Abstract
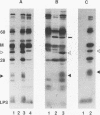
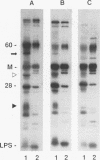
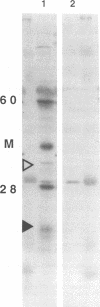
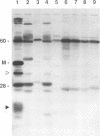
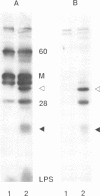
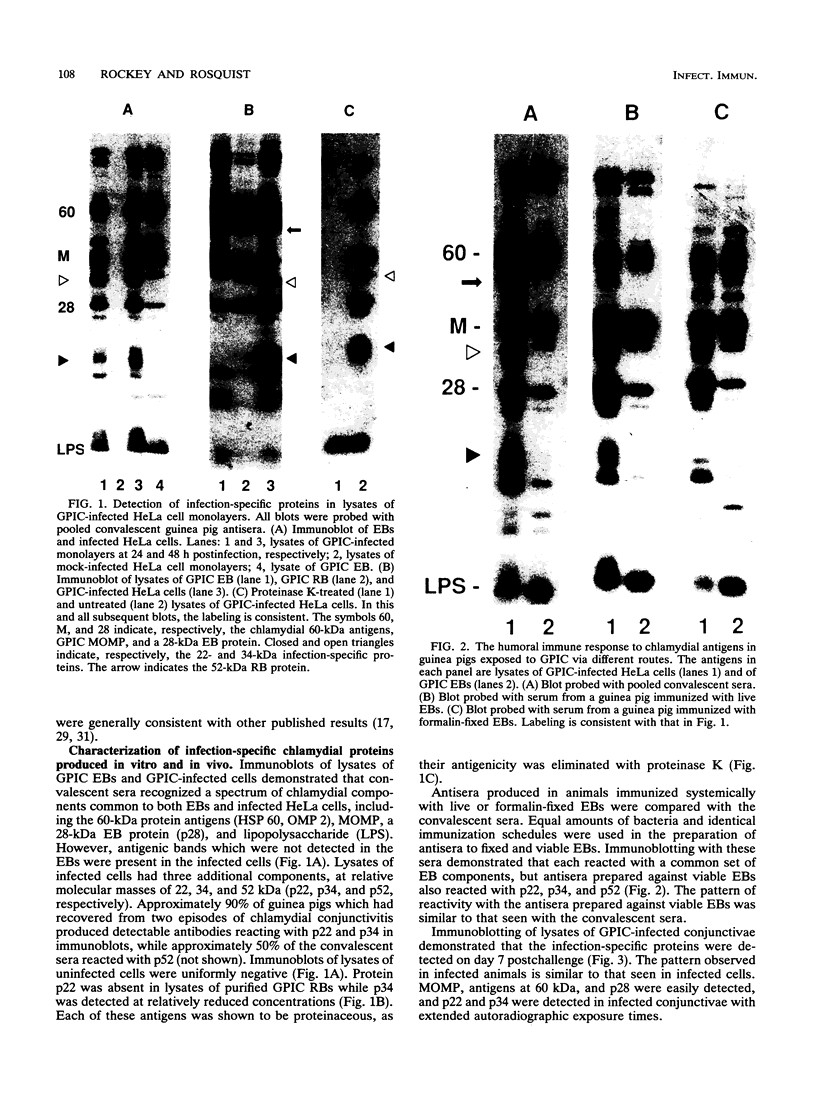
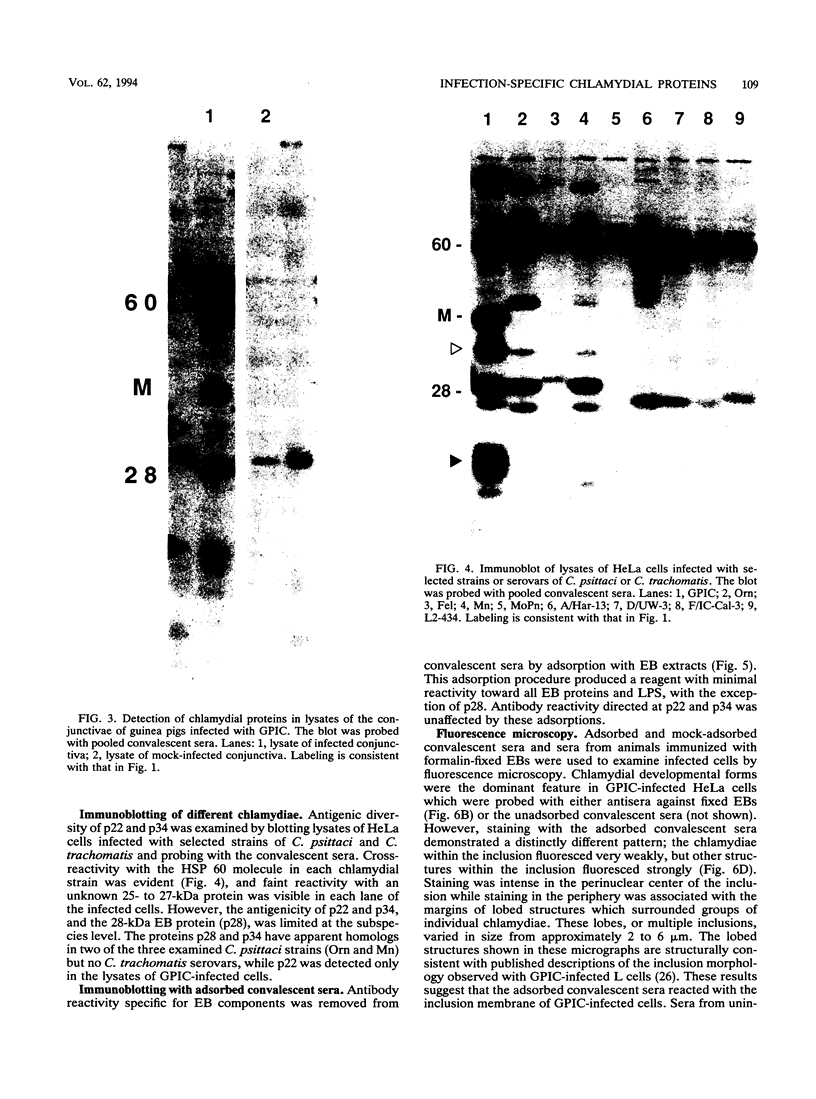
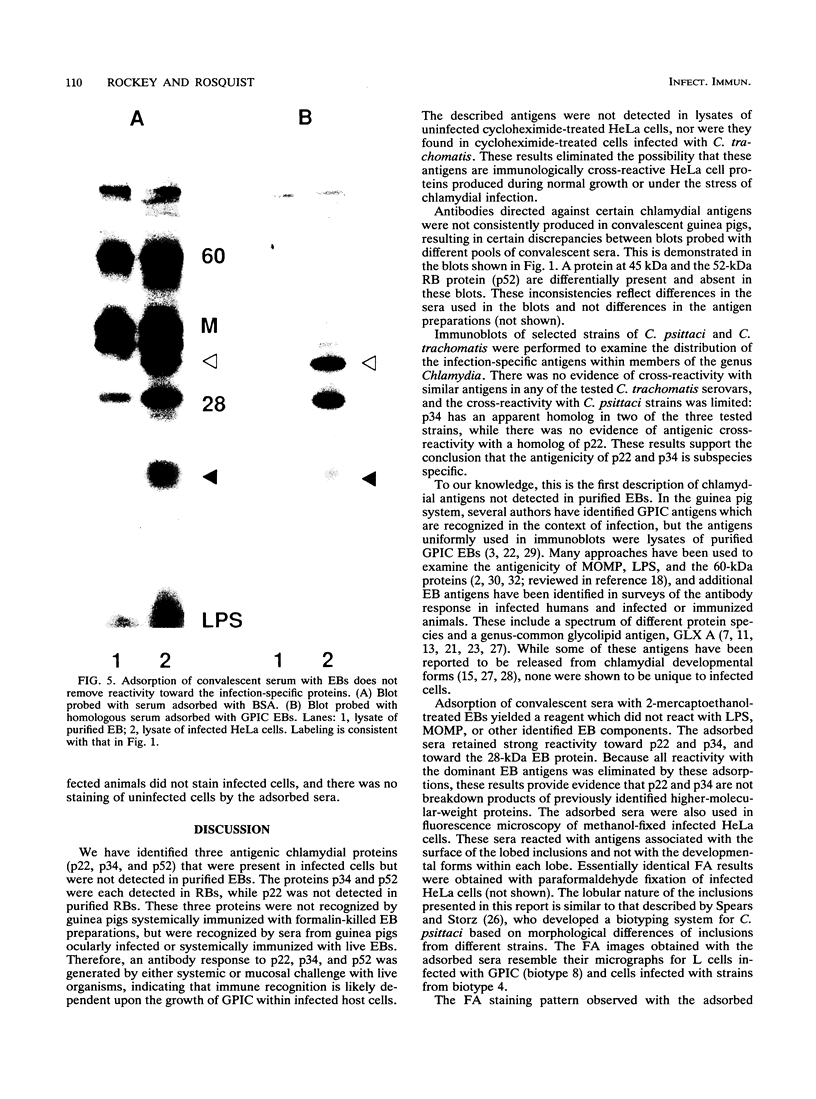
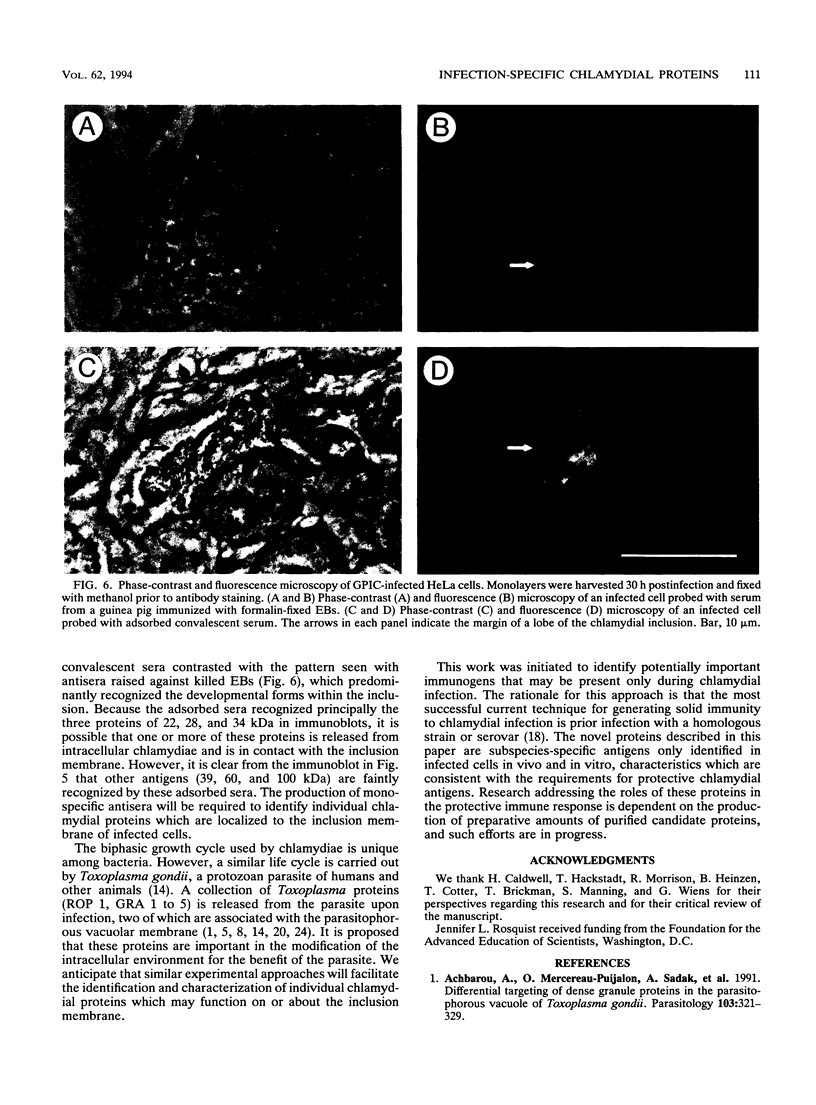
Ocular infection of guinea pigs with the guinea pig inclusion conjunctivitis (GPIC) strain of Chlamydia psittaci produces a clinical condition representative of acute chlamydial conjunctivitis in humans. Guinea pigs which had recovered from two challenges with GPIC were used as a source of sera for the identification of antigens present in GPIC-infected tissue culture cells but absent in the infectious elementary body (EB). Immunoblots of lysates of infected HeLa cells probed with the convalescent-phase sera identified protein antigens of 22, 34, and 52 kDa (p22, p34, and p52, respectively) that were not detected in lysates of purified EB or in uninfected HeLa cells. Protein p22 was also not detected in lysates of purified reticulate bodies. Immunoblotting of lysates of HeLa cells infected with other chlamydiae demonstrated that the antigenicity of p22 and p34 was subspecies specific. Immunoblotting was also used to detect p22 and p34 in lysates of the conjunctivae of infected guinea pigs. Adsorption of convalescent-phase sera with GPIC EB produced a reagent with dominant reactivity toward p22, p34, and a 28-kDa EB protein. Immunofluorescent staining of GPIC-infected HeLa cells demonstrated that these adsorbed sera labeled the inclusion and inclusion membrane, with no apparent reactivity toward EB or reticulate bodies. Collectively, these data identify non-EB chlamydial components which may be released into the inclusion during intracellular growth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achbarou A., Mercereau-Puijalon O., Sadak A., Fortier B., Leriche M. A., Camus D., Dubremetz J. F. Differential targeting of dense granule proteins in the parasitophorous vacuole of Toxoplasma gondii. Parasitology. 1991 Dec;103(Pt 3):321–329. doi: 10.1017/s0031182000059837. [DOI] [PubMed] [Google Scholar]
- Allen J. E., Stephens R. S. An intermolecular mechanism of T cell help for the production of antibodies to the bacterial pathogen, Chlamydia trachomatis. Eur J Immunol. 1993 May;23(5):1169–1172. doi: 10.1002/eji.1830230529. [DOI] [PubMed] [Google Scholar]
- Batteiger B. E., Rank R. G. Analysis of the humoral immune response to chlamydial genital infection in guinea pigs. Infect Immun. 1987 Aug;55(8):1767–1773. doi: 10.1128/iai.55.8.1767-1773.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berche P., Gaillard J. L., Sansonetti P. J. Intracellular growth of Listeria monocytogenes as a prerequisite for in vivo induction of T cell-mediated immunity. J Immunol. 1987 Apr 1;138(7):2266–2271. [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Stewart S., Johnson S., Taylor H. Tear and serum antibody response to Chlamydia trachomatis antigens during acute chlamydial conjunctivitis in monkeys as determined by immunoblotting. Infect Immun. 1987 Jan;55(1):93–98. doi: 10.1128/iai.55.1.93-98.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubremetz J. F., Achbarou A., Bermudes D., Joiner K. A. Kinetics and pattern of organelle exocytosis during Toxoplasma gondii/host-cell interaction. Parasitol Res. 1993;79(5):402–408. doi: 10.1007/BF00931830. [DOI] [PubMed] [Google Scholar]
- Eissenberg L. G., Wyrick P. B., Davis C. H., Rumpp J. W. Chlamydia psittaci elementary body envelopes: ingestion and inhibition of phagolysosome fusion. Infect Immun. 1983 May;40(2):741–751. doi: 10.1128/iai.40.2.741-751.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg L. G., Wyrick P. B. Inhibition of phagolysosome fusion is localized to Chlamydia psittaci-laden vacuoles. Infect Immun. 1981 May;32(2):889–896. doi: 10.1128/iai.32.2.889-896.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths P. C., Philips H. L., Dawson M., Clarkson M. J. Antigenic and morphological differentiation of placental and intestinal isolates of Chlamydia psittaci of ovine origin. Vet Microbiol. 1992 Feb;30(2-3):165–177. doi: 10.1016/0378-1135(92)90111-6. [DOI] [PubMed] [Google Scholar]
- Harty J. T., Bevan M. J. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J Exp Med. 1992 Jun 1;175(6):1531–1538. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourihan J. T., Rota T. R., MacDonald A. B. Isolation and purification of a type-specific antigen from Chlamydia trachomatis propagated in cell culture utilizing molecular shift chromatography. J Immunol. 1980 May;124(5):2399–2404. [PubMed] [Google Scholar]
- Joiner K. A., Dubremetz J. F. Toxoplasma gondii: a protozoan for the nineties. Infect Immun. 1993 Apr;61(4):1169–1172. doi: 10.1128/iai.61.4.1169-1172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald A. B. Antigens of Chlamydia trachomatis. Rev Infect Dis. 1985 Nov-Dec;7(6):731–736. doi: 10.1093/clinids/7.6.731. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992 Jan;174(1):1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnickendam M. A., Darougar S., Treharne J. D., Tilbury A. M. Development of chronic conjunctivitis with scarring and pannus, resembling trachoma, in guinea-pigs. Br J Ophthalmol. 1980 Apr;64(4):284–290. doi: 10.1136/bjo.64.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991 Mar;55(1):143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S., Fielder T. J., Peterson E. M., de la Maza L. M. Analysis of the immune response in mice following intrauterine infection with the Chlamydia trachomatis mouse pneumonitis biovar. Infect Immun. 1993 Feb;61(2):772–776. doi: 10.1128/iai.61.2.772-776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Batteiger B. E., Soderberg L. S. Susceptibility to reinfection after a primary chlamydial genital infection. Infect Immun. 1988 Sep;56(9):2243–2249. doi: 10.1128/iai.56.9.2243-2249.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D. L., MacDonald A. B. Isolation of a type-specific antigen from Chlamydia trachomatis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol. 1979 Jan;122(1):136–139. [PubMed] [Google Scholar]
- Sokolovic Z., Goebel W. Synthesis of listeriolysin in Listeria monocytogenes under heat shock conditions. Infect Immun. 1989 Jan;57(1):295–298. doi: 10.1128/iai.57.1.295-298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears P., Storz J. Biotyping of Chlamydia psittaci based on inclusion morphology and response to diethylaminoethyl-dextran and cycloheximide. Infect Immun. 1979 Apr;24(1):224–232. doi: 10.1128/iai.24.1.224-232.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart E. S., Tirrell S. M., MacDonald A. B. Characterization of an antigen secreted by Chlamydia-infected cell culture. Immunology. 1987 Aug;61(4):527–533. [PMC free article] [PubMed] [Google Scholar]
- Stuart E. S., Wyrick P. B., Choong J., Stoler S. B., MacDonald A. B. Examination of chlamydial glycolipid with monoclonal antibodies: cellular distribution and epitope binding. Immunology. 1991 Dec;74(4):740–747. [PMC free article] [PubMed] [Google Scholar]
- Treharne J. D., Shallal A. The antigenic specificity of the humoral immune response to primary and repeated ocular infections of the guinea pig with the GPIC agent (Chlamydia psittaci). Eye (Lond) 1991;5(Pt 3):299–304. doi: 10.1038/eye.1991.47. [DOI] [PubMed] [Google Scholar]
- Tuffrey M., Alexander F., Conlan W., Woods C., Ward M. Heterotypic protection of mice against chlamydial salpingitis and colonization of the lower genital tract with a human serovar F isolate of Chlamydia trachomatis by prior immunization with recombinant serovar L1 major outer-membrane protein. J Gen Microbiol. 1992 Aug;138(Pt 8):1707–1715. doi: 10.1099/00221287-138-8-1707. [DOI] [PubMed] [Google Scholar]
- Watkins N. G., Hadlow W. J., Moos A. B., Caldwell H. D. Ocular delayed hypersensitivity: a pathogenetic mechanism of chlamydial-conjunctivitis in guinea pigs. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7480–7484. doi: 10.1073/pnas.83.19.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin S. S., Jeremias J., Toth M., Ledger W. J. Cell-mediated immune response to the recombinant 57-kDa heat-shock protein of Chlamydia trachomatis in women with salpingitis. J Infect Dis. 1993 Jun;167(6):1379–1383. doi: 10.1093/infdis/167.6.1379. [DOI] [PubMed] [Google Scholar]