Urotensin II (U‐II) is a vasoactive peptide that has been highly conserved throughout evolution. U‐II has been described as the most potent vasoconstrictor yet identified.1 The identification in humans of immune reactive U‐II in plasma, the U‐II receptor, and U‐II expression in the myocardium, arterial system, and kidney and the elevation of U‐II in important disease states such as hypertension and congestive heart failure suggest that U‐II may be an important mediator in the regulation of cardiovascular function.1,2,3,4
U‐II has never been investigated in children with congenital heart disease (CHD) and in those undergoing cardiac surgery requiring cardiopulmonary bypass (CPB). The objective of this study was twofold. Firstly, we compared plasma U‐II in children with CHD with concentrations in healthy children; and secondly, we examined the 24 hour profile of U‐II in children undergoing open heart surgery.
PATIENTS AND METHODS
Forty children undergoing surgery for CHD were recruited, of whom 20 had low or normal preoperative pulmonary blood flow (LNF group; undergoing valve repairs, relief of outflow tract obstruction, tetralogy of Fallot repair, Fontan operations, and arterial switch operation) and 20 had high preoperative pulmonary blood flow (HF group; undergoing closure of septal defects). In the HF patients, preoperative angiotensin converting enzyme inhibitors were being taken by five children, diuretics by seven, and digoxin by one child. Seventeen of the children with CHD underwent modified ultrafiltration (MUF) after CPB, according to institutional protocols. Twenty children without cardiopulmonary or renal disease who were undergoing day case surgical procedures were also recruited for the study (controls).
In controls, a single blood sample was taken after induction of anaesthesia. In patients with CHD, samples were taken after induction of anaesthesia (baseline), after cross clamp removal, and at 4, 8, and 24 hours after CPB. Samples were centrifuged and supernatant plasma was stored at −80° C. U‐II was measured by radioimmunoassay as previously described.4
Within group data were compared by analysis of variance for repeated measures and by the Bonferroni method for post hoc analysis. Between group data were compared by Student's t test or the Mann‐Whitney rank sum test. Correlations were analysed with the Spearman rank correlation coefficient. Values are expressed as mean (SD) or median (absolute range).
RESULTS
The median age of controls was greater than that of children with CHD. Baseline U‐II in children with CHD was higher than in controls (p < 0.001). Age and U‐II were not correlated in controls (p = 0.8) and U‐II was negatively correlated with age in children with CHD (r = −0.37, p = 0.02). When controls were age matched with children with CHD, U‐II was greater in those with CHD (2.09 (0.72) pmol/l for age matched patients with CHD and 0.85 (0.39) pmol/l for controls, p < 0.001).
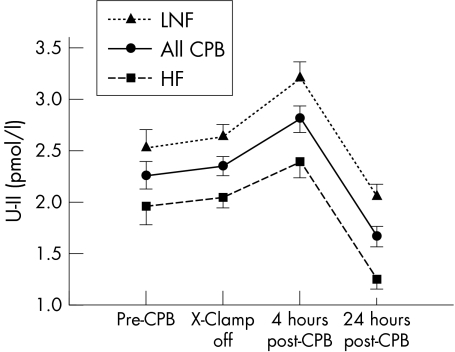
In all patients with CHD, U‐II increased early after CPB, peaked at four hours, then fell to concentrations below baseline at 24 hours (fig 1).
Figure 1 Twenty four hour profile of urotensin II (U‐II) in children with congenital heart disease. U‐II concentrations at four hours after separation from cardiopulmonary bypass (CPB) were higher than at baseline and at 24 hours were lower than at baseline for all children with congenital heart disease. At all time points, U‐II was higher in children with low or normal preoperative pulmonary flow (LNF) than in children with high flow (HF). X‐Clamp, aortic cross clamp.
MUF did not influence U‐II. U‐II at four hours was 2.83 (0.79) pmol/l for patients undergoing MUF and 2.79 (0.8) pmol/l for those not undergoing MUF (p = 0.87); at 24 hours U‐II was 1.6 (0.66) pmol/l and 1.7 (0.58) pmol/l for MUF and non‐MUF groups, respectively (p = 0.74).
Preoperative oxygen saturation was lower in the LNF than in the HF group and was negatively correlated with baseline U‐II (r = −0.421, p = 0.008). U‐II was higher in the LNF patients at all time points and LNF patients required longer CPB than did HF patients (p = 0.002) (table 1).
Table 1 Baseline details for participants in the study.
| All CHD (n = 39) | HF (n = 19) | LNF (n = 20) | p value (HF v LNF) | Controls (n = 20) | p Value (CHD v controls) | |
|---|---|---|---|---|---|---|
| Age (months) | 15.9 (0.16–152.7) | 18.6 (0.89–151.5) | 14.8 (0.16–152.7) | 0.978 | 60 (16.7–188) | <0.001 |
| Oxygen saturation (%) | ||||||
| Preoperative | 98 (61–100) | 100 (94–100) | 89.5 (61–99) | <0.001 | 99 (95–100) | 0.134 |
| Postoperative | 97.7 (4.4) | 98.6 (2.1) | 96.7 (5.8) | 0.447 | NA | NA |
| Baseline U‐II (pmol/l) | 2.26 (0.81) | 1.96 (0.76) | 2.53 (0.79) | 0.031 | 0.85 (0.39) | <0.001 |
| CPB time (min) | 114 (54–288) | 87 (54–281) | 136 (68–288) | 0.002 | NA | NA |
| Cross clamp time (min) | 69 (0–224) | 53 (24–213) | 85.5 (0–224) | 0.077 | NA | NA |
Data for urotensin II (U‐II) concentrations are expressed as mean (SD); other data are expressed as median (absolute range).
CHD, congenital heart disease; CPB, cardiopulmonary bypass; HF, high preoperative pulmonary blood flow; LNF, low or normal preoperative pulmonary blood flow; NA, not applicable.
DISCUSSION
In this study, which is the first to investigate immune reactive U‐II in children with CHD, we made three important findings. We have shown, firstly, that U‐II is raised in children with CHD; secondly, that surgery with CPB results in an early increase in U‐II, which is not affected by MUF; and thirdly, that U‐II is higher in patients with cyanotic CHD than in those with CHD and normal saturation.
The recent identification of the human U‐II isoform, its receptor, and expression of its pre‐pro peptide mRNA have generated considerable interest in a possible role of U‐II in the pathophysiology of cardiovascular disease.1 Douglas et al2 showed that myocardial U‐II expression and urotensin receptor expression is increased in congestive cardiac failure, and that expression is proportional to the degree of left ventricular dysfunction. Richards et al,4 who have been pivotal in developing radioimmunoassays to U‐II, reported raised concentrations in severe heart failure.
In our study, baseline U‐II in CHD children was more than double that of controls. Lower preoperative oxygen saturation was associated with higher U‐II; and U‐II was higher at all time points in children with LNF than in children with HF lesions. In a model of pulmonary hypertension secondary to chronic hypoxia, the U‐II content of right ventricular myocardium almost doubled and that of the left ventricle increased by one third.5 The association between cyanosis and U‐II has not previously been explored in humans but future studies will address myocardial U‐II expression in children with cyanotic and non‐cyanotic CHD.
U‐II values were raised in all children early after CPB, with a characteristic peak at four hours and concentrations falling to below baseline after 24 hours. The mechanism underlying this rise and its significance are uncertain, but three recent laboratory investigations have shown important axes through which U‐II may influence the cardiovascular dysfunction that typically occurs in the first 12 hours after paediatric CPB. Firstly, U‐II activates mitogen activated protein kinases,6 which have a role in the genesis of changes in vascular permeability, cytokine production, vasomotor function, and reperfusion injury, all of which accompany CPB. Secondly, changes in mitogen activated protein kinase pathways have also been shown to mediate coronary microcirculatory dysfunction after CPB.7 Lastly, evidence suggests that U‐II stimulates interleukin 6 expression from urotensin receptor expressing cardiomyocytes.8
We were interested to find that MUF did not influence U‐II. MUF has previously been shown to lower concentrations of endothelin 1 and other inflammatory mediators early after CPB.9 Although MUF may mitigate some of the bypass related cardiovascular dysfunction after CPB, this is not a panacea, and our findings may point to an important role of U‐II in this phenomenon.
Our study has shown that the pathways leading to the release of U‐II are activated in children with CHD and are further stimulated by CPB. Further studies examining the tissue expression of U‐II in children with CHD and in models of CPB will give us a better understanding of the mechanisms underlying the release of U‐II.
In conclusion, plasma U‐II is raised in children with CHD and concentrations increase further early after CPB. Although its exact role is still not established in this population, U‐II may be an important mediator in the cardiovascular dysfunction that affects children with CHD early after CPB.
ACKNOWLEDGEMENTS
We thank A/Prof Tim Yandle and the team in the Christchurch Cardioendocrine Research Group, Christchurch School of Medicine and Health Sciences, New Zealand for performing the urotensin II assays. Without their help this study would not have been possible.
Abbreviations
CHD - congenital heart disease
CPB - cardiopulmonary bypass
HF - high preoperative pulmonary blood flow
LNF - low or normal preoperative pulmonary blood flow
MUF - modified ultrafiltration
U‐II - urotensin II
Footnotes
†Also the Paediatric Intensive Care Unit, The Royal Children's Hospital, Melbourne, Australia
Financial support: Dr C Simpson is supported by a postgraduate medical research scholarship from the National Health and Medical Research Council of Australia.
Competing interests: None declared
Ethics approval: This study was approved by the Ethics in Humans Research Committee of The Royal Children's Hospital, Melbourne, Australia. EHRC No 24013A; approved 1 April 2004.
References
- 1.Ames R S, Sarau H M, Chambers J K.et al Human urotensin‐II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature 1999401282–286. [DOI] [PubMed] [Google Scholar]
- 2.Douglas S A, Tayara L, Ohlstein E H.et al Congestive heart failure and expression of myocardial urotensin II. Lancet 20023591990–1997. [DOI] [PubMed] [Google Scholar]
- 3.Cheung B M, Leung R, Man Y B.et al Plasma concentration of urotensin II is raised in hypertension. J Hypertens 2004221341–1344. [DOI] [PubMed] [Google Scholar]
- 4.Richards A M, Nicholls M G, Lainchbury J G.et al Plasma urotensin II in heart failure. Lancet 2002360545–546. [DOI] [PubMed] [Google Scholar]
- 5.MacLean M R, Alexander D, Stirrat A.et al Contractile responses to human urotensin‐II in rat and human pulmonary arteries: effect of endothelial factors and chronic hypoxia in the rat. Br J Pharmacol 2000130201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onan D, Pipolo L, Yang E.et al Urotensin II promotes hypertrophy of cardiac myocytes via mitogen‐activated protein kinases. Mol Endocrinol 2004182344–2354. [DOI] [PubMed] [Google Scholar]
- 7.Khan T A, Bianchi C, Araujo E G.et al Activation of pulmonary mitogen‐activated protein kinases during cardiopulmonary bypass. J Surg Res 200311556–62. [DOI] [PubMed] [Google Scholar]
- 8.Johns D G, Ao Z, Naselsky D.et al Urotensin‐II‐mediated cardiomyocyte hypertrophy: effect of receptor antagonism and role of inflammatory mediators. Naunyn Schmiedebergs Arch Pharmacol 2004370238–250. [DOI] [PubMed] [Google Scholar]
- 9.Gaynor J W. The effect of modified ultrafiltration on the postoperative course in patients with congenital heart disease. Semin Thorac Cardiovasc Surg Pediatr Card Surg 20036128–139. [DOI] [PubMed] [Google Scholar]