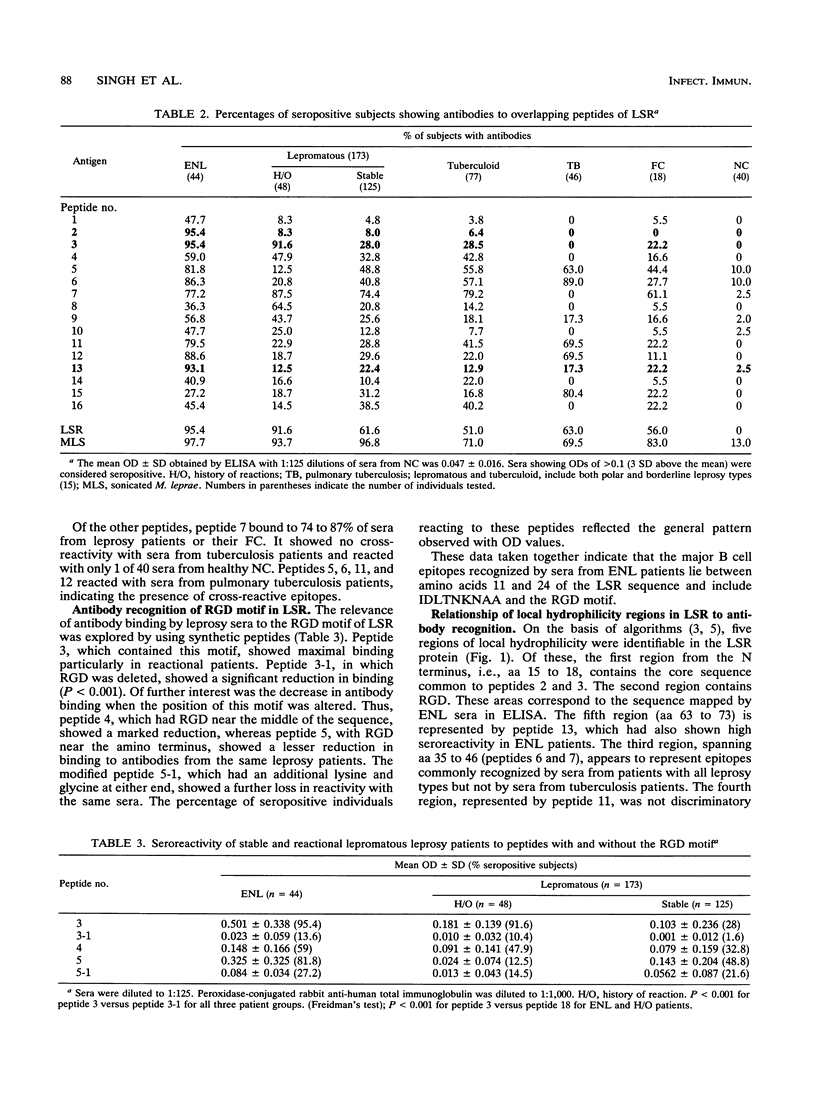
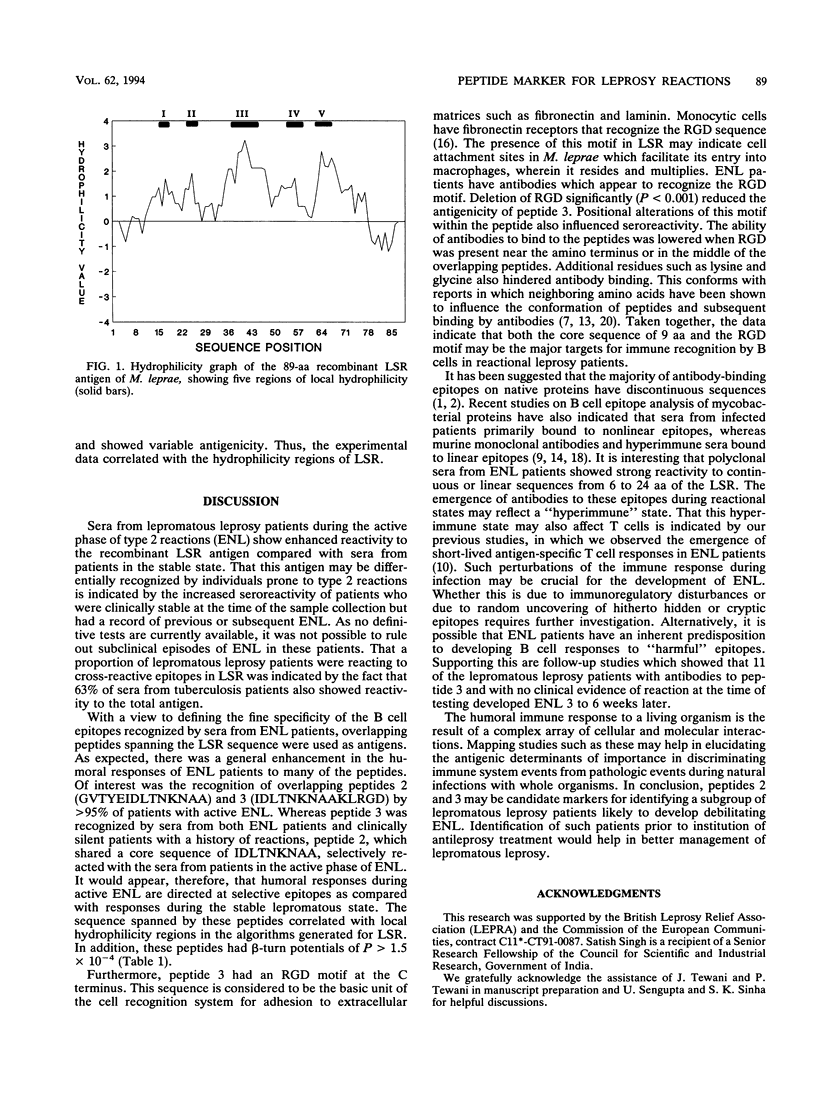
Abstract
Type 2 reactions (erythema nodosum leprosum [ENL]) are episodic, reactional states causing significant morbidity in lepromatous leprosy patients. With a view to defining the immunological differences between the stable and reactional forms of lepromatous leprosy, we determined antibody responses to LSR, a recombinant protein of Mycobacterium leprae previously described by us (S. Laal, Y.D. Sharma, H.K. Prasad, A. Murtaza, S. Singh, S. Tangri, R. S. Mishra, and I. Nath, Proc. Natl. Acad. Sci. USA 88:1054-1058, 1991), as well as to 10- to 15-mer overlapping peptides synthesized on the basis of the LSR amino acid sequence. We report here the selective recognition of B cell epitopes by sera from patients with ENL as compared with a control group with nonreactional lepromatous leprosy. Peptides 2 and 3, with the sequences GVTYEIDLTNKNAA and IDLTNKNAAKLRGD, respectively, were recognized by > 95% of sera from patients with active ENL. Peptide 3 in addition showed reactivity with sera taken from 91.6% of lepromatous leprosy patients who were apparently stable but who were recorded to have had ENL several weeks before or after the sample collection. The core sequence IDLTNKNAA common to both these peptides may be a major target of humoral responses in ENL. In addition, the RGD motif at the C terminus appeared to influence the antigenicity of peptide 3 in enzyme-linked immunosorbent assay. It would appear that humoral responses during ENL are directed to selective antigenic determinants of the leprosy bacillus. The use of such serological markers to identify lepromatous leprosy patients with a high risk for developing ENL would be of clinical and predictive value.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow D. J., Edwards M. S., Thornton J. M. Continuous and discontinuous protein antigenic determinants. Nature. 1986 Aug 21;322(6081):747–748. doi: 10.1038/322747a0. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A. Intrinsic and extrinsic factors in protein antigenic structure. Science. 1985 Sep 6;229(4717):932–940. doi: 10.1126/science.2410982. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Jacobs W. R., Docherty M. A., Ritchie L. R., Curtiss R., 3rd Molecular analysis of DNA and construction of genomic libraries of Mycobacterium leprae. J Bacteriol. 1985 Mar;161(3):1093–1102. doi: 10.1128/jb.161.3.1093-1102.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W., Sander C. On the use of sequence homologies to predict protein structure: identical pentapeptides can have completely different conformations. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1075–1078. doi: 10.1073/pnas.81.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Cohn Z. A. The immunobiology of leprosy. Int Rev Exp Pathol. 1986;28:45–78. [PubMed] [Google Scholar]
- Klatser P. R., De Wit M. Y., Kolk A. H., Hartskeerl R. A. Characterization of murine B-cell epitopes on the Mycobacterium leprae proline-rich antigen by use of synthetic peptides. Infect Immun. 1991 Jan;59(1):433–436. doi: 10.1128/iai.59.1.433-436.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laal S., Bhutani L. K., Nath I. Natural emergence of antigen-reactive T cells in lepromatous leprosy patients during erythema nodosum leprosum. Infect Immun. 1985 Dec;50(3):887–892. doi: 10.1128/iai.50.3.887-892.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laal S., Sharma Y. D., Prasad H. K., Murtaza A., Singh S., Tangri S., Misra R. S., Nath I. Recombinant fusion protein identified by lepromatous sera mimics native Mycobacterium leprae in T-cell responses across the leprosy spectrum. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1054–1058. doi: 10.1073/pnas.88.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlin R. L., Mehra V., Jordan R., Bloom B. R., Rea T. H. In situ and in vitro characterization of the cellular immune response in erythema nodosum leprosum. J Immunol. 1986 Feb 1;136(3):883–886. [PubMed] [Google Scholar]
- Peake P. W., Britton W. J., Davenport M. P., Roche P. W., McKenzie K. R. Analysis of B-cell epitopes in the variable C-terminal region of the Mycobacterium leprae 70-kilodalton heat shock protein. Infect Immun. 1993 Jan;61(1):135–141. doi: 10.1128/iai.61.1.135-141.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Sela S., Thole J. E., Ottenhoff T. H., Clark-Curtiss J. E. Identification of Mycobacterium leprae antigens from a cosmid library: characterization of a 15-kilodalton antigen that is recognized by both the humoral and cellular immune systems in leprosy patients. Infect Immun. 1991 Nov;59(11):4117–4124. doi: 10.1128/iai.59.11.4117-4124.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbon A., Hartskeerl R. A., Kolk A. H. Murine and human B cell epitope mapping of the Mycobacterium tuberculosis 10-kD heat shock protein using overlapping peptides. Clin Exp Immunol. 1991 Oct;86(1):6–12. doi: 10.1111/j.1365-2249.1991.tb05765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemambu S. N., Turk J. L., Waters M. F., Rees R. J. Erythema nodosum leprosum: a clinical manifestation of the arthus phenomenon. Lancet. 1969 Nov 1;2(7627):933–935. doi: 10.1016/s0140-6736(69)90592-3. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Haft D. H., Getzoff E. D., Tainer J. A., Lerner R. A., Brenner S. Identical short peptide sequences in unrelated proteins can have different conformations: a testing ground for theories of immune recognition. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5255–5259. doi: 10.1073/pnas.82.16.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Kaufmann S. H., Hermans P. W., Thole J. E. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992 Jan;6(2):133–145. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]
- Young R. A., Mehra V., Sweetser D., Buchanan T., Clark-Curtiss J., Davis R. W., Bloom B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature. 1985 Aug 1;316(6027):450–452. doi: 10.1038/316450a0. [DOI] [PubMed] [Google Scholar]



