed the statistics presented in this paper. Lesley McFarlane and Valerie Godfrey analysed the plasma samples.
Abbreviations
ACIP - Asymptomatic Cardiac Ischemia Pilot
BNP - B‐type natriuretic peptide
ETT - exercise tolerance testing
EUROASPIRE - European action on Secondary Prevention through Intervention to Reduce Events
IHD - ischaemic heart disease
IQR - interquartile range
LV - left ventricular
LVEF - left ventricular ejection fraction
LVMI - left ventricular mass index
Footnotes
This research was funded by the Chief Scientist Office, Scottish Department of Health
Competing interests: None declared
References
- 1.Vreede‐Swagemakers J J, Gorgels A P, Dubois‐Arbouw W I.et al Out‐of‐hospital cardiac arrest in the 1990's: a population‐based study in the Maastricht area on incidence, characteristics and survival. J Am Coll Cardiol 1997301500–1505. [DOI] [PubMed] [Google Scholar]
- 2.Chen H H, Burnett J C., Jr The natriuretic peptides in heart failure: diagnostic and therapeutic potentials. Proc Assoc Am Physicians 1999111406–416. [DOI] [PubMed] [Google Scholar]
- 3.Stanek B, Frey B, Hulsmann M.et al Prognostic evaluation of neurohumoral plasma levels before and during beta‐blocker therapy in advanced left ventricular dysfunction. J Am Coll Cardiol 200138436–442. [DOI] [PubMed] [Google Scholar]
- 4.Goetze J P, Christoffersen C, Perko M.et al Increased cardiac BNP expression associated with myocardial ischemia. FASEB J 2003171105–1107. [DOI] [PubMed] [Google Scholar]
- 5.Hopkins W E, Chen Z, Fukagawa N K.et al Increased atrial and brain natriuretic peptides in adults with cyanotic congenital heart disease: enhanced understanding of the relationship between hypoxia and natriuretic peptide secretion. Circulation 20041092872–2877. [DOI] [PubMed] [Google Scholar]
- 6.Weber M, Dill T, Arnold R.et al N‐terminal B‐type natriuretic peptide predicts extent of coronary artery disease and ischemia in patients with stable angina pectoris. Am Heart J 2004148612–620. [DOI] [PubMed] [Google Scholar]
- 7.Davidson N C, Pringle S D, Pringle T H.et al Right coronary artery stenosis is associated with impaired cardiac endocrine function during exercise. Eur Heart J 1997181749–1754. [DOI] [PubMed] [Google Scholar]
- 8.Asada J, Tsuji H, Iwasaka T.et al Usefulness of plasma brain natriuretic peptide levels in predicting dobutamine‐induced myocardial ischemia. Am J Cardiol 200493702–704. [DOI] [PubMed] [Google Scholar]
- 9.Sadanandan S, Cannon C P, Chekuri K.et al Association of elevated B‐type natriuretic peptide levels with angiographic findings among patients with unstable angina and non‐ST‐segment elevation myocardial infarction. J Am Coll Cardiol 200444564–568. [DOI] [PubMed] [Google Scholar]
- 10.Bibbins‐Domingo K, Ansari M, Schiller N B.et al B‐type natriuretic peptide and ischemia in patients with stable coronary disease: data from the heart and soul study. Circulation 20031082987–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruce R A. Exercise testing in patients with coronary artery disease. Ann Clin Res 19713323–332. [PubMed] [Google Scholar]
- 12.Mark D B, Hlatky M A, Harrell F E., Jret al Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med 1987106793–800. [DOI] [PubMed] [Google Scholar]
- 13.Shaw L J, Peterson E D, Shaw L K.et al Use of a prognostic treadmill score in identifying diagnostic coronary disease subgroups. Circulation 1998981622–1630. [DOI] [PubMed] [Google Scholar]
- 14.Devereux R B, Alonso D R, Lutas E M.et al Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 198657450–458. [DOI] [PubMed] [Google Scholar]
- 15.Bhalla M A, Chiang A, Epshteyn V A.et al Prognostic role of B‐type natriuretic peptide levels in patients with type 2 diabetes mellitus. J Am Coll Cardiol 2004441047–1052. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa S, Takeuchi K, Ito S. Plasma BNP levels in the treatment of type 2 diabetes with pioglitazone. J Clin Endocrinol Metab 2003883993–3996. [DOI] [PubMed] [Google Scholar]
- 17.De Lemos J A, Morrow D A, Bentley J H.et al The prognostic value of B‐type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med 20013451014–1021. [DOI] [PubMed] [Google Scholar]
- 18.Wang T J, Larson M G, Levy D.et al Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 2004350655–663. [DOI] [PubMed] [Google Scholar]
- 19.De Lemos J A, Morrow D A. Brain natriuretic peptide measurement in acute coronary syndromes: ready for clinical application? Circulation 20021062868–2870. [DOI] [PubMed] [Google Scholar]
- 20.Bazzino O, Fuselli J J, Botto F.et al Relative value of N‐terminal probrain natriuretic peptide, TIMI risk score, ACC/AHA prognostic classification and other risk markers in patients with non‐ST‐elevation acute coronary syndromes. Eur Heart J 200425859–866. [DOI] [PubMed] [Google Scholar]
- 21.Gaede P, Hildebrandt P, Hess G.et al Plasma N‐terminal pro‐brain natriuretic peptide as a major risk marker for cardiovascular disease in patients with type 2 diabetes and microalbuminuria. Diabetologia 200548156–163. [DOI] [PubMed] [Google Scholar]
- 22.Tarnow L, Hildebrandt P, Hansen B V.et al Plasma N‐terminal pro‐brain natriuretic peptide as an independent predictor of mortality in diabetic nephropathy. Diabetologia 200548149–155. [DOI] [PubMed] [Google Scholar]
- 23.Anderson H V, Talley J D, Black A J.et al Usefulness of coronary angioplasty in asymptomatic patients. Am J Cardiol 19906535–39. [DOI] [PubMed] [Google Scholar]
- 24.Bergin P, Myler R K, Shaw R E.et al Transluminal coronary angioplasty in the treatment of silent ischemia. Cathet Cardiovasc Diagn 198815223–228. [DOI] [PubMed] [Google Scholar]
- 25.Bourassa M G, Pepine C J, Forman S A.et al Asymptomatic cardiac ischemia pilot (ACIP) study: effects of coronary angioplasty and coronary artery bypass graft surgery on recurrent angina and ischemia. The ACIP investigators. J Am Coll Cardiol 199526606–614. [DOI] [PubMed] [Google Scholar]
- 26.EUROASPIRE Study Group EUROASPIRE. A European Society of Cardiology survey of secondary prevention of coronary heart disease: principal results, EUROASPIRE study group. European action on secondary prevention through intervention to reduce events. Eur Heart J 1997181569–1582. [DOI] [PubMed] [Google Scholar]
- 27.Hall L M, Jung R T, Leese G P. Controlled trial of effect of documented cardiovascular risk scores on prescribing. BMJ 2003326251–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaede P, Vedel P, Larsen N.et al Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003348383–393. [DOI] [PubMed] [Google Scholar]
- 29.Bacci S, Villella M, Villella A.et al Screening for silent myocardial ischaemia in type 2 diabetic patients with additional atherogenic risk factors: applicability and accuracy of the exercise stress test. Eur J Endocrinol 2002147649–654. [DOI] [PubMed] [Google Scholar]
- 30.Rutter M K, Wahid S T, McComb J M.et al Significance of silent ischemia and microalbuminuria in predicting coronary events in asymptomatic patients with type 2 diabetes. J Am Coll Cardiol 20024056–61. [DOI] [PubMed] [Google Scholar]
- 31.Bruins S, Fokkema M R, Romer J W.et al High intraindividual variation of B‐type natriuretic peptide (BNP) and amino‐terminal proBNP in patients with stable chronic heart failure. Clin Chem 2004502052–2058. [DOI] [PubMed] [Google Scholar]