Abstract
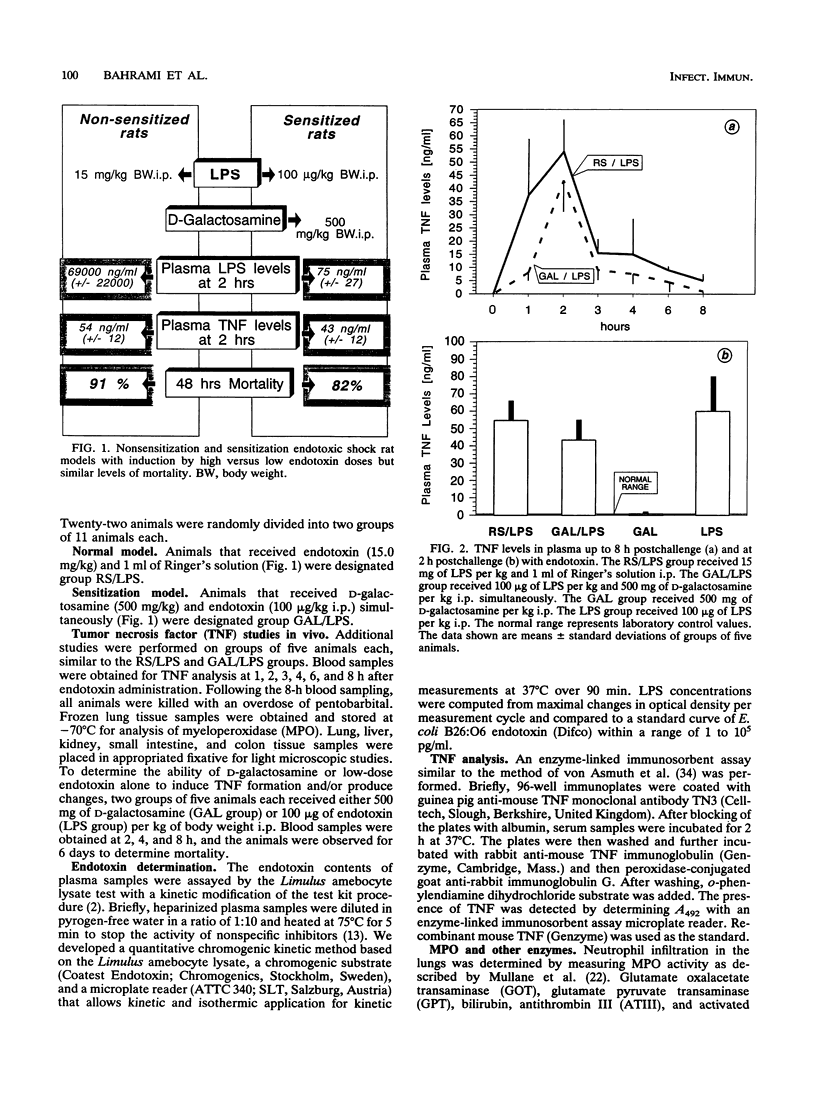
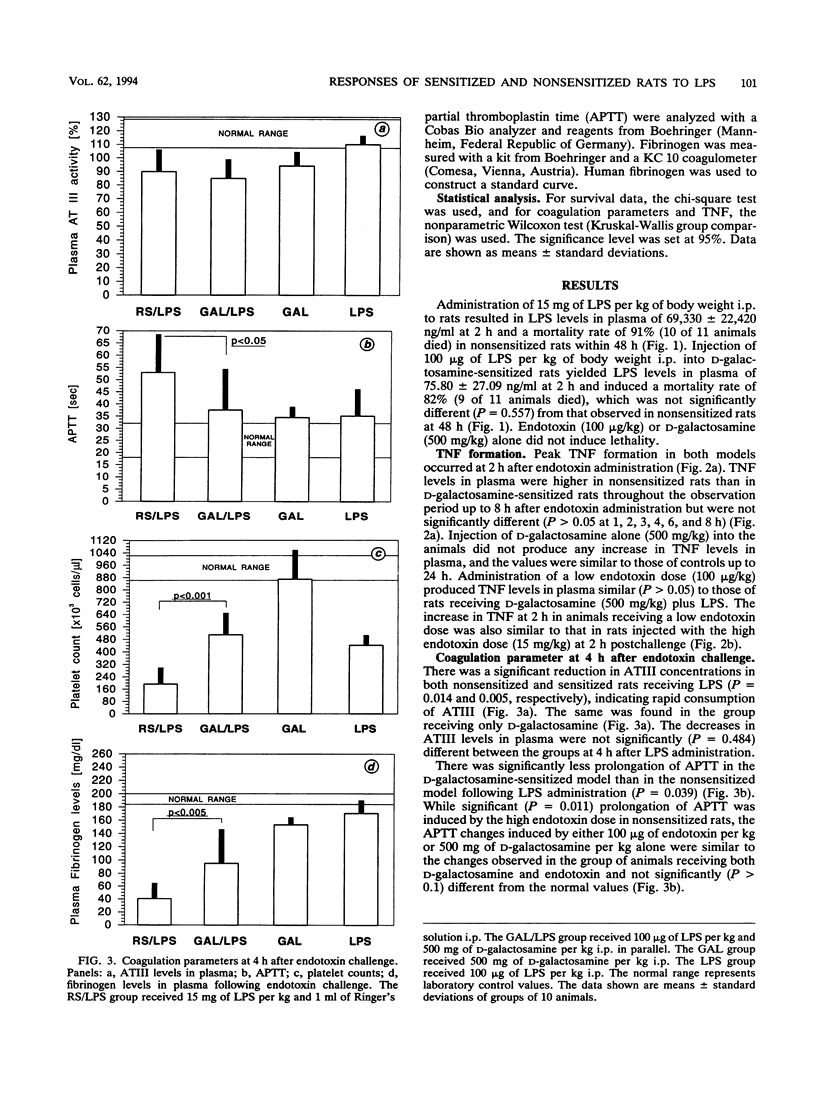
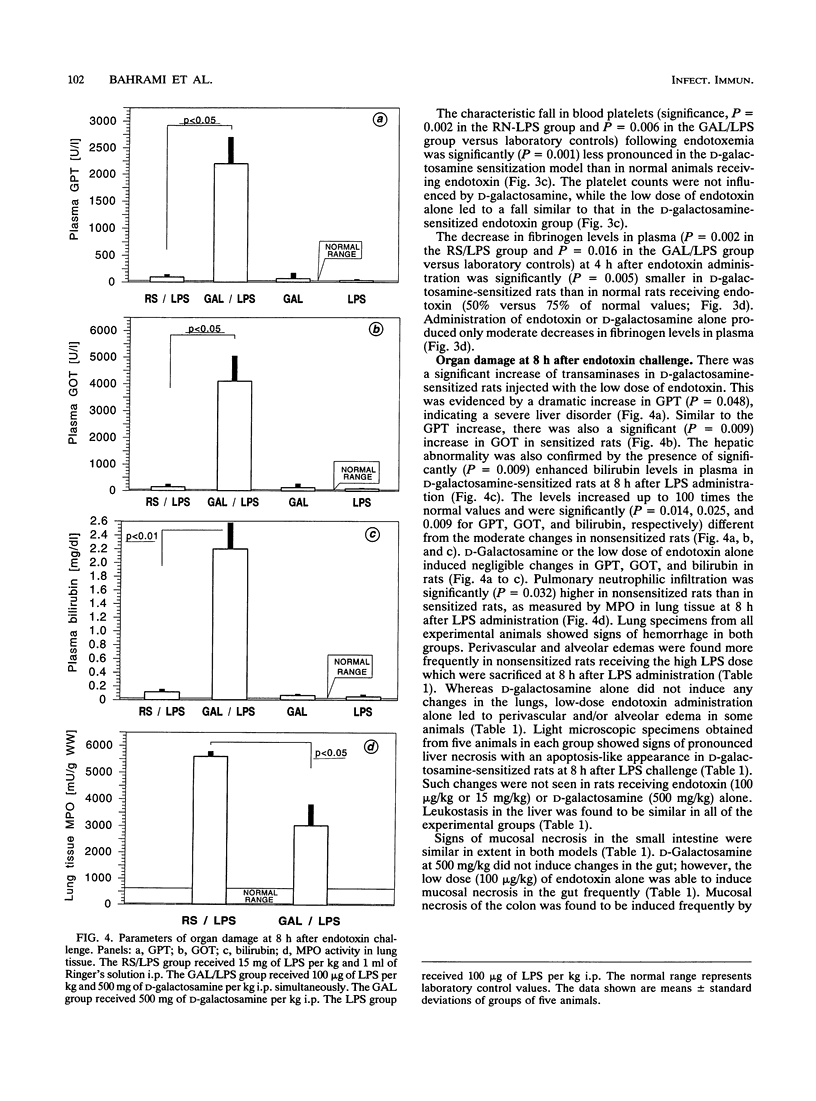
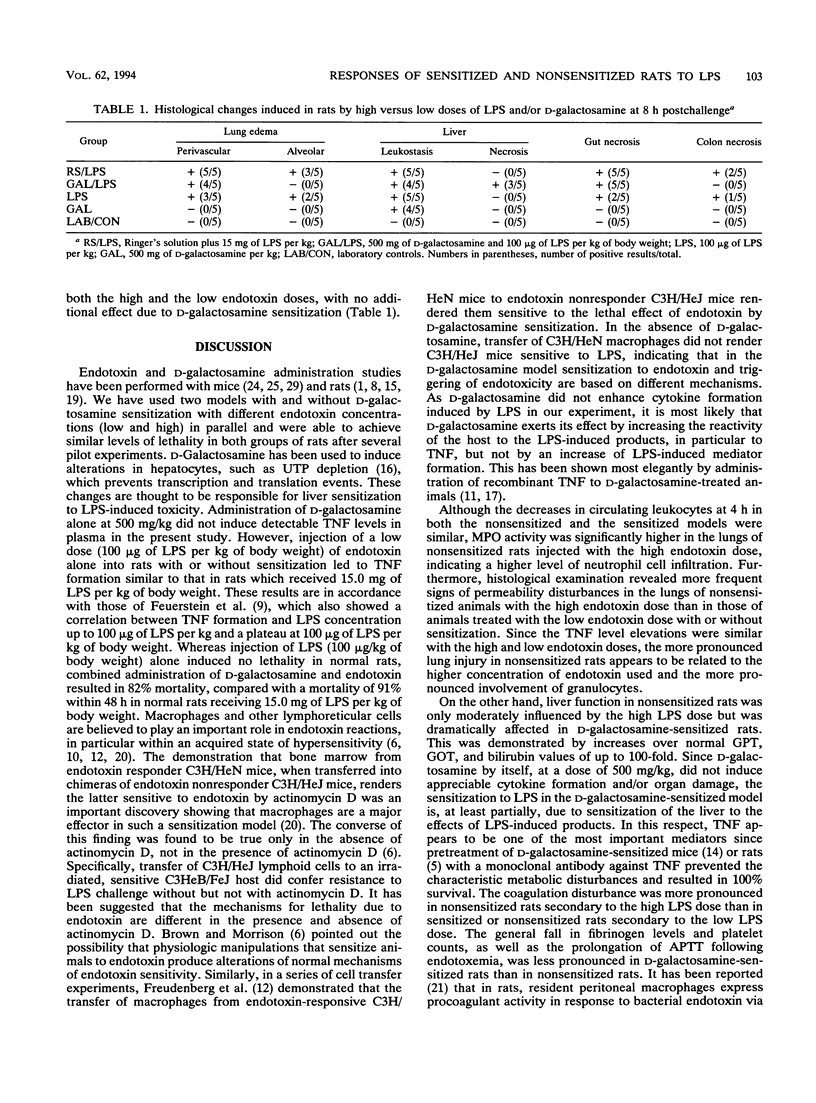
To compare cytokine release and coagulation disturbances induced by administration of high versus low doses of endotoxin (lipopolysaccharide [LPS]), we used two endotoxin test systems similar in mortality but different in the degree of endotoxemia. One group of rats (n = 11) randomly received endotoxin (15.0 mg/kg of body weight intraperitoneally [i.p.]) and 1 ml of Ringer's solution (nonsensitized animals). The second group (n = 11) received 1 ml of D-galactosamine (500 mg/kg i.p.) and endotoxin (100 micrograms/kg i.p.) simultaneously (sensitized animals). Endotoxin levels in the plasma of nonsensitized rats were 1,000-fold higher than those in the plasma of sensitized rats (69.33 x 10(3) +/- 22.42 x 10(3) versus 75.8 +/- 27.08 ng of LPS per ml), leading to a mortality of 91% in nonsensitized rats versus 82% in the sensitized-rat model within 48 h postendotoxemia. Serum transaminase activity increased up to 100-fold in sensitized rats as a sign of hepatocyte damage. Despite the large difference in LPS levels in plasma, the time courses of the plasma tumor necrosis factor (TNF) increase were similar in the two groups, with a peak at 2 h (54 +/- 12 ng/ml in nonsensitized rats versus 43 +/- 12 ng/ml in sensitized rats), and also similar to that of a group of nonsensitized rats (n = 5) that received a low dose of LPS (100 micrograms/kg) only (52 +/- 21 ng/ml), while D-galactosamine alone did not induce TNF release. Despite similar TNF levels, a more pronounced coagulation disorder was observed at 4 h in nonsensitized rats (with the high LPS dose) as measured by platelet counts, plasma fibrinogen levels, and activated partial thromboplastin time prolongation (191 x 10(3) +/- 107 x 10(3) cells per microliter, 40 +/- 24 mg/dl, and 53 +/- 15 s, respectively) than in rats with the low LPS dose either sensitized (495 x 10(3) +/- 153 x 10(3), 95 +/- 49, and 38 +/- 16, respectively) or nonsensitized (439 x 10(3) +/- 62 x 10(3), 170 +/- 18, and 35 +/- 11, respectively).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann W., Harms E., Hassels B., Henninger H., Reuitter W. Studies on rat liver plasma membrane. Altered protein and phospholipid metabolism after injection of D-galactosamine. Biochem J. 1977 Sep 15;166(3):455–462. doi: 10.1042/bj1660455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami S., Paul E., Redl H., Schlag G. Therapeutic modalities to ameliorate endotoxin induced DIC in the rats. Prog Clin Biol Res. 1989;308:977–982. [PubMed] [Google Scholar]
- Brown D. E., Morrison D. C. Possible alteration of normal mechanisms of endotoxin toxicity in vivo by actinomycin D. J Infect Dis. 1982 Dec;146(6):746–750. doi: 10.1093/infdis/146.6.746. [DOI] [PubMed] [Google Scholar]
- Conway E. M., Bach R., Rosenberg R. D., Konigsberg W. H. Tumor necrosis factor enhances expression of tissue factor mRNA in endothelial cells. Thromb Res. 1989 Feb 1;53(3):231–241. doi: 10.1016/0049-3848(89)90098-4. [DOI] [PubMed] [Google Scholar]
- Czaja M. J., Flanders K. C., Biempica L., Klein C., Zern M. A., Weiner F. R. Expression of tumor necrosis factor-alpha and transforming growth factor-beta 1 in acute liver injury. Growth Factors. 1989;1(3):219–226. doi: 10.3109/08977198908997998. [DOI] [PubMed] [Google Scholar]
- Feuerstein G., Hallenbeck J. M., Vanatta B., Rabinovici R., Perera P. Y., Vogel S. N. Effect of gram-negative endotoxin on levels of serum corticosterone, TNF alpha, circulating blood cells, and the survival of rats. Circ Shock. 1990 Mar;30(3):265–278. [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Induction of tolerance to lipopolysaccharide (LPS)-D-galactosamine lethality by pretreatment with LPS is mediated by macrophages. Infect Immun. 1988 May;56(5):1352–1357. doi: 10.1128/iai.56.5.1352-1357.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Tumor necrosis factor alpha mediates lethal activity of killed gram-negative and gram-positive bacteria in D-galactosamine-treated mice. Infect Immun. 1991 Jun;59(6):2110–2115. doi: 10.1128/iai.59.6.2110-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Keppler D., Galanos C. Requirement for lipopolysaccharide-responsive macrophages in galactosamine-induced sensitization to endotoxin. Infect Immun. 1986 Mar;51(3):891–895. doi: 10.1128/iai.51.3.891-895.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friberger P., Knös M., Mellstam L. A quantitative endotoxin assay utilizing LAL and a chromogenic substrate. Prog Clin Biol Res. 1982;93:195–206. [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler D. O., Pausch J., Decker K. Selective uridine triphosphate deficiency induced by D-galactosamine in liver and reversed by pyrimidine nucleotide precursors. Effect on ribonucleic acid synthesis. J Biol Chem. 1974 Jan 10;249(1):211–216. [PubMed] [Google Scholar]
- Lehmann V., Freudenberg M. A., Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-galactosamine-treated mice. J Exp Med. 1987 Mar 1;165(3):657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura M., Galanos C. Induction of hypersensitivity to endotoxin and tumor necrosis factor by sublethal infection with Salmonella typhimurium. Infect Immun. 1990 Apr;58(4):935–937. doi: 10.1128/iai.58.4.935-937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuschak G. M., Pinsky M. R., Klein E. C., Van Thiel D. H., Rinaldo J. E. Effects of D-galactosamine-induced acute liver injury on mortality and pulmonary responses to Escherichia coli lipopolysaccharide. Modulation by arachidonic acid metabolites. Am Rev Respir Dis. 1990 May;141(5 Pt 1):1296–1306. doi: 10.1164/ajrccm/141.5_Pt_1.1296. [DOI] [PubMed] [Google Scholar]
- Michalek S. M., Moore R. N., McGhee J. R., Rosenstreich D. L., Mergenhagen S. E. The primary role of lymphoreticular cells in the mediation of host responses to bacterial endotoxim. J Infect Dis. 1980 Jan;141(1):55–63. doi: 10.1093/infdis/141.1.55. [DOI] [PubMed] [Google Scholar]
- Moore J. N., Cook J. A., Morris D. D., Halushka P. V., Wise W. C. Endotoxin-induced procoagulant activity, eicosanoid synthesis, and tumor necrosis factor production by rat peritoneal macrophages: effect of endotoxin tolerance and glucan. Circ Shock. 1990 Jul;31(3):281–295. [PubMed] [Google Scholar]
- Mullane K. M., Kraemer R., Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985 Nov;14(3):157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- Revhaug A., Michie H. R., Manson J. M., Watters J. M., Dinarello C. A., Wolff S. M., Wilmore D. W. Inhibition of cyclo-oxygenase attenuates the metabolic response to endotoxin in humans. Arch Surg. 1988 Feb;123(2):162–170. doi: 10.1001/archsurg.1988.01400260042004. [DOI] [PubMed] [Google Scholar]
- Schade U. F. Pentoxifylline increases survival in murine endotoxin shock and decreases formation of tumor necrosis factor. Circ Shock. 1990 Jun;31(2):171–181. [PubMed] [Google Scholar]
- Schlayer H. J., Laaff H., Peters T., Woort-Menker M., Estler H. C., Karck U., Schaefer H. E., Decker K. Involvement of tumor necrosis factor in endotoxin-triggered neutrophil adherence to sinusoidal endothelial cells of mouse liver and its modulation in acute phase. J Hepatol. 1988 Oct;7(2):239–249. doi: 10.1016/s0168-8278(88)80488-4. [DOI] [PubMed] [Google Scholar]
- Schleef R. R., Bevilacqua M. P., Sawdey M., Gimbrone M. A., Jr, Loskutoff D. J. Cytokine activation of vascular endothelium. Effects on tissue-type plasminogen activator and type 1 plasminogen activator inhibitor. J Biol Chem. 1988 Apr 25;263(12):5797–5803. [PubMed] [Google Scholar]
- Suffredini A. F., Fromm R. E., Parker M. M., Brenner M., Kovacs J. A., Wesley R. A., Parrillo J. E. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med. 1989 Aug 3;321(5):280–287. doi: 10.1056/NEJM198908033210503. [DOI] [PubMed] [Google Scholar]
- Suffredini A. F., Shelhamer J. H., Neumann R. D., Brenner M., Baltaro R. J., Parrillo J. E. Pulmonary and oxygen transport effects of intravenously administered endotoxin in normal humans. Am Rev Respir Dis. 1992 Jun;145(6):1398–1403. doi: 10.1164/ajrccm/145.6.1398. [DOI] [PubMed] [Google Scholar]
- Tiegs G., Wolter M., Wendel A. Tumor necrosis factor is a terminal mediator in galactosamine/endotoxin-induced hepatitis in mice. Biochem Pharmacol. 1989 Feb 15;38(4):627–631. doi: 10.1016/0006-2952(89)90208-6. [DOI] [PubMed] [Google Scholar]
- Zabel P., Wolter D. T., Schönharting M. M., Schade U. F. Oxpentifylline in endotoxaemia. Lancet. 1989 Dec 23;2(8678-8679):1474–1477. doi: 10.1016/s0140-6736(89)92929-2. [DOI] [PubMed] [Google Scholar]
- van Deventer S. J., Büller H. R., ten Cate J. W., Aarden L. A., Hack C. E., Sturk A. Experimental endotoxemia in humans: analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood. 1990 Dec 15;76(12):2520–2526. [PubMed] [Google Scholar]
- van Hinsbergh V. W., Kooistra T., van den Berg E. A., Princen H. M., Fiers W., Emeis J. J. Tumor necrosis factor increases the production of plasminogen activator inhibitor in human endothelial cells in vitro and in rats in vivo. Blood. 1988 Nov;72(5):1467–1473. [PubMed] [Google Scholar]
- van der Poll T., Büller H. R., ten Cate H., Wortel C. H., Bauer K. A., van Deventer S. J., Hack C. E., Sauerwein H. P., Rosenberg R. D., ten Cate J. W. Activation of coagulation after administration of tumor necrosis factor to normal subjects. N Engl J Med. 1990 Jun 7;322(23):1622–1627. doi: 10.1056/NEJM199006073222302. [DOI] [PubMed] [Google Scholar]
- van der Poll T., Levi M., Büller H. R., van Deventer S. J., de Boer J. P., Hack C. E., ten Cate J. W. Fibrinolytic response to tumor necrosis factor in healthy subjects. J Exp Med. 1991 Sep 1;174(3):729–732. doi: 10.1084/jem.174.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Asmuth E. J., Maessen J. G., van der Linden C. J., Buurman W. A. Tumour necrosis factor alpha (TNF-alpha) and interleukin 6 in a zymosan-induced shock model. Scand J Immunol. 1990 Oct;32(4):313–319. doi: 10.1111/j.1365-3083.1990.tb02925.x. [DOI] [PubMed] [Google Scholar]


