Abstract
Objective
To analyse the short and long term prognostic significance of admission glycaemia in a large registry of non‐diabetic patients with acute myocardial infarction.
Methods
Assessment of short and long term prognostic significance of admission blood glucose in a consecutive population of 1604 non‐diabetic patients admitted to intensive care units in France in November 2000 for a recent (⩽ 48 hours) myocardial infarction.
Results
In‐hospital mortality, compared with that of patients with admission glycaemia below the median value of 6.88 mmol/l (3.7%), rose gradually with each of the three upper sextiles of glycaemia: 6.5%, 12.5% and 15.2%. Conversely, one year survival decreased from 92.5% to 88%, 83% and 75% (p < 0.001). Admission glycaemia remained an independent predictor of in‐hospital and one year mortality after multivariate analyses accounting for potential confounders. Increased admission glycaemia also was a predictor of poor outcome in all clinical subsets studied: patients without heart failure on admission, younger and older patients, patients with or without reperfusion therapy, and patients with or without ST segment elevation.
Conclusion
In non‐diabetic patients, raised admission blood glucose is a strong and independent predictor of both in‐hospital and long term mortality.
Keywords: glycaemia, acute myocardial infarction, mortality, left ventricular failure
The outcome of diabetic patients sustaining a myocardial infarction is poor, compared with that of non‐diabetic patients.1 In diabetic patients, glycaemic control at the acute stage is an important determinant of outcomes.2,3 The role of admission glycaemia in non‐diabetic patients with acute myocardial infarction (AMI), however, has been less extensively studied.4,5 In particular, very limited data are available regarding the long term prognostic influence of admission glycaemia in these patients.5 A recent review of several studies including diabetic and non‐diabetic patients, and using variable thresholds to define stress hyperglycaemia, showed that patients with raised concentrations of blood glucose on admission were at increased risk of in‐hospital death, irrespective of their diabetic status.6 A strong correlation between glycaemia and shock or development of heart failure has also been reported.7 However, the impact of admission glycaemia on in‐hospital and long term outcomes in different subsets of non‐diabetic patients, particularly those without evidence of heart failure on admission, remain poorly documented. The purpose of the present study was to analyse the short and long term prognostic significance of admission glycaemia in a large registry of patients with myocardial infarction admitted to an intensive care unit in France in November 2000, permitting adequate subgroup analyses, with a specific focus on patients without heart failure.
PATIENTS AND METHODS
The patient population and methods of the USIC 2000 registry have been described in detail elsewhere.8,9 Briefly, the objective of the study was to gather complete and representative data on the management and outcome of patients admitted to intensive care units for definite AMI over a one month period in France, irrespective of the type of institution to which the patients were admitted (that is, university hospitals, public hospitals or private clinics). Of the 443 centres that treated patients with AMI at that time, 369 participated in the study (83%). One physician responsible for the study was recruited in each centre and filled in a case record form for each patient meeting the inclusion criteria and admitted to the intensive care unit during the study recruitment period. The physicians in charge of the patients took care of them according to their usual practice and independently of the study. The methods used for this prospective registry were similar to those of a previous survey carried out in France five years earlier,10 although more data were collected in the most recent registry.
Patients
All consecutive patients admitted to the participating centres from 1 November through 30 November 2000 were included in the registry if they had (1) raised serum markers of myocardial necrosis higher than twice the upper limit of normal for creatine kinase, creatine kinase MB fraction or troponins, and (2) any or all of symptoms compatible with AMI for at least 30 minutes, ECG changes on at least two contiguous leads with pathological Q waves (at least 0.04 seconds) and persistent ST elevation or depression > 0.1 mV. The time from the beginning of symptoms to admission to the intensive care unit had to be < 48 hours.
For the present analysis, all patients with glycaemia measured on admission and who had no history of or treatment for diabetes mellitus at entry were included. Diabetics diagnosed during the hospital stay were excluded. Of a total population of 2320 patients, 1833 had no known or recognised diabetes mellitus. From this group, the concentration of admission blood glucose was recorded in 1604 patients (88%), who formed the study population.
Data collection
The patients' cardiovascular history, their medications at the time of admission, their risk factors, their in‐hospital clinical course, including maximum Killip class, and the initial diagnostic and therapeutic management were recorded for each patient. Furthermore, left ventricular ejection fraction, when assessed at any time during the first five days, was recorded.
Statistical analysis
We compared initial and outcome data according to sextiles of blood glucose concentrations at admission. However, as the baseline characteristics and outcomes in the patients in the first three sextiles were similar, the results presented here regroup all three first sextiles into a single category (that is, those with admission glycaemia below the median value). All continuous variables are described as their mean (SD). All categorical variables are described in terms of absolute and relative frequency distributions. Groups were compared by one way analysis of variance for continuous variables and χ2 tests for discrete variables. Multiple logistic regression analysis was used to determine independent correlates of in‐hospital mortality and Cox multivariate regression analysis was used to assess predictors of one year outcome. Variables with p < 0.10 on univariate analyses were included in the models. Survival curves were generated by the Kaplan–Meier method and compared by log rank tests. For all tests p < 0.05 was considered significant.
RESULTS
Baseline characteristics
Mean (SD) admission glycaemia was 7.60 (2.7) mmol/l and the median value for admission glycaemia was 6.88 mmol/l. Table 1 describes the baseline characteristics of the population according to admission glycaemia. Patients with a higher concentration of admission blood glucose were older, more of them were women and they were more likely to have signs of left ventricular failure on admission. They also had a higher prevalence of ST elevation myocardial infarction (STEMI), concomitant with a higher rate of reperfusion therapy, and they were more commonly admitted to hospital within three hours of symptom onset.
Table 1 Baseline characteristics, risk factors and medical history of patients without diabetes mellitus.
| Glycaemia sextile (mmol/l) | p Value | ||||
|---|---|---|---|---|---|
| ⩽6.88 (n = 796) | 6.88–7.88 (n = 275) | 7.88–9.27 (n = 264) | >9.27 (n = 269) | ||
| Age (years) | 63 (15) | 64 (16) | 66 (14) | 67 (15) | 0.001 |
| Body mass index (kg/m2) | 26 (4) | 26 (4) | 26 (4) | 26 (4) | NS |
| Systolic BP (mm Hg) | 132 (24) | 135 (28) | 134 (26) | 132 (31) | NS |
| Diastolic BP (mm Hg) | 76 (14) | 78 (17) | 78 (16) | 76 (17) | NS |
| Heart rate (beats/min) | 76 (17) | 77 (18) | 79 (20) | 82 (22) | 0.001 |
| Glycaemia (mmol/l) | 5.88 (0.7) | 7.33 (0.3) | 8.49 (0.4) | 11.9 (0.4) | 0.001 |
| Women | 174 (22%) | 71 (26%) | 59 (22%) | 88 (33%) | 0.004 |
| Hypertension | 296 (37%) | 120 (44%) | 113 (43%) | 127 (47%) | 0.017 |
| Hyperlipidaemia | 325 (41%) | 94 (35%) | 102 (39%) | 109 (41%) | NS |
| Current smoking | 306 (39%) | 81 (30%) | 98 (37%) | 81 (30%) | 0.012 |
| Previous MI | 123 (15%) | 41 (15%) | 43 (16%) | 51 (19%) | NS |
| History of CHF | 33 (4%) | 17 (6%) | 17 (6%) | 28 (10%) | 0.002 |
| Previous stroke | 21 (3%) | 13 (5%) | 7 (3%) | 17 (6%) | 0.024 |
| Previous CABG | 33 (4%) | 9 (3%) | 7 (3%) | 11 (4%) | NS |
| Previous PCI | 77 (10%) | 18 (7%) | 14 (5%) | 21 (8%) | NS |
| Peripheral vascular disease | 63 (8%) | 18 (7%) | 13 (5%) | 23 (9%) | NS |
| History of renal insufficiency | 27 (3%) | 9 (3%) | 10 (4%) | 8 (3%) | NS |
| STEMI | 637 (80%) | 241 (88%) | 231 (87%) | 232 (86%) | 0.002 |
| Anterior MI | 262 (33%) | 93 (34%) | 108 (41%) | 16 (43%) | 0.006 |
| Admission Killip class I | 686 (87%) | 229 (83%) | 200 (76%) | 180 (67%) | 0.001 |
| Time to admission ⩽3 h | 220 (27%) | 91 (33%) | 105 (40%) | 89 (33%) | 0.057 |
| Reperfusion therapy in STEMI | 308 (48%) | 136 (56%) | 145 (63%) | 129 (55%) | |
| Thrombolysis | 169 (26%) | 82 (34%) | 96 (42%) | 73 (31%) | 0.001 |
| Primary PCI | 139 (22%) | 54 (22%) | 49 (21%) | 56 (24%) | NS |
Data are mean (SD).
BP, blood pressure; CABG, coronary artery bypass graft; CHF, congestive heart failure; MI, myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST elevation myocardial infarction.
In‐hospital and one year outcomes
In‐hospital complications were more common in patients with raised admission glycaemia, and the increase in complication rates was linear in the three upper sextiles of the population (table 2). In addition, among the group without cardiogenic shock on admission, more patients with raised glycaemia subsequently developed cardiogenic shock (table 2).
Table 2 In‐hospital complications in patients without diabetes mellitus.
| Glycaemia sextile (mmol/l) | p Value | ||||
|---|---|---|---|---|---|
| ⩽6.88 | 6.88–7.88 | 7.88–9.27 | >9.27 | ||
| Five day mortality | 18 (2.3%) | 13 (4.7%) | 24 (9.1%) | 32 (11.9%) | 0.001 |
| In‐hospital mortality | 27 (3.7%) | 18 (6.5%) | 33 (12.5%) | 41 (15.2%) | 0.001 |
| Atrial fibrillation | 43 (5.5%) | 20 (7.3%) | 17 (6.5%) | 48 (17.8%) | 0.001 |
| Ventricular fibrillation | 11 (1.4%) | 7 (2.6%) | 17 (6.5%) | 18 (6.7%) | 0.001 |
| 2nd–3rd degree AV block | 22 (2.8%) | 8 (2.9%) | 12 (4.6%) | 23 (8.6%) | 0.001 |
| Development of cardiogenic shock | 15 (1.9%) | 11 (4.0%) | 17 (6.4%) | 14 (5.2%) | 0.002 |
| Killip deterioration (⩾2 classes) | 25 (3.1%) | 9 (3.3%) | 17 (6.4%) | 20 (7.4%) | 0.007 |
| Stroke | 2 (0.3%) | 2 (0.7%) | 3 (1.1%) | 2 (0.7%) | NS |
| LVEF ⩽35% | 57 (8%) | 27 (11%) | 28 (12%) | 46 (20%) | 0.001 |
Data are mean (SD).
AV, atrioventricular; LVEF, left ventricular ejection fraction.
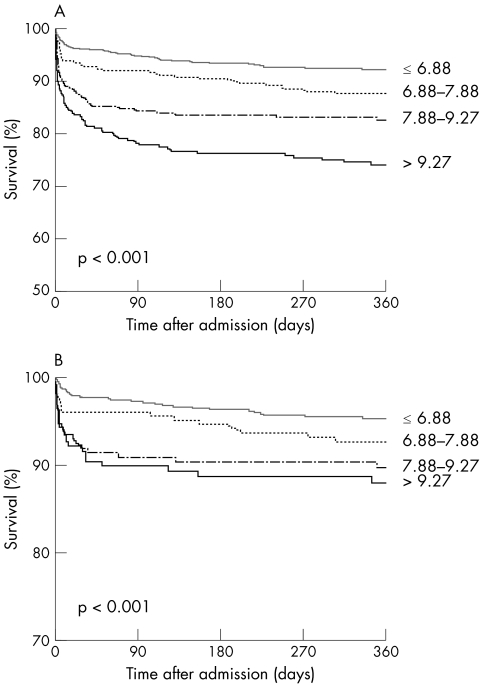
More patients in the highest blood glucose sextile had an ejection fraction measured during the hospital stay of ⩽ 35%. In‐hospital mortality was 3.7% in patients with admission glycaemia < 6.88 mmol/l, compared with 6.5% in patients in the fourth sextile, 12.5% in those in the fifth sextile and 15.2% in those in the upper sextile of admission glycaemia (p < 0.001). One year survival was 92.5% versus 88%, 83% and 75%, respectively (p < 0.001) (fig 1).
Figure 1 One year survival according to the presence of raised blood glucose (in mmol/l) on admission in the whole population (panel A) and in patients in Killip class I (panel B).
Prognostic role of admission blood glucose concentration according to Killip class on admission
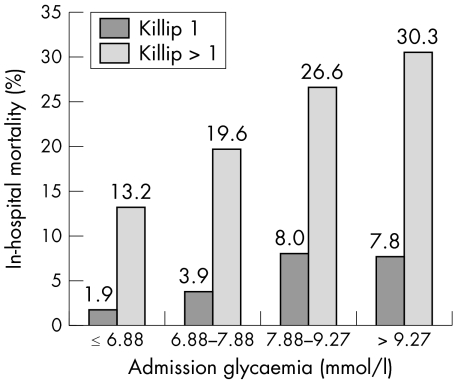
A strong interaction was found between blood glucose concentration and presence of left ventricular failure on admission (mean blood glucose: 7.33 (2.3), 8.16 (3.2), 8.71 (3.6), 11.1 (5.3) mmol/l for Killip classes I to IV, respectively, p < 0.001). Admission glycaemia was related to in‐hospital mortality in patients both with and without signs of heart failure on admission (fig 2). Likewise, in both groups, initial blood glucose concentrations strongly predicted one year survival.
Figure 2 In‐hospital mortality according to admission glycaemia in patients with or without signs of heart failure on admission.
Prognostic role of admission blood glucose in patient subgroups
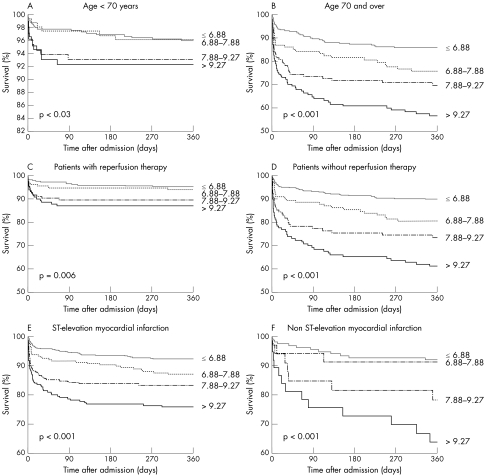
Initial blood glucose concentrations predicted one year outcomes in patients both ⩾ 70 and < 70 years old (fig 3). Blood glucose concentration was also a determinant of mortality regardless of whether they received reperfusion therapy at the acute stage. Lastly, blood glucose concentration was related to increased mortality in patients with STEMI as well as in those with non‐STEMI.
Figure 3 One year survival in subgroups of patients according to age, use of reperfusion therapy and type of infarction.
Predictors of 30 day and one year mortality by multivariate analysis
Multivariate analyses showed that admission blood glucose beyond the median value of 6.88 mmol/l was an independent and potent predictor of both in‐hospital and one year mortality in the whole population (table 3).
Table 3 Predictors of in‐hospital and one year mortality by multivariate regression analysis in patients without diabetes mellitus.
| In‐hospital mortality | Odds ratio | 95% CI | p Value |
|---|---|---|---|
| Age ⩾70 years | 3.41 | 1.75 to 6.65 | 0.001 |
| Killip class on admission (v class I) | 0.001 | ||
| II or III | 2.80 | 1.62 to 4.83 | |
| IV | 6.11 | 2.03 to 18.4 | |
| Admission glycaemia (v 3 first sextiles) | 0.001 | ||
| 4th sextile | 2.24 | 1.03 to 4.91 | |
| 5th sextile | 3.42 | 1.70 to 6.88 | |
| 6th sextile | 3.37 | 1.68 to 6.76 | |
| Admission SBP (v 1st tertile) | 0.009 | ||
| 2nd tertile | 0.50 | 0.26 to 0.94 | |
| 3rd tertile | 0.41 | 0.22 to 0.77 | |
| Anterior MI | 1.80 | 1.08 to 3.00 | 0.025 |
| Current smoking | 0.37 | 0.15 to 0.91 | 0.029 |
| One year mortality | Hazard ratio | 95% CI | p Value |
|---|---|---|---|
| Age ⩾ 70 years | 3.22 | 2.14 to 4.87 | 0.001 |
| Killip class on admission (v class I) | 0.001 | ||
| II or III | 2.96 | 2.05 to 4.28 | |
| IV | 6.76 | 3.57 to 12.8 | |
| Admission glycaemia (v 3 first sextiles) | 0.001 | ||
| 4th sextile | 1.64 | 1.00 to 2.71 | |
| 5th sextile | 1.81 | 1.14 to 2.87 | |
| 6th sextile | 2.29 | 1.49 to 3.51 | |
| Admission SBP (v 1st tertile) | 0.001 | ||
| 2nd tertile | 0.61 | 0.41 to 0.91 | |
| 3rd tertile | 0.45 | 0.29 to 0.69 | |
| Reperfusion therapy | 0.49 | 0.33 to 0.73 | 0.001 |
| History of PVD | 1.89 | 1.20 to 2.99 | 0.006 |
| Low BMI (1st quintile) | 1.65 | 1.17 to 2.33 | 0.004 |
| Anterior MI | 1.58 | 1.13 to 2.21 | 0.007 |
BMI, body mass index; MI, myocardial infarction; PVD, peripheral vascular disease; SBP, systolic blood pressure.
Increased blood glucose concentration was also an independent predictor of one year mortality in patients in Killip class I on admission; hazard ratios and 95% confidence intervals (CIs) for one year mortality versus admission blood glucose below the median value were for the fourth sextile, 1.50 (95% CI 0.74 to 3.05), for the fifth sextile, 2.25 (95% CI 1.16 to 4.33), and for the sixth sextile, 2.37 (95% CI 1.19 to 4.72). In patients who were discharged alive from the hospital, one year survival was 96% in patients with blood glucose below median, 94% and 95% in those in the fourth and fifth sextiles, and 88% in those in the sixth sextile (p < 0.0005). Cox multivariate analysis including baseline characteristics, ejection fraction and drugs at discharge showed that admission blood glucose in the upper sextile was associated with an increased risk of death (hazard ratio 1.90, 95% CI 1.13 to 3.22; p = 0.02), whereas only a non‐significant trend was noted for the fourth and fifth sextiles (hazard ratios 1.06 and 1.13, respectively).
DISCUSSION
In diabetic patients, acute hyperglycaemia during acute coronary syndromes is associated with adverse outcomes and with higher incidences of death and congestive heart failure.3,4,6,11 To our knowledge, our series is the largest to date describing the impact of admission glycaemia on short and long term outcomes in non‐diabetic patients with AMI. It documents that the association between increased blood glucose concentrations and outcomes is not confined to patients with diabetes and that raised blood glucose concentrations on admission are potent predictors of both early and late mortality. The increased mortality in our patients was observed only beyond a threshold of 6.88 mmol/l, which was the median admission glycaemia, and it appeared linear beyond this concentration. Importantly, the deleterious prognostic significance of increased admission glycaemia was observed in a variety of subsets of our population: patients with or without signs of left ventricular failure on admission, older and younger patients, patients with STEMI or non‐STEMI, or those receiving or not receiving reperfusion therapy. In addition, increased blood glucose concentration on admission was associated with the most severe complications at the acute stage, such as ventricular fibrillation, atrial fibrillation or the development of cardiogenic shock.
Glycaemia and short term outcomes
In a systematic review and meta‐analysis of 15 studies in AMI populations with and without diabetes, Capes et al6 showed that in diabetic and non‐diabetic patients stress hyperglycaemia was associated with an increased risk of in‐hospital death. In addition, glucose concentrations of 8–10 mmol/l were associated with a higher risk of developing heart failure or cardiogenic shock. Similar findings were recently reported in a registry of patients with myocardial infarction where hyperglycaemia was associated with an increased risk of developing cardiogenic shock during the initial hospital stay.7 The populations included in the meta‐analysis,6 however, were heterogeneous, with few patients treated with reperfusion therapy or currently recommended medications, and various definitions of hyperglycaemia (measured on admission or fasting blood samples) were used. Suleiman et al12 analysed the additive prognostic value of admission glycaemia and fasting blood glucose in a population of 735 non‐diabetic patients admitted for AMI. They showed that fasting blood glucose concentration was a potent indicator of 30 day mortality and appeared more discriminant than admission blood glucose; no long term data were reported. The early mortality figures in this comparatively young population (59 to 64 years), however, were very high (29% 30 day mortality in patients with fasting blood glucose concentrations > 7.71 mmol/l, not taking into account patients dying before fasting blood glucose could be measured). However, from the clinical standpoint, fasting blood glucose and admission blood glucose concentrations provide different indications, as the fasting blood glucose cannot be used to take specific therapeutic measures during the first hours of acute ischaemia.
Conversely, Foo et al13 in a cohort of 2127 patients presenting with acute coronary syndromes, including only a minority with STEMI, analysed major complications by quartiles of admission blood glucose concentrations. Although admission glycaemia was related to in‐hospital mortality by univariate analysis, its prognostic significance disappeared when left ventricular failure was included in the statistical models. In contrast, in our patients, admission glycaemia was an independent and powerful predictor of in‐hospital and late mortality in the presence or absence of left ventricular failure and whatever the type of infarction (STEMI or non‐STEMI), as shown by the subgroup and multivariate analyses.
Glycaemia and long term outcomes
Recently, Stranders et al5 in a retrospective study of 737 non‐diabetic patients with AMI found that a 1 mmol/l increase in blood glucose was associated with a 4% increase in long term mortality. In this study, however, in‐hospital mortality was very low (5%), particularly when considering that the patients were admitted from 1989 to 1996 and that only 2% of them underwent primary angioplasty; in addition, and at variance with all other studies, in‐hospital mortality was not different according to the initial blood glucose concentration. This suggests that the population studied may have been selected and not representative of most patients admitted with AMI. In contrast, our registry had a broad national coverage, with 83% of the institutions admitting patients with AMI participating.
Potential mechanisms involved
The reasons why increased blood glucose concentrations may increase mortality remain partly speculative. Several mechanisms may be involved. Firstly, raised blood glucose may correspond to a pre‐diabetic state unmasked under stressful conditions. Many of the non‐diabetic patients with raised blood glucose have undiagnosed diabetes. Norhammar et al14 found that 65% of non‐diabetic patients with glycaemia < 11 mmol/l had undiagnosed diabetes or impaired glucose tolerance. Likewise, in the study from Suleiman et al,12 admission blood glucose concentrations were correlated with fasting blood glucose. Diabetic patients may have worse outcomes for many reasons, including more severe coronary artery disease, diabetic cardiomyopathy, autonomic dysfunction and decreased endogenous fibrinolytic activity.15,16,17 Secondly, there is strong experimental and clinical evidence that hyperglycaemia per se may be detrimental. Acute hyperglycaemia attenuates endothelium dependent vasodilatation in humans in vivo, abolishes the effect of ischaemic preconditioning through attenuation of mitochondrial ADP regulated potassium channel activation, and induces oxidative stress affecting platelet function coagulation and fibrinolysis.18,19,20,21 At the acute stage of myocardial infarction, hyperglycaemia is a predictor of impaired coronary flow before reperfusion therapy.22 In addition, in patients treated with primary PCI for STEMI, Iwakura et al,23 by using myocardial contrast echocardiography, also showed a strong association between admission blood glucose concentrations and the occurrence of a no‐reflow phenomenon after angioplasty, which has a documented deleterious impact on clinical outcome. Lastly, admission hyperglycaemia may be not only the cause of more severe myocardial damage but also its consequence. Large infarcts are more likely to cause catecholamine release, which affects fatty acid and glucose homeostasis. The catecholamine response is proportional to the severity of the infarct, as confirmed by the correlation between admission blood glucose and heart rate or Killip class on admission.24 In a study by Oswald et al25 concentrations of cortisol, epinephrine and norepinephrine were the main determinants of plasma glucose concentration measured in non‐diabetic patients with AMI. The fact that hyperglycaemia was a prognostic indicator in our patients without heart failure, however, suggests that it is a true determinant of outcome, rather than a simple consequence of a larger infarct size.
Conclusion
The present study, which to our knowledge is the largest to date in such a population, emphasises the major prognostic significance of blood glucose concentration on admission in patients with AMI. Even in non‐diabetic patients, hyperglycaemia on admission is independently associated with a higher risk of developing acute left ventricular failure, as well as with a higher risk of in‐hospital and long term mortality. These findings suggest that adequate metabolic control of blood glucose would be an important treatment target, even in non‐diabetic patients, to limit the deleterious effect of increased blood glucose in the setting of acute myocardial ischaemia. The best therapeutic methods to achieve such a goal, however, remain to be determined.
Abbreviations
AMI - acute myocardial infarction
CI - confidence interval
STEMI - ST elevation myocardial infarction
Footnotes
The USIC 2000 was made possible through an unrestricted grant from Laboratoire Sanofi‐Aventis
Several of the authors received honoraria from Sanofi‐Aventis for participating in the steering committee of the USIC registry
References
- 1.Malmberg K, Ryden L. Myocardial infarction in patients with diabetes mellitus. Eur Heart J 19889256–264. [DOI] [PubMed] [Google Scholar]
- 2.Malmberg K, Norhammar A, Wedel H.et al Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction. Long‐term results from the diabetes and insulin‐glucose infusion in acute myocardial infarction (DIGAMI) study. Circulation 1999992626–2632. [DOI] [PubMed] [Google Scholar]
- 3.Malmberg K, Ryden L, Wedel H.et al Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J 200526650–661. [DOI] [PubMed] [Google Scholar]
- 4.Norhammar A M, Ryden L, Malmberg K.et al Admission plasma glucose: independent risk factor for long term prognosis after myocardial infarction even in non diabetic patients. Diabetes Care 1999221827–1831. [DOI] [PubMed] [Google Scholar]
- 5.Stranders I, Diamant M, van Gelder R E.et al Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus. Arch Intern Med 2004164982–988. [DOI] [PubMed] [Google Scholar]
- 6.Capes S, Hunt D, Malmberg K.et al Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 2000355773–778. [DOI] [PubMed] [Google Scholar]
- 7.Zeller M, Cottin Y, Brindisi M C.et al Impaired fasting glucose and cardiogenic shock in patients with acute myocardial infarction. Eur Heart J 200425308–312. [DOI] [PubMed] [Google Scholar]
- 8.Hanania G, Cambou J P, Guéret P.et al for the USIC 2000 investigators. Management and in‐hospital outcome of patients with acute myocardial infarction admitted to intensive care units at the turn of the century: results from the French nationwide USIC 2000 registry, Heart 2004901404–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danchin N, Blanchard D, Steg P G.et al Impact of prehospital thrombolysis for acute myocardial infarction on 1‐year outcome: results from the French nationwide USIC 2000 registry. Circulation 20041101909–1915. [DOI] [PubMed] [Google Scholar]
- 10.Danchin N, Vaur L, Genès N.et al Management of acute myocardial infarction in intensive care units in 1995: a nationwide French survey of practice and early hospital results. J Am Coll Cardiol 1997301598–1605. [DOI] [PubMed] [Google Scholar]
- 11.Fava S, Aquilina O, Azzopardi J.et al The prognostic value of blood glucose in diabetic patients with acute myocardial infarction. Diabetic Med 19961380–83. [DOI] [PubMed] [Google Scholar]
- 12.Suleiman M, Hammerman H, Boulos M.et al Fasting glucose is an important independent risk factor for 30‐day mortality in patients with acute myocardial infarction: a prospective study. Circulation 2005111754–760. [DOI] [PubMed] [Google Scholar]
- 13.Foo K, Cooper J, Deaner A.et al A single serum glucose measurement predicts adverse outcome across the whole range of acute coronary syndromes. Heart 200389512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norhammar A, Tenerz A, Nilsson G.et al Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet 20023592140–2144. [DOI] [PubMed] [Google Scholar]
- 15.Ledru F, Ducimetière P, Battaglia S.et al New diagnostic criteria for diabetes and coronary artery disease: insights from an angiographic study. J Am Coll Cardiol 2001371543–1550. [DOI] [PubMed] [Google Scholar]
- 16.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med 19883181315–1321. [DOI] [PubMed] [Google Scholar]
- 17.Davi G, Catalano I, Averna M.et al Thromboxane biosynthesis and platelet function in type II diabetes mellitus. N Engl J Med 19903221769–1774. [DOI] [PubMed] [Google Scholar]
- 18.Kersten J R, Schmeling T J, Orth K G.et al Acute hyperglycaemia abolishes ischemic preconditioning in vivo. Am J Physiol Heart Circ Physiol 1998275H721–H725. [DOI] [PubMed] [Google Scholar]
- 19.Kersten J R, Toller W G, Tessmer J P.et al Hyperglycaemia reduces coronary blood flow through a nitric oxide‐mediated mechanism. Am J Physiol Heart Circ Physiol 2001281H2097–H2104. [DOI] [PubMed] [Google Scholar]
- 20.Di Carli M F, Janisse J, Grunberger G.et al Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol 2003411387–1393. [DOI] [PubMed] [Google Scholar]
- 21.Shechter M, Merz M B, Paul‐Labrador M J.et al Blood glucose and platelet‐dependent thrombosis in patients with coronary artery disease. J Am Coll Cardiol 200035300–307. [DOI] [PubMed] [Google Scholar]
- 22.Timmer J R, Ottervanger J P, de Boer M J.et al Hyperglycemia is an important predictor of impaired coronary flow before reperfusion therapy in ST‐segment elevation myocardial infarction. J Am Coll Cardiol 200545999–1002. [DOI] [PubMed] [Google Scholar]
- 23.Iwakura K, Ito H, Ikushima M.et al Association between hyperglycemia and the no‐reflow phenomenon in patients with acute myocardial infarction. J Am Coll Cardiol 2003411–7. [DOI] [PubMed] [Google Scholar]
- 24.Karlsberg R P, Cryer P E, Roberts R.et al Serial plasma catecholamine response early in the course of clinical acute myocardial infarction: relation to infarct extent and mortality. Am Heart J 198110224–29. [DOI] [PubMed] [Google Scholar]
- 25.Oswald G A, Smith C C T, Betteridge D J.et al Determinants and importance of stress hyperglycemia in non‐diabetic patients with myocardial infarction. BMJ 1986293917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]