Abstract
Objective
To study the cardiac geometric changes after transcatheter closure of large atrial septal defects (ASDs) according to patient age at the time of the procedure.
Design
Prospective echocardiographic follow‐up study.
Setting
Tertiary referral centre.
Patients and intervention
25 asymptomatic patients younger than 16 years (median 8 years; group 1) and 21 asymptomatic adults (median 38 years; group 2) underwent percutaneous closure of large ASD with the Amplatzer septal occluder device (mean 25 (SD 7) mm).
Main outcome measures
Cardiac remodelling was assessed by M mode and two dimensional echocardiography one and six months after ASD closure.
Results
By six months, right atrial volume decreased from 31 (15) to 19 (5) ml/m2 (p < 0.001) and right ventricular (RV) transverse diameter decreased from 29.8 (8.6) to 23.2 (5.6) mm/m2 (p < 0.001). Conversely, left atrial volume did not change significantly (from 18 (6) to 20 (6) ml/m2, NS) and left ventricular (LV) transverse diameter increased from 27.8 (6.4) to 31.8 (7.3) mm/m2 (p < 0.05). Ventricular remodelling resulted in an RV:LV diameter ratio decrease from 1.1 (0.2) to 0.7 (0.1) (p < 0.001). The magnitude and time course of cardiac remodelling did not differ significantly between the age groups. Indeed, right atrial volume decreased by 33 (26)% versus 37 (23)%, RV diameter decreased by 26 (10)% versus 20 (13)%, LV diameter increased by 17 (15)% versus 15 (10)%, and RV:LV diameter ratio decreased by 36 (8)% versus 27 (15)% in groups 1 and 2, respectively.
Conclusions
Cardiac remodelling after percutaneous ASD closure seems to be independent of the patient's age at the time of the procedure up to early adulthood. Thus, postponing ASD closure for a few years may be a reasonable option for potentially suitable asymptomatic children.
Keywords: atrial septal defect, device, echocardiography, cardiac remodelling
Atrial septal defect (ASD) is a leading cause of chronic right chamber volume overload.1,2 Progressive right chamber dilatation is a major predictor of long term arrhythmias and left ventricular (LV) functional impairment, affecting cardiac performance even in asymptomatic adult patients.3,4,5,6,7 It is widely accepted that the more prolonged the volume overload, the less complete the cardiac remodelling after ASD closure. Thus, early treatment of this malformation during infancy has been suggested.8 Over time, the transcatheter approach evolved as a reliable and safe alternative to surgery in both paediatric and adult patients.9,10 Although this approach may be safely and successfully performed even in very young children, its failure rate may not be negligible in this subset of patients.11,12,13 In addition, the long term mechanical impact of a relatively stiff prosthesis inside a growing heart is not completely known and is being extensively investigated.14,15 Lastly, postponing the procedure for a few years may make the difference between the transcatheter and the surgical option in younger, smaller patients. To date, no study has specifically addressed age related cardiac remodelling after percutaneous closure of large ASDs, comparing the extent and time course of these changes between asymptomatic paediatric and young adult patients. These data may help clinical decision making on the timing of percutaneous ASD treatment in the era of device closure.
METHODS
Patient population
Between March 2000 and March 2004, 46 patients with a large ASD (that is, stretched diameter > 20 mm or invasive pulmonary to systemic flow ratio > 1.5:1) underwent transcatheter closure at our institution. On the basis of patient age at the time of the procedure, two groups (group 1, patients < 16 years, n = 25; group 2, adults, n = 21) were identified. Table 1 summarises clinical and haemodynamic data of both the whole population and the age subgroups. Neither paediatric nor adult patients were symptomatic, having had the ASD diagnosed during routine screening.
Table 1 Clinical and haemodynamic data according to the patients' age at the procedure.
| Variable | Total population | Group 1 | Group 2 | p Value* |
|---|---|---|---|---|
| Age (years) | 22 (18) | 9 (4) | 38 (15) | NA |
| Body surface area (m2) | 1.4 (0.4) | 1.2 (0.2) | 1.7 (0.1) | <0.001 |
| ASD stretched diameter (mm) | 23 (7) | 20 (6) | 27 (6) | <0.05 |
| Mean PA pressure (mm Hg) | 22 (6) | 23 (6) | 21 (6) | NS |
| Qp:Qs | 2.2 (0.9) | 2.2 (0.9) | 2.2 (1.0) | NS |
Data are mean (SD).
*Group 1 v group 2.
ASD, atrial septal defect; NA, not applicable; PA, pulmonary artery; Qp, pulmonary blood flow; Qs, systemic blood flow.
Interventional procedure
All patients or children's parents provided informed consent for the interventional procedure. ASDs were closed under continuous transoesophageal echocardiographic monitoring with a 5 MHz multiplane probe (Acuson Sequoia; Acuson Corporation, Mountain View, California, USA). In all instances, the Amplatzer septal occluder device (AGA Medical Corporation, Golden Valley, Minnesota, USA) was used. The ASD was sized by the pulling technique (large occlusion balloon; Meditech, Boston Scientific Cork, Cork, Ireland), with the occluding device chosen to be within 2 mm of the balloon stretched diameter. After a complete haemodynamic evaluation, the device was delivered through its specific sheath as previously described.9,10,16,17,18 Aspirin treatment (3–5 mg/kg) was started 24 hours before ASD closure and continued for six months.
Echocardiographic data collection
All echocardiographic studies were performed by two experienced observers (MP, SC) with a Sequoia C256 system (Acuson Corporation) equipped with a 3V2c or 7V3c probe before and one and six months after device implantation. Images were acquired and digitally stored for offline analysis at the end of each study. The observers were blinded to the previous ultrasound data. Echocardiographic measurements were averaged from three consecutive beats. Right ventricular (RV) and LV dimensions were assessed by M mode in the parasternal long axis view and by two dimensional B mode in the four chamber view, according to the recommendations of the American Society of Echocardiography.19 Values were indexed for body surface area. The RV to LV diameter ratio (RV:LV) was calculated from both M mode and two dimensional measurements. LV ejection fraction was derived by the Teichholz formula.20 Left atrial anteroposterior dimension was assessed at end systole by M mode in the parasternal long axis view. In the four chamber view, both right atrial and left atrial dimensions were obtained by two dimensional echocardiography as end systolic mediolateral and superoinferior diameters. Atrial volume was then derived by a length–diameter ellipsoid method and indexed for body surface area.3,21 Echocardiographic data and the time course of cardiac remodelling after ASD closure were analysed both pooled and after the population was divided into two subgroups according to patient age at the time of the procedure.
Statistical analysis
Data were statistically analysed by SPSS for Windows release 11.0 (SPSS, Chicago, Illinois, USA). Results are expressed as mean (SD). Groups were compared by the χ2 test. Multiple stages were compared by one way analysis of variance. When differences were found, interstage data were compared by the Bonferroni test. Age subgroups were compared by the independent sample t test. Differences were considered significant at p < 0.05 (two sided). Linear regression analysis and partial correlation test by Pearson's method were done to assess univariate relationships.
RESULTS
Transcatheter ASD closure was successfully performed in all patients with the Amplatzer septal occluder device (mean diameter 25 (SD 7) mm, range 11–38, median 24), achieving a complete occlusion in 100% of patients at six months after the procedure.
Echocardiographic changes
ASD closure caused pronounced early cardiac remodelling (fig 1), with a trend that steadily continued over the next few months (table 2). By six months, both right atrial volume and RV diameters had decreased significantly, whereas left atrial volume had not changed and LV diameters had increased. Ventricular remodelling resulted in a similar and significant decrease of the RV:LV diameter ratio on two dimensional (from 1.1 (0.2) to 0.7 (0.1), p < 0.001) and M mode analyses (from 0.9 (0.2) to 0.6 (0.1), p < 0.001). At six months, RV dimension had not decreased in three patients (two patients < 16 years, one patient > 16 years) as derived by either two dimensional or M mode echocardiography. After ASD closure, LV ejection fraction increased from 62 (15)% to 68 (10)% (NS).
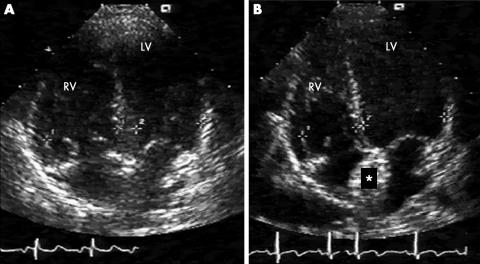
Figure 1 Cardiac volumetric unloading after percutaneous atrial septal defect (ASD) closure. (A) Right to left volumetric unbalance is clearly evident before ASD closure and (B) is almost completely reverted six months after the procedure. LV, left ventricle; RV, right ventricle. *Amplatzer septal occluder device.
Table 2 Echocardiographic data before and after atrial septal defect closure in the whole population.
| Variable | Before closure | After closure | p Value* | |
|---|---|---|---|---|
| 1 month | 6 months | |||
| RA volume (ml/m2) | 31 (15) | 20 (7) | 19 (5) | <0.001 |
| LA volume (ml/m2) | 18 (6) | 18 (5) | 20 (6) | NS |
| RV diameter (mm/m2) | ||||
| 2D | 29.8 (8.6) | 25.9 (6.4) | 23.2 (5.6) | <0.001 |
| M mode | 24.8 (6.6) | 19.2 (4.6) | 18.1 (4.1) | <0.001 |
| LV diameter (mm/m2) | ||||
| 2D | 27.8 (6.4) | 31.9 (7.3) | 31.8 (7.3) | <0.05 |
| M mode | 28.6 (6.0) | 33.4 (7.9) | 33.3 (7.3) | <0.01 |
| RV:LV | ||||
| 2D | 1.1 (0.2) | 0.8 (0.1) | 0.7 (0.1) | <0.001 |
| M mode | 0.9 (0.2) | 0.6 (0.1) | 0.6 (0.1) | <0.001 |
| LVEF (%) | 62 (15) | 68 (9) | 68 (10) | NS |
Data are mean (SD).
*Six months v before.
2D, by two dimensional echocardiography; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; M mode, by M mode echocardiography; RA, right atrial; RV, right ventricular
Age related cardiac remodelling
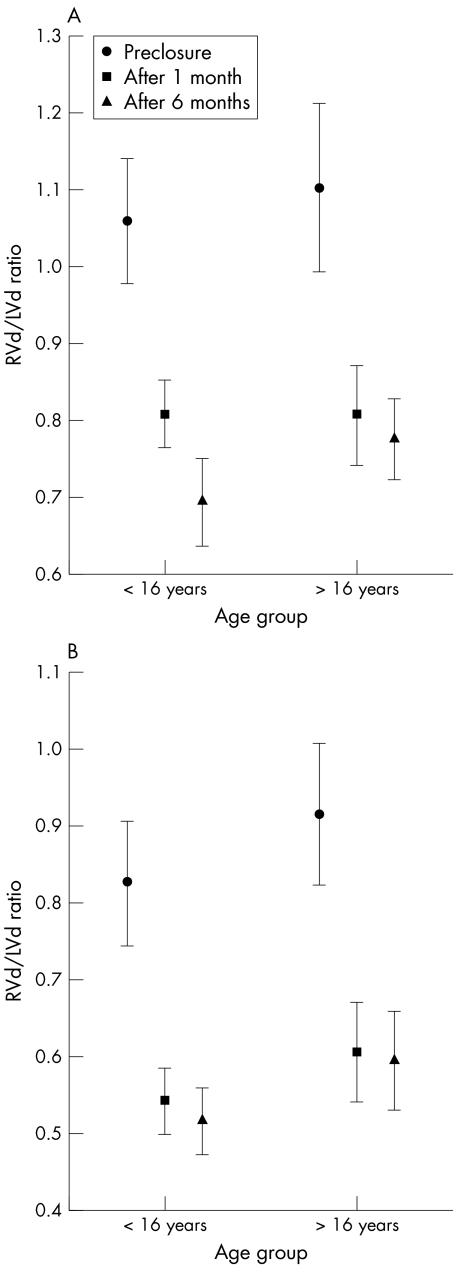
The extent of right chamber volume overload relief did not differ significantly between paediatric and adult patients, with chamber size reverting to normal within a few weeks (table 3).22 By six months, right atrial volume had decreased by 33 (26)% in group 1 (p < 0.001 v before closure) and by 37 (23)% in group 2 (p < 0.001 v before closure), RV transverse diameter had decreased by 26 (10)% in group 1 (p < 0.001 v before closure) and 20 (13)% in group 2 (p < 0.001 v before closure), and LV transverse diameter had increased by 17 (15)% in group 1 (p < 0.05 v before closure) and 15 (10)% in group 2 (p < 0.05 v before closure). These changes resulted in a decrease in RV:LV diameter ratio by 36 (8)% in group 1 (p < 0.001 v before closure) and by 27 (15)% in group 2 (p < 0.001 v before closure; NS between groups). In addition, no difference between groups was found in the time course of RV diameter index and RV:LV diameter ratio decrease (fig 2) after ASD closure. Lastly, a similar correlation between the preprocedural RV:LV diameter ratio and its decrease after shunt disappearance was found in both of the age groups either on M mode (group 1: y = 0.0826 × −0.377, r2 = 0.778; group 2: y = 0.735 × −0.362, r2 = 0.712) and two dimensional analysis (group 1: y = 0.575 × −0.241, r2 = 0.488; group 2: y = 0.925 × −0.685, r2 = 0.912).
Table 3 Echocardiographic measurements according to the patient age at the time of the procedure.
| Variable | Group 1 | Group 2 | p Value* |
|---|---|---|---|
| RA volume (ml/m2) | |||
| Before | 30 (18) | 32 (11) | |
| 1 month | 18 (6) | 22 (9) | |
| 6 months | 20 (6) | 19 (7) | |
| Decrease (%) | 33 (26) | 37 (23) | NS |
| p Value (within groups) | <0.05 | <0.01 | |
| LA volume (ml/m2) | |||
| Before | 18 (6) | 20 (5) | |
| 1 month | 18 (5) | 20 (6) | |
| 6 months | 20 (5) | 19 (7) | |
| p Value (within groups) | NS | NS | |
| RV diameter index (2D) (mm/m2) | |||
| Before | 34.2 (9.4) | 25.1 (3.8) | |
| 1 month | 29.6 (6.1) | 21.3 (2.8) | |
| 6 months | 25.7 (6.6) | 20.2 (1.7) | |
| Decrease (%) | 26 (10) | 20 (13) | NS |
| p Value (within groups) | <0.01 | <0.001 | |
| RV diameter index (M mode) (mm/m2) | |||
| Before | 26.3 (8.0) | 22.9 (3.7) | |
| 1 month | 20.4 (5.1) | 17.4 (3.3) | |
| 6 months | 19.1 (4.3) | 16.9 (3.3) | |
| Decrease (%) | 23 (15) | 30 (16) | NS |
| p Value (within groups) | <0.001 | <0.001 | |
| LV diameter index (2D) (mm/m2) | |||
| Before | 31.9 (6.3) | 23.1 (2.2) | |
| 1 month | 36.4 (6.6) | 26.3 (2.6) | |
| 6 months | 36.3 (7.1) | 26.3 (1.8) | |
| Increase (%) | 17 (15) | 15 (10) | NS |
| p Value (within groups) | <0.01 | <0.01 | |
| LV diameter index (M mode) (mm/m2) | |||
| Before | 31.6 (6.5) | 25.1 (2.7) | |
| 1 month | 37.4 (8.0) | 28.1 (3.0) | |
| 6 months | 37.6 (7.3) | 28.1 (3.2) | |
| Increase (%) | 22 (13) | 11 (8) | NS |
| p Value (within groups) | <0.001 | <0.01 | |
| RV:LV (2D) | |||
| Before | 1.1 (0.2) | 1.1 (0.2) | |
| 1 month | 0.8 (0.1) | 0.8 (0.1) | |
| 6 months | 0.7 (0.1) | 0.8 (0.1) | |
| Decrease (%) | 36 (8) | 27 (15) | NS |
| p Value (within groups) | <0.001 | <0.001 | |
| RV:LV ratio (M mode) | |||
| Before | 0.8 (0.2) | 0.9 (0.2) | |
| 1 month | 0.6 (0.1) | 0.6 (0.2) | |
| 6 months | 0.5 (0.1) | 0.6 (0.1) | |
| Decrease (%) | 37 (13) | 33 (17) | NS |
| p Value (within groups) | <0.001 | <0.001 | |
| LVEF increase (%) | |||
| Before | 63 (13) | 61 (18) | |
| 1 month | 69 (8) | 67 (11) | |
| 6 months | 70 (6) | 64 (15) | NS |
| p Value (within groups) | <0.001 | NS | |
Data are mean (SD).
*Between groups.
2D, by two dimensional echocardiography; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; M mode, by M mode echocardiography; RA, right atrial; RV, right ventricular
Figure 2 Time course of age related changes in the ratio of right ventricular (RVd) to left ventricular dimension (LVd) on (A) two dimensional four chamber view and (B) M mode long axis view echocardiography.
DISCUSSION
Natural history studies have shown that right chamber volume overload caused by atrial shunt tends to increase progressively over time, thereby affecting cardiac performance even in asymptomatic patients.4,5,6,7 Thus, the target of ASD closure is either to treat symptoms or to prevent this progressive cardiac enlargement in asymptomatic patients. There is no doubt that symptomatic patients should undergo ASD closure as soon as possible, regardless of age. Asymptomatic patients should be treated as well, because of a favourable functional impact of ASD closure on cardiac geometry and performance.6,7 The timing of ASD closure in this subset of patients, however, needs to be readdressed in the device era for several reasons. Firstly, the benefits of early ASD closure in childhood over young adulthood are less clear in the asymptomatic patient, in which case the benefits have to be weighed against the potential risks associated with device implantation. In fact, although percutaneous closure may be safe and successful even in very young children, the failure rate of this option may be as high as 16% in this subset of patients.11,12,13 In addition, the long term mechanical impact of a quite rigid device inside a growing heart is poorly understood. A recent retrospective study showed that a high device diameter to patient size ratio was an independent risk factor for the development of atrioventricular block after percutaneous ASD closure with the Amplatzer septal occluder device.15 Secondly, to date no studies have specifically shown an increased risk of late arrhythmias or heart failure in patients undergoing ASD closure in early adulthood compared with childhood. Moreover, although early striking volumetric changes have been consistently reported, very few and conflicting data are available on cardiac remodelling after percutaneous ASD closure in children and young adults depending on volume overload duration.7,16,17,18,23 Indeed, Du et al16 reported only a weak negative correlation between age and RV dimensional changes 24 hours after ASD closure. Kort et al18 found that age at the time of the procedure had no effect on RV remodelling and only a weak effect on right atrial remodelling at the 12 month follow up. On the basis of these scanty and conflicting data, postponing the percutaneous ASD closure for a few years may be advisable for younger patients.
In agreement with previous studies, our data showed that transcatheter closure caused early and striking cardiac volumetric changes.7,16,17,18,23 By six months, right chamber size reverted to normal and this cardiac remodelling resulted in a 12% increase of LV ejection fraction. In our series, the extent and time course of these changes were not affected by the patient's age at the time of the procedure. In fact, we found a similar and significant relationship between pre‐closure volume overload and six month cardiac remodelling in both age groups. The time course was also age independent, with most of the cardiac remodelling developing within one month after ASD closure. Our data partially contradict previous studies showing an age dependence of the cardiac remodelling potential, after either surgical or percutaneous ASD closure.16,17,18,24,25,26,27 These studies did not, however, detail the demographic and haemodynamic data of the adult groups. Thus, it is possible that the mean age, as well as the pulmonary artery pressure, of their “adult” populations was higher than that of ours. In fact, the cardiac remodelling potential may be partially lost in very old patients due to a progressive myocardial fibrosis seen with ageing or longlasting pulmonary hypertension.28 This has been found in experimental models as well as in patients with combined pressure–volume overload by using back scattering ultrasound analysis.28,29 This hypothesis is also in agreement with previous echocardiographic studies that found a significant impairment of cardiac remodelling capacity in very old patients compared with younger adults after either surgical or percutaneous ASD closure.27,30 Conversely, a normal remodelling potentiality is expected in patients with ASD without pulmonary hypertension up to early adulthood, as highlighted by the lack of cardiac ultrastructural abnormalities seen in back scattering ultrasound analysis of pure right chamber volume overload.29 Thus, the cardiac remodelling capacity conceivably would not be affected by the volume overload itself but by combined volume and pressure overload resulting from a progressive pulmonary vasculopathy complicating a longlasting atrial shunt.
Study limitations
The study goal of improving the timing of percutaneous ASD closure in asymptomatic children may be hampered by two major theoretical limitations. Firstly, non‐randomised patient enrolment introduced a potentially significant selection bias—that is, the older patients may have had a more favourable disease course than that of patients undergoing device closure at a younger age. This thesis is disproved by the lack of symptoms, as well as a similar haemodynamic burden, in both our age groups. Secondly, this study focused on geometric cardiac remodelling after ASD closure, which is only one of the reasons for treatment. It did not address the potential risk of pulmonary vascular disease and atrial arrhythmias, which can be associated with treatment deferral. As inferred from natural history studies, however, the potential of these chronic complications is negligible up to early adulthood and they should be weighed against the functional consequences of implantation of large devices in small patients or even of surgical closure.
Conclusions
Our study shows that positive cardiac remodelling occurs very early after percutaneous closure of large ASDs, irrespective of the magnitude and duration of volume overload. Our results raise questions about whether the historically accepted approach of early treatment of large ASD should be revised in the era of transcatheter closure. Our results suggest that percutaneous ASD closure may be deferred until later in life in potentially suitable but small patients, perhaps during adolescence, when growth has progressed. This may be a wise approach, awaiting more conclusive data about the long term effects of ASD devices on the growing heart. Longer term studies of larger cohorts, as well as using more specific functional and biochemical end points, are still required, however, before any definitive conclusion may be drawn.31,32,33,34
Abbreviations
ASD - atrial septal defect
LV - left ventricular
RV - right ventricular
References
- 1.Porter C J, Feldt R H, Edward W D.et al Atrial septal defects. In: Emmanouilides GC, Riemenschneider TA, Allen HD, et al, eds. Heart disease in infants, children and adolescents, including the fetus and young adults. 5th ed. Baltimore: Williams & Wilkins, 1995687–703.
- 2.Campbell M. Natural history of atrial septal defect. Br Heart J 197032820–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morton J B, Sanders P, Vohra J K.et al Effect of chronic right atrial stretch on atrial electrical remodeling in patients with an atrial septal defect. Circulation 20031071775–1782. [DOI] [PubMed] [Google Scholar]
- 4.Ascah K J, King M E, Gillam L D.et al The effects of right ventricular hemodynamics on left ventricular configuration. Can J Cardiol 1990699–106. [PubMed] [Google Scholar]
- 5.Walker R E, Moran A M, Gavreau K.et al Evidence of adverse ventricular interdependence in patients with atrial septal defects. Am J Cardiol 2004931374–1377. [DOI] [PubMed] [Google Scholar]
- 6.Brochu M C, Baril J F, Dore A.et al Improvement in exercise capacity in asymptomatic and mildly symptomatic adults after atrial septal defect percutaneous closure. Circulation 20021061821–1826. [DOI] [PubMed] [Google Scholar]
- 7.Giardini A, Donti A, Formigari R.et al Determinants of cardiopulmonary functional improvement after transcatheter atrial septal defect closure in asymptomatic adults. J Am Coll Cardiol 2004431886–1891. [DOI] [PubMed] [Google Scholar]
- 8.Rocchini A P. Transcatheter closure of atrial septal defect: past, present and future. Circulation 1990821044–1045. [DOI] [PubMed] [Google Scholar]
- 9.Thomson J D, Aburawi E H, Watterson K G.et al Surgical and transcatheter (Amplatzer) closure of atrial septal defects: a prospective comparison of results and costs. Heart 200287466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du Z D, Hijazi Z M, Kleinman C S.et al Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol 2002391836–1844. [DOI] [PubMed] [Google Scholar]
- 11.Vogel M, Berger F, Dahnert I.et al Treatment of atrial septal defects in symptomatic children aged less than 2 years of age using the Amplatzer septal occluder. Cardiol Young 200010534–537. [DOI] [PubMed] [Google Scholar]
- 12.Bjornstad P G, Holmstrom H, Smevik B.et al Transcatheter closure of atrial septal defects in the oval fossa: is the method applicable in small children? Cardiol Young 200212352–356. [DOI] [PubMed] [Google Scholar]
- 13.Butera G, De Rosa G, Chessa M.et al Transcatheter closure of atrial septal defect in young children: results and follow‐up. J Am Coll Cardiol 200342241–245. [DOI] [PubMed] [Google Scholar]
- 14.Lange A, Coleman D M, Palka P.et al Effect of catheter device closure of atrial septal defect on diastolic mitral annular motion. Am J Cardiol 200391104–108. [DOI] [PubMed] [Google Scholar]
- 15.Suda K, Raboisson M J, Piette E.et al Reversible atrio‐ventricular block associated with closure of atrial septal defect using the Amplatzer device. J Am Coll Cardiol 2004431677–1682. [DOI] [PubMed] [Google Scholar]
- 16.Du Z D, Cao Q L, Koenig P.et al Speed of normalization of right ventricular volume overload after transcatheter closure of atrial septal defect in children and adults. Am J Cardiol 2001881450–1453. [DOI] [PubMed] [Google Scholar]
- 17.Veldtman G R, Razack V, Siu S.et al Right ventricular form and function after percutaneous atrial septal defect device closure. J Am Coll Cardiol 2001372108–2113. [DOI] [PubMed] [Google Scholar]
- 18.Kort H W, Balzer D T, Johnson M C. Resolution of right heart enlargement after closure of secundum atrial septal defect with transcatheter technique. J Am Coll Cardiol 2001381528–1532. [DOI] [PubMed] [Google Scholar]
- 19.American Society of Echocardigraphy American Society of Echocardiography committee on standards, subcommittee on quantitation of two‐dimensional echocardiography. Recommendation for quantitation of the left ventricular by two‐dimensional echocardiography. J Am Soc Echocardiogr 19892358–367. [DOI] [PubMed] [Google Scholar]
- 20.Teichholz L E, Kreulen T, Herman M V.et al Problems in echocardiographic volume determinations: echocardiographic‐angiographic correlations in the presence or absence of asynergy. Am J Cardiol 1976377–11. [DOI] [PubMed] [Google Scholar]
- 21.Pritchett A M, Jacobsen S J, Mahoney D W.et al Left atrial volume as an index of left atrial size: a population‐based study. J Am Coll Cardiol 2003411036–1043. [DOI] [PubMed] [Google Scholar]
- 22.Schnittger I, Gordon E P, Fitzgerald P J.et al Standardized intracardiac measurements of two dimensional echocardiography. J Am Coll Cardiol 19832934–938. [DOI] [PubMed] [Google Scholar]
- 23.Santoro G, Pascotto M, Sarubbi B.et al Early electrical and geometric changes after percutaneous closure of large atrial septal defect. Am J Cardiol 200493876–880. [DOI] [PubMed] [Google Scholar]
- 24.Pearlman A S, Borer J S, Clark C E.et al Abnormal right ventricular size and ventricular septal motion after atrial septal defect closure: etiology and functional significance. Am J Cardiol 197841295–301. [DOI] [PubMed] [Google Scholar]
- 25.Meyer R A, Korfhageh J C, Covitz W.et al Long‐term follow‐up study after closure of secundum atrial septal defect in children: an echocardiographic study. Am J Cardiol 198250143–148. [DOI] [PubMed] [Google Scholar]
- 26.Ning S B, Fazal H, Cook D.et al Right ventricular size and ventricular septal motion after repair of atrial septal defect in children. Can J Surg 198427395–398. [PubMed] [Google Scholar]
- 27.Ghosh S, Chatterjee S, Black E.et al Surgical closure of atrial septal defects in adults: effect of age at operation on outcome. Heart 200288485–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bishop J E, Rhodes S, Laurent G J.et al Increased collagen synthesis and decreased collagen degradation in right ventricular hypertrophy induced by pressure overload. Cardiovasc Res 1994281581–1585. [DOI] [PubMed] [Google Scholar]
- 29.Pacileo G, Limongelli G, Verrengia M.et al Backscatter evaluation of myocardial functional and textural findings in children with right ventricular pressure and/or volume overload. Am J Cardiol 200493594–597. [DOI] [PubMed] [Google Scholar]
- 30.Swan L, Varma C, Yip J W.et al Favorable right heart remodeling after atrial septal defect device closure even in the over‐60 age group. J Am Coll Cardiol 200341(6 suppl B)473 [abstract] [Google Scholar]
- 31.Weidemann F, Eyskens B, Mertens L.et al Quantification of regional right and left ventricular function by ultrasonic strain rate and strain indexes after surgical repair of tetralogy of Fallot. Am J Cardiol 200290133–138. [DOI] [PubMed] [Google Scholar]
- 32.Di Salvo G, Pacileo G, Caso P.et al A new echocardiographic approach to evaluate myocardial function in congenital cardiopathies: strain and strain rate imaging. Ital Heart J 20034375–382. [PubMed] [Google Scholar]
- 33.Humpl T, Campbell R, Stephens D.et al Levels of exhaled nitric oxide before and after surgical and transcatheter device closure of atrial septal defects in children. J Thorac Cardiovasc Surg 2002124806–810. [DOI] [PubMed] [Google Scholar]
- 34.Groundstroem K W, Iivainen T E, Lahtela J T.et al Natriuretic peptide and echocardiography after operation of atrial septal defect. Int J Cardiol 20038945–52. [DOI] [PubMed] [Google Scholar]