There is increasing confusion regarding the results and benefits of catheter and surgical ablation for atrial fibrillation (AF). This is because of rapidly evolving techniques and a wide range of opinion regarding its efficacy. Just as the balloon and stent enthusiasts of the 1980s were viewed with some suspicion by many doctors treating coronary atherosclerosis,w1 the practice of curative ablation of AF has been critically reviewed by cardiologists.w2 w3 This article aims to demonstrate why an apparently chaotic heart rhythm is amenable to cure, critically review the currently employed surgical and catheter techniques, and provide some guidelines as to the appropriate referral of patients for these procedures.
PAROXYSMAL, PERSISTENT, AND PERMANENT AF
AF is usually prefixed by a temporal descriptive term such as paroxysmal or chronic which has implications for the most suitable treatment strategy. A consensus on nomenclature has now been achieved1 in which an AF event is either the first detected or a recurrent episode. Paroxysmal AF describes episodes that terminate spontaneously within seven days. AF is persistent if it lasts longer than seven days or requires cardioversion by any means to restore sinus rhythm. Permanent AF is reserved for when either attempts to cardiovert have failed or not been attempted.
MECHANISMS UNDERLYING AF
The mechanism of AF is not clearly understood. The predominant theory of the 20th century was that chaotic multiple re‐entry circuits, following constantly varying lines of conduction block, perpetuate AF (fig 1).w4 w5 This concept was challenged, however, by the observation that in some patients without cardiac structural abnormalities, an ECG pattern of AF can be seen even when there is a definite focal source such as a rapidly firing atrial tachycardia emerging from a pulmonary vein (PV).2 A single wavefront propagating across the atria is vulnerable to being split and turned by anatomical obstacles and by spatial variation in the conduction properties of the atrial myocardium such that if the focus depolarises rapidly the rest of the atria may not be able to conduct 1:1, thus forming multiple wavefronts. This is described as fibrillatory conduction (fig 1).3
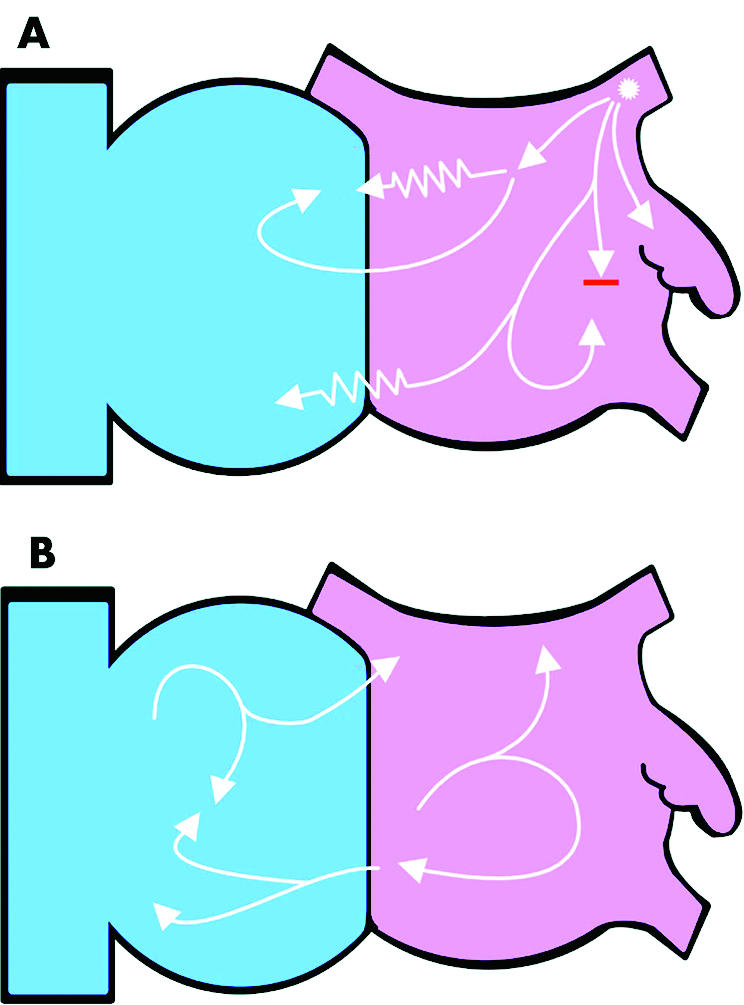
Figure 1 A schematic view of the interior surface of the right (blue) and left (pink) atria. The white arrows represent wavefronts of electrical depolarisation. (A) An ectopic focus (asterisk) from within the left superior pulmonary vein generates repetitive wavefronts at a high frequency. The rest of the atrial myocardium cannot propagate these wavefronts uniformly because of the heterogeneity of its conduction properties and anatomical obstacles. The zigzag lines represent slowed conduction and the red line conduction block. The wavefronts are consequently broken down into multiple wavefronts which manifest as atrial fibrillation on the ECG. If the focal trigger stops firing the fibrillation will terminate. (B) Multiple wavelet re‐entry. The wavefronts are turned and split by colliding with the anatomical structures (fixed block) and other wavefronts (functional block). The number of wavefronts is determined by the size and conduction properties of the atria. These wavefronts self propagate and are not dependent on a focal trigger.
These two mechanisms should not be considered as separate entities though. Patients with paroxysmal AF and relatively normal hearts are more likely to have a focal mechanism, in which the episode only persists as long as the source of the wavefronts is present. Over time, however, because of the recurrent tachycardia episodes, remodelling of the atrial tissue (altering both the conduction and structural properties) will occur, such that the atria dilate and can then maintain multiple re‐entry wavefronts without the need for the focal source.4 This pathological progression (remodelling of the atria) is mirrored by the well recognised clinical observation that paroxysmal AF becomes permanent, particularly if untreated.w6 w7 It is also possible that even in patients with permanent AF, focal drivers may sustain AF. Although there is evidence to support this in animal models,3 there have been little data regarding this in humans.
BRINGING ORDER TO CHAOS
Curative ablation of AF then has two mechanistic goals: to remove all potential triggers that may initiate or perpetuate AF; and the alteration of the conduction properties of the atria (substrate modification) so that AF cannot be sustained even when triggered. It is recognised that particular AF mechanisms may result in a particular clinical substrate and thus allow the procedure to be tailored so that, for example, in paroxysmal AF only isolation of triggers is attempted, whereas in persistent AF additional and more complex substrate modification may be performed.
The most common sources of triggers are the PVs; however, up to 20% may be non‐PV in origin such as the superior vena cava, coronary sinus, or crista terminalis.5 The trigger sites are isolated from the rest of the atria by either destroying the muscular connections that link them to the atria or by creating a continuous line of conduction block in the atria that surrounds and completely encloses them.
There are two approaches to substrate modification. Firstly, it can be achieved by creating transmural linear lesions that connect two anatomical structures and form barriers to conduction, thus interrupting the re‐entry circuits that perpetuate AF. The alternative approach (also known as atrial debulking) is to reduce the amount of atrial tissue available to form re‐entry wavefronts, either by enclosing and isolating large areas of atrial tissue (typically done around the PVs) or to ablate widely all over the atria thus reducing the amount of viable tissue. A possible downside to substrate modification is the reduction in the contractile potential of the atria which may prevent recovery of mechanical function.
CLINICAL OBJECTIVES OF ABLATION OF AF
It is important to remember what the clinical objectives are when treating AF in this invasive way with its potential risks. The main goals are to abolish or reduce symptoms, to improve left ventricular function by restoring both electrical and mechanical atrial systole, and finally to reduce the risk of stroke. Evidence from large multicentre trials have shown us that the success rates of conventional treatments for restoring and maintaining sinus rhythm are low and that attempts to restore sinus rhythm may do more harm than good, particularly if anticoagulation is not prescribed appropriately.6,7 Unfortunately these data do not help us decide whether a strategy of restoring and maintaining sinus rhythm is a worthwhile one and to date there is no evidence that treatment of AF by ablation improves mortality, although there are uncontrolled data suggesting that this may be the case.8 Therefore, asymptomatic patients should not be offered curative ablation of AF, except in the case of those patients undergoing cardiac surgery who may benefit from surgical ablation of their AF as an adjunctive procedure. There is also evidence that patients with heart failure have significant improvements in left ventricular function following successful catheter ablation of AF. This result is not explained by better ventricular rate control and is particularly notable if they do not have another cause for their impaired cardiac function (for example, ischaemic heart disease).9
SURGICAL ABLATION
The Maze procedure
Cardiac surgeons were the pioneers of curative ablation of AF, and in 1992 Cox's Maze‐III procedure evolved from five years accumulated worldwide surgical experience and carefully conducted animal and human mapping studies.10 Initially the lesions were created by a “cut and sew” method through a median sternotomy. This has the huge advantage of introducing lesions under direct vision in which the transmurality of the lesions is certain and therefore the mechanistic goals described above are definitely achieved. As a consequence this technique is extremely effective, with maintenance of sinus rhythm reported by Cox at greater than 97%w8 w9 and at 84.9% in a systematic review of 1553 patients in all published series up to 2004.11 In addition both left atrial (LA) mechanical functionw8 w10 w11 and left ventricular function have been shown to improve.w12 Another important feature of the surgical approach is the removal or closure of the LA appendage which leads to a very low operative (0.5%) and follow up stroke rate (0.3% at 12 years in one study).11w13
Limitations of the surgical Maze
If the prevalence of AF is so high and the Maze III is so effective, why has it not been widely embraced by cardiac surgeons? In its original form the operation was technically challenging with Cox himself describing its difficulty as being “9.5 on a scale to 10”.w14 As a result few centres in the world have been able to replicate the original Cox results. In addition there is a mortality and morbidity associated with the procedure which may be too high for the treatment of an arrhythmia considered by many (albeit wrongly) to be benign. From the large series the 30 day mortality rates vary from 0–7.2% (mean 2.1%); however, many of these deaths have occurred in patients undergoing concomitant surgery.11 Other complications are sinus node dysfunction with requirement for permanent pacing (5.8%), bleeding caused by the multiple incisions (4.9%), and stroke (0.5%).11
Newer techniques of surgical ablation
The key to the success of any catheter or surgical ablation of AF is the correct choice of lesions (as described above) and the production of transmural lesions (fig 2).12w15 w16 To make AF surgery more attractive, particularly as a stand alone procedure, techniques have developed allowing it to become minimally invasive and reduce the length of the procedure. Progress has been made on two fronts.
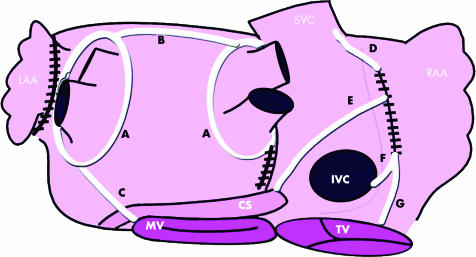
Figure 2 A schematic view of the posterior aspect of the left and right atria demonstrating a typical lesion set for surgical ablation of AF as an adjunct to mitral valve surgery at our institution. This is based upon that used by Sie et al.w51 Irrigated radiofrequency ablation can be delivered by a bipolar clamp or endocardially using a monopolar pen. The mitral valve (MV), tricuspid valve (TV), coronary sinus (CS), superior vena cava (SVC), inferior vena cava (IVC), and left (LAA) and right (RAA) atrial appendages are labelled. The four pulmonary veins are visible on the left atrium. The black hatched lines are incisions and the white lines ablation lesions. (A) The pulmonary veins are isolated as ipsilateral pairs by applying the clamp epicardially. The LA is entered via an incision adjacent to the posterior intra atrial groove. (B) This line of conduction block is extended by ablation to reach the left pulmonary veins. The LAA is excised and a line of ablation extended from it to the left pulmonary vein line. (C) A line of ablation extends from the left pulmonary veins to the MV annulus. The right atrium is entered via a lateral incision. (D) A line is extended from this incision superiorly to the SVC and (E–G) lines to the IVC, CS, and TV to produce conduction block in the TV–IVC isthmus.
Firstly, there has been a search for the minimum lesion set needed to achieve the mechanistic goals described above. The Maze‐III procedure was designed to interrupt all possible re‐entry circuits that could exist in AF and it remains the gold standard; however, it appears the LA is usually the source of AF wavefronts with the right atrium as a bystander.w17–19 It is not surprising then that similar results have been found for ablation in the LA only, compared to ablation performed in both atria.11 A lesion set that isolates all pulmonary veins, a line that links the isolated PV line to the mitral valve annulus (and ideally encircles the coronary sinus at that point) and a right atrial line across the tricuspid valve–inferior vena cava isthmus may be sufficient on its own to give high success rates in most patients. Cox has described this as the Minimaze.w14 Lesion sets limited to just isolating the PVs are not effective for permanent AF. w15 w20
Secondly, new energy sources for ablation have been developed as an alternative to cut and sew. Cryoablation and radiofrequency are the most common, which use hand held probes applied endocardially by direct vision. Alternatively, clamp devices hold the atrial wall between two jaws, either using small incisions in the atria or by surrounding the PV antrum epicardially, and deliver radiofrequency energy to produce a complete lesion.w20 Newer energy sources such as laser, microwave,w21 and ultrasound have the potential to produce transmural lesions even when applied epicardially. A further development from this is the use of a limited thoracic incision and thorascopically guided procedure.w22 The inevitable goal is the development of a closed chest, robotically assisted procedure performed on the beating heart.w23
As is the case with catheter ablation studies, the variety of surgical techniques, the heterogeneity of the patients treated, and the different antiarrhythmic regimens make comparing studies difficult. However, a comprehensive review of 2279 patients who underwent these newer surgical methods found 78.3% maintenance of sinus rhythm at follow up with an operative mortality of 4.2%11; 98.4% of these operations were performed alongside other cardiac surgery, predominantly mitral valve surgery. Meta‐analysis of surgical AF ablation studies reveals that the results are very similar regardless of the technique used.
CATHETER ABLATION
The potential for catheter ablation of AF was awakened by the discovery that ectopic atrial activation emerging from pulmonary veins could trigger AF and that ablation of these sites prevents recurrence of AF in previously highly symptomatic patients.2 There has been an exponential growth in the number of catheter AF ablations performed since then.w24
Isolation of the triggers
The PVs are the predominant source of triggers and the minimum catheter ablation procedure involves isolating them from the rest of the atria. Potential non‐PV triggers can also be isolated at the same procedure if they are spontaneously active.5 PV isolation is the usual procedure performed for symptomatic paroxysmal AF. Initial attempts were made to identify from which PV the triggers were arising and ablate the culprits only. It was then recognised, however, that AF may have multiple triggers of which many will be silent during the ablation procedure; consequently the current approach aims to ablate all PVs. Two main techniques that have been developed for total PV isolation and their relative merits are among the most controversial issues of invasive arrhythmia management.
The Bordeaux group pioneered destroying the connections of the PV to the LA—also known as segmental ostial ablation.2,13 Via two transseptal punctures an ablation catheter and PV mapping catheter are introduced into the LA. The PV catheter is an adjustable circumferential catheter which is positioned at the ostia of each of the veins and allows activation mapping of the PV. For the left sided PVs it can be difficult to distinguish between the potentials of the PV and those of the immediately adjacent muscular LA appendage. This can be overcome by pacing either from the distal coronary sinus or the LA appendage, which then separates the two potentials by advancing the LA appendage signal. If the patient is in sinus rhythm the technique is to identify from the PV catheter at which segment the PV activates initially. The ablation catheter is moved to this site and positioned 1 cm proximal to the PV–LA junction. Energy is delivered at this site until the signal recorded at the ablation signal is attenuated or the activation pattern in the PV changes. This process is repeated, moving the ablation catheter to new sites until one of two end points is reached; either abolition of all PV potentials (figs 3 and 4) or the PV potentials become dissociated from the rest of the LA. Ablation of the PV can also be performed during AF with the end point as abolition of all signals.w25 w26 The outcomes of segmental ostial isolation are excellent (51–100% freedom from AF); however, the methods and reporting of observational studies have varied greatly (table 1).
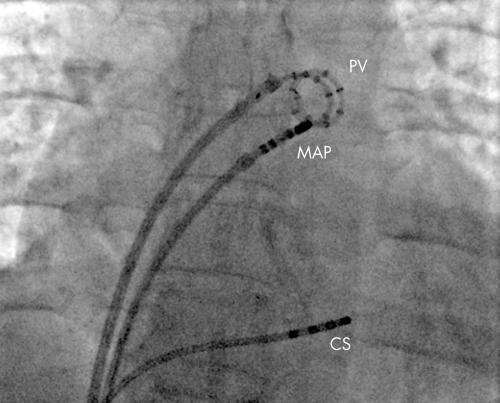
Figure 3 Electrical isolation of the left superior pulmonary vein. A fluoroscopic image of the heart viewed in the anterior posterior projection. The pulmonary vein catheter (PV) has 14 electrodes in a spiral and is positioned in the left superior pulmonary vein recording the electrograms inside the vein. The ablation catheter (MAP) is at the ostium of the vein where it joins the left atrium. Through this catheter radiofrequency energy is delivered to ablate the connections between the left atrium and pulmonary vein until electrical isolation of a pulmonary vein is achieved. A catheter is also seen in the coronary sinus (CS) which can be used to pace to separate the pulmonary vein potential form the far field left atrial potential.
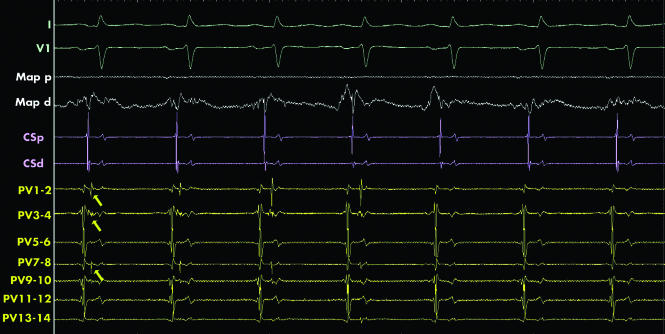
Figure 4 Electrical isolation of the left superior pulmonary vein. The surface and intracardiac electrograms recorded during ablation at the ostium of the left superior pulmonary vein. The signals shown are (from top to bottom); the surface ECG leads (I and V1, green), ablation catheter (Map, white), the coronary sinus (CS, pink) and the pulmonary vein catheter (PV, yellow). A double potential is recorded on some of the bipoles of the PV catheter (marked by yellow arrows). The first potential is the far field left atrial signal and the second the local PV potential. From the fifth sinus beat onwards the local PV potential disappears indicating the vein has become electrically isolated from the rest of the left atrium.
Table 1 Results of peer reviewed observational studies of paroxysmal AF ablation.
| Study | Patients (n) | SR (%) | Follow up (months) Mean (SD) | Type of AF (%) | Structural heart disease (%) | Technology used | Repeat procedures for quoted success (%) | How SR assessed | Use of antiarrhythmic drugs for quoted success* (%) | Serious complication rate† (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Segmental ostial isolation of 4 pulmonary veins | ||||||||||
| Haissaguerre et al 2000w28 | 90 | 71 | 8 (5) | 100 paroxysmal | 19 | Circular PV catheter | 54 | Symptoms & “Holter recordings” | 0 | 4.4 |
| 4 mm RFA | ||||||||||
| Fluoroscopy only | ||||||||||
| Deisenhofer et al 2003w52 | 75 | 51 | 7.6 (4.4) | 92 paroxysmal.8 persistent | 56 | Circular PV catheter | 40 | Repeated 7 day Holters | Not clearly stated | 10.7 |
| 4 mm RFA | ||||||||||
| Fluoroscopy only | ||||||||||
| Marrouche et al 2002w53 | 211 | 79–100 | 4 (2)–10 (3) | 54 paroxysmal,16 persistent,30 permanent | 24 | Circular PV catheter | 0 | “Holter recordings” | Not clearly stated | 3.5 |
| 4 mm, 8 mm and cooled RFA | “Event recorder considered if symptomatic” | |||||||||
| Fluoroscopy only | Recurrences <3 weeks ignored | |||||||||
| Tse et al 2003w54 | 52 | 56 | 12 (6) | 87 paroxysmal,13 persistent | 46 | Circular PV catheter | 0 | Event recorders | 38 | 7.7 |
| Cryoablation | ||||||||||
| Fluoroscopy only | ||||||||||
| Oral et al 2002w34 | 70 | 71 paroxysmal,25 persistent | 4.9 (2.6) | 83 paroxysmal,17 persistent | 7 | Circular PV catheter | 9 | Event recorder if symptomatic | 0 | 1.4 |
| 4 mm RFA | ||||||||||
| Fluoroscopy only | ||||||||||
| Macle et al 2002w45 | 136 | 66 | 8.8 (5.3) | 90 paroxysmal,10 persistent | 17 | Circular PV catheter | 49 | Not stated | 0 | 0.7 |
| Cooled RFA | ||||||||||
| Fluoroscopy only | ||||||||||
| Wide area circumference ablation of pulmonary veins | ||||||||||
| Pappone et al 200114 | 251 | 80 | 10.4 (4.5) | 71 paroxysmal,29 permanent | 14 | Electroanatomical mapping | Not stated | Monthly Holter monitoring | 5 | 0.8 |
| RFA (size not stated) | ||||||||||
| Pappone et al 2004w31 | 280 | 76 | Not stated | 66 paroxysmal,34 permanent | 42 | Electroanatomical mapping | Not stated | Daily trans telephonic monitoring | Not clearly stated | 0.7 |
| 8 mm RFA | Monthly Holter monitoring | |||||||||
| Recurrences <6 weeks ignored | ||||||||||
| Combination of wide area circumferential ablation and isolation of PV catheter | ||||||||||
| Ouyang et al 200417 | 41 | 95 | 6 (1) | 100% paroxysmal | Not clearly stated | 2 Circular PV catheters | 22 | Transtelephonic monitoring for asymptomatic patients and regular Holter monitoring | 0 | 0 |
| Cooled RFA | ||||||||||
| Electroanatomical mapping | ||||||||||
| Verma et al 200518 | 700 | 86 paroxysmal,73 non‐paroxysmal | 15.8 (7.8) | 39% paroxysmal,61 non‐paroxysmal | 44 | Circular PV catheter | 0 | Transtelephonic monitoring and regular 48 Holter monitoring Recurrences <2 months ignored | 0‡ | Not stated |
| 8 mm RFA | ||||||||||
| Electroanatomical mapping | ||||||||||
| Intracardiac echo | ||||||||||
*Vaughn‐Williams group 1 and 3 only.
†Death, stroke, tamponade, arterial–venous fistula needing repair, pulmonary embolism, phrenic nerve palsy, or ⩾1 pulmonary vein stenosis >50% (or causing symptoms).
‡All patients started on sotalol, propafenone, flecainide, or dofetilide for first 2 months then all stopped.
AF, atrial fibrillation; PV, pulmonary vein; RFA, radiofrequency ablation; SR, sinus rhythm.
The alternative strategy is to create a continuous line of ablation in the LA that surrounds and completely encloses the PVs in ipsilateral pairs—also known as wide area circumferential ablation. Placement of multiple lesions as a contiguous line in such a complex three dimensional structure is much easier if a non‐fluoroscopic guidance system is utilised. These make use of magnetic fields (Carto), low amplitude electrical fields (Ensite NavX), or a non‐contact mapping balloon array (Ensite Array). They all enable catheters to be viewed without fluoroscopy and enable construction of a computer generated model of the LA onto which anatomical structures and ablation lesions can be superimposed (fig 5). The technique as originally described is an empiric anatomic one with no attempt made to demonstrate that the PVs are electrically isolated. In one study using an end point of voltage reduction to < 1 mV within a coalescent line of ablation around the pulmonary veins, 45% of PVs remained electrically connected to the LA.w27 Despite this being contrary to our understanding of AF mechanisms, it has delivered excellent results with 80% freedom from AF (table 1).14
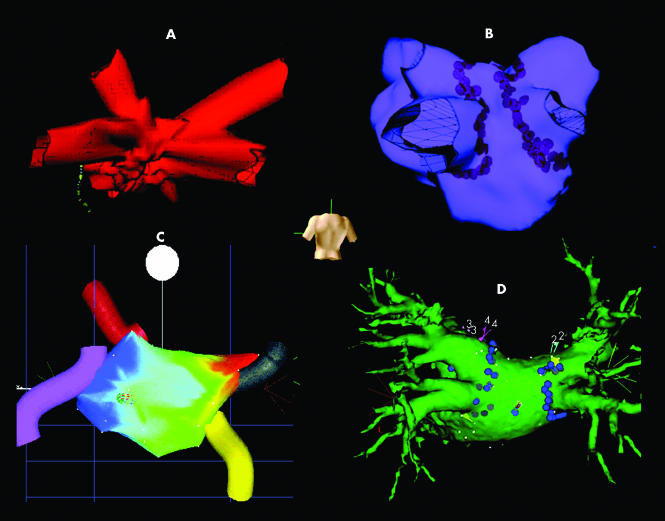
Figure 5 The non‐fluoroscopic catheter navigation systems used for catheter ablation of AF. A reconstructed geometry of the left atrium is visualised from the posterior perspective as indicated by the human torso. (A) Ensite Navx uses electrical fields to locate the catheters. Four pulmonary veins are clearly visible and a diagnostic decapolar catheter positioned in the coronary sinus is also visible. (B) Ensite Array (non‐contact mapping) uses electrical signals to locate the catheters. Four pulmonary veins are visible and the brown markers are lesions of radiofrequency ablation. (C) Carto (electroanatomical mapping) uses magnetic fields to locate the ablation catheter. The coloured cylinders represent the pulmonary veins. The colour depicts the timing of the atrial electrograms recorded; in this case the earliest activation (red) is the result of a right upper pulmonary vein tachycardia. (D) Cartomerge integrates a three dimensional computed tomographic reconstruction of the patient's left atrium into the Carto geometry giving much greater anatomical definition than seen in (C). The mapping catheter can be seen in the centre of the image. The blue markers represent lesions of radiofrequency ablation.
The advantage of segmental ostial isolation is that a definite, measurable electrophysiological end point is achieved. There is conflicting evidence from observational studies that isolation of the PVs is both a predictor of,w28 and not necessary for,w29 a successful outcome. Even randomised controlled trials which have compared the two techniques have had opposite results.15,16 What is clear, however, is that wide area circumferential ablation virtually eliminates the risk of PV stenosis at the cost of an incidence of macroreentrant atrial tachycardias as high as 24%,w30 although this may be reduced by the addition of linear lesions on the posterior wall of the LA.w31 A technique that combines the merits of these rival strategies is to both perform wide area circumferential ablation and check for PV isolation using a circular mapping catheter. Such a technique attacks all the possible mechanisms of paroxysmal AF—PV triggers, microreentry in the PV antrum, and denervation of the parasympathetic inputs surrounding the PVs. Freedom from paroxysmal AF is high using this technique (86–95%).17,18
Risks and benefits of catheter ablation
Success 60–90% depending on paroxysmal versus permanent AF
Success rate reduced in permanent AF in patients with longstanding AF (> 5 years)
Many patients (approximately 40%) (particularly permanent AF) will need more than one procedure to achieve drug‐free sinus rhythm
Serious complication rate 2%—the most common is pericardial tamponade then stroke (approximately 0.5–1%)
Substrate modification
It is clear from observational studies that PV isolation alone is not as effective for patients with persistent or permanent rather than paroxysmal AF,w32–34 which is consistent with the current understanding of the mechanisms described above. Initial attempts at delivering long lines of radiofrequency ablation aimed at mimicking the lines of the surgical Maze, although successful, led to high complication rates.w35 w36 Delivering lines to the right atrium only is safe but of low efficacy, particularly in permanent AF.w37–39 Ablation of the cavotricuspid isthmus, however, is a simple adjunctive procedure to ablation in the LA, that may reduce the incidence of typical atrial flutter.w40
Substrate modification of the LA has evolved as electrophysiologists have become comfortable ablating around the PVs. The simplest approach of ablating around the PVs (wide area circumferential ablation) excludes a large area of the LA which is then not available to support AF. Following this procedure Pappone reported 68% freedom from permanent AF and Verma 73% from non‐paroxysmal AF.14,18 Further improvements have required a strategy closer to the surgical Maze—that is, lines to connect the ipsilateral pairs of the PVs and a line to link the left pulmonary vein to the mitral valve annulus, which can be described as the catheter Maze. Such lines further improve the outcomes of paroxysmal AF w31 w41 and have produced good results for permanent AF ablation as well.w42 It is clear that producing lines with proven transmural conduction block leads to a smaller recurrence of AF; however, achieving this is technically challenging and requires long, arduous procedures.12w43 w44 In addition gaps in these lines may promote macroreentrant (left) atrial tachycardia.
A novel approach has been the ablation of all fractionated electrograms in the right and left atrium, with the hypothesis being that these are consistent sites where fibrillating wavefronts turn or split. By ablating these areas the propagating random wavefronts are progressively restricted until the atria can no longer support AF. Nademanee demonstrated 70% freedom from AF following a single procedure for permanent AF patients.19 In another study it was shown that in addition to wide area circumferential ablation, targeting fractionated potentials reduced intraoperative inducibilty of AF from 90% to 40%.w41 It is not clear from either of these studies though whether the success of this technique is related simply to debulking myocardium or to targeting the critically positioned fractionated electrograms.
Complications of catheter ablation
For the 9000 patients reported in the worldwide survey of AF ablation there was a mortality of 0.05% and an overall complication rate of 5.9%.w24 Some of the largest interventional centres, however, did not contribute to this study and published single centre studies report the incidence of complications at < 1% for paroxysmal AF (table 1).14,17w45 When linear ablation is attempted a higher rate is expected.12
Ablation of atrial fibrillation (AF): key points
Who to refer for consideration of AF ablation
Patients experiencing symptomatic AF who have failed conventional treatment—for example, antiarrhythmic drugs and/or cardioversion (for catheter ablation)
Patients with AF undergoing cardiothoracic surgery for other reasons (for surgical ablation)
A less proven indication is AF associated with heart failure
It is important that patients are fully counselled as to the risks of ablation and are prepared to take them
Who not to refer for AF ablation
Patients wishing to come off anticoagulation—there is still no randomised controlled data demonstrating that patient's stroke risk is reduced by ablation
Patients hoping that complications and death associated with AF will be avoided
Stroke and pericardial tamponade are understandable risks of a procedure that involves multiple transseptal punctures and the delivery of radiofrequency energy within the systemic circulation. These can be minimised though by good technique and training (table 2). Iatrogenic PV stenosis is a clinical condition that became apparent as a result of delivering too much energy within the PVs. Symptoms of progressive dyspnoea, cough, and haemoptysis, however, are insidious and can be wrongly diagnosed as respiratory pathology. Remarkably patients are usually asymptomatic unless more than a single PV is occluded or severely stenosed (fig 6).w46 Although it can be successfully treated by angioplasty,w47 it is best avoided (table 2). The development of an atrio‐oesophageal fistula is a very rare but lethal complication. Presentation is with pericarditis, sepsis (suspected endocarditis), or massive haemotemesis.w48 Even prompt surgical intervention may not be life saving and therefore this complication must be avoided (table 2).
Table 2 Complications of catheter ablation.
| Complication | Incidencew24 | How to minimise risk |
|---|---|---|
| Stroke/transient ischaemic attack | 1% | • Warfarin substituted for clexane during perioperative period |
| • Preoperative transoesophageal echocardiography | ||
| • Heparin infusion to maintain activated clotting time >300 s throughout case | ||
| • Heparin–saline irrigated ablation catheters | ||
| • Transseptal sheaths in right side of heart when possible | ||
| • Fastidious technique when removing/exchanging catheters | ||
| Tamponade | 1.2% | • Competency in transseptal puncture |
| • Intracardiac echo to monitor microbubbles and venting (indicating potential cavitation of lesion) | ||
| • Competency in emergency pericardial aspiration | ||
| • Rapid access to cardiothoracic surgical assistance | ||
| >50% pulmonary vein stenosis | 1.3% | • Ablation on atrial aspect of LA‐PV junction or outside vein |
| • Low power (20–30 W) radiofrequency ablation near PV | ||
| • Cryoablation causes less PV stenosis but longer procedure | ||
| • Symptoms non‐specific—therefore need low suspicion to investigate | ||
| Atrio‐oesophageal fistula | Few cases worldwide | • Where possible avoid lesions in posterior LA |
| • Reduced power (20–30 W) if ablating at posterior LA | ||
| • Fluoroscopic location of oesophagus using probe |
LA, left atrium; PV, pulmonary vein.
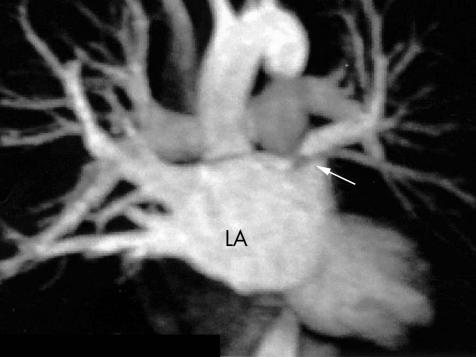
Figure 6 A contrast enhanced magnetic resonance image of the left atrium (LA) showing a severe ostial stenosis of the left superior pulmonary vein. The body of the left atrium is viewed in the anteroposterior (AP) projection. The right superior and inferior pulmonary veins are visible and the arrow indicates the stenosis. This patient was asymptomatic; however, reduced perfusion to the left lung was demonstrated by a VQ scan and a successful balloon angioplasty of this vessel was performed.
Who to refer for catheter ablation
One of the difficulties with counselling patients regarding AF ablation is that the published studies are very difficult to compare and interpret because they vary in the proportion of patients with persistent or permanent AF, prevalence of structural heart disease, the length of follow up, how sinus rhythm is assessed, the use of antiarrhythmic drugs, and the need for repeat procedures. Studies often emerge from single centres with polarised views on the mechanisms of AF and the best technique of ablation. The reader should be aware of such bias and it is often easier to recommend to patients that they ask the results of an individual centre when they are assessed for AF ablation before they make a final decision as to how they wish to proceed. We have given a summary of the results that most good high volume centres are likely to achieve as a guide (table 1).
CONCLUSION
For many patients with a previously untreatable heart rhythm, ablation has dramatically improved their symptoms by restoring and maintaining sinus rhythm. The rapid development of these treatments has made this an exciting time to be involved in cardiac electrophysiology. Despite this we cannot be sure that catheter or surgical ablation offers any advantages over any other form of treatment as there have been only three small randomised controlled trials comparing surgical or catheter ablation to conventional therapy.20w49 w50 This is primarily because ablation is used most often in patients who have already failed conventional treatment. There is therefore still a need for well conducted multicentre randomised controlled trials using standardised and verifiable criteria of inclusion, procedure, and follow up. This would give us a better idea of the impact that ablation may have on patients' mortality and morbidity, most important of which is the risk of stroke.
Additional references appear on the Heart website—http://www.heartjnl.com/supplemental
Additional references appear on the Heart website—http://www.heartjnl.com/supplemental
Supplementary Material
ACKNOWLEDGEMENTS
Dr Dominic Abrams contributed the original artwork for fig 1 and 2.
Footnotes
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article
Additional references appear on the Heart website—http://www.heartjnl.com/supplemental
References
- 1.Levy S, Camm A J, Saksena S.et al International consensus on nomenclature and classification of atrial fibrillation: a collaborative project of the working group on arrhythmias and the working group of cardiac pacing of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. J Cardiovasc Electrophysiol 200314443–445.This is an important consensus for standardising the terms we use to describe the temporal aspect of atrial fibrillation—that is, paroxysmal, persistent, and permanent. [DOI] [PubMed] [Google Scholar]
- 2.Haissaguerre M, Jais P, Shah D C.et al Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998339659–666.A landmark paper that ignited the current enthusiasm for catheter ablation of AF. An observational study of 45 patients where careful mapping of the atria revealed that runs of atria tachycardia from pulmonary veins triggered paroxysms of AF and that ablation of these foci reduced the incidence of AF episodes. [DOI] [PubMed] [Google Scholar]
- 3.Jalife J, Berenfeld O, Mansour M. Mother rotors and fibrillatory conduction: a mechanism of atrial fibrillation. Cardiovasc Res 200254204–216.A review by the chief proponent of the concept that high frequency re‐entry circuits drive AF. [DOI] [PubMed] [Google Scholar]
- 4.Wijffels M C, Kirchhof C J, Dorland R.et al Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995921954–1968.A landmark experiment supporting the concept of remodelling of the electrical properties of the atria caused by AF. [DOI] [PubMed] [Google Scholar]
- 5.Chen S A, Tai C T. Catheter ablation of atrial fibrillation originating from the non‐pulmonary vein foci. J Cardiovasc Electrophysiol 200516229–232.Not all foci driving paroxysmal AF are from the pulmonary veins and this review explains the approach to ablation of alternative foci. [DOI] [PubMed] [Google Scholar]
- 6.The AFFIRM Investigators A comparison of rate control and rhythm control in atrial fibrillation. N Engl J Med 20023471825–1833. [DOI] [PubMed] [Google Scholar]
- 7.Van Gelder I C, Hagens V E, Bosker H A.et al A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 20023471834–1840.These two studies have generated a great deal of debate regarding whether restoration of sinus rhythm is a worthwhile goal for patients with AF. Their most important messages, however, are that antiarrhythmic drugs are toxic and proarrhythmic and that anticoagulation with warfarin is the cornerstone of AF management. Only very few patients had AF ablation. [DOI] [PubMed] [Google Scholar]
- 8.Pappone C, Rosanio S, Augello G.et al Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: outcomes from a controlled nonrandomized long‐term study. J Am Coll Cardiol 200342185–197.Carlo Pappone has performed by far the greatest number of AF ablations worldwide using his technique of wide area circumferential ablation. Although non‐randomised, this study highlights the superiority of this approach in his hands. [DOI] [PubMed] [Google Scholar]
- 9.Hsu L F, Jais P, Sanders P.et al Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med 20043512373–2383.This is another very important study from Bordeaux, which shows that successful ablation of AF improves left ventricular function in heart failure patients. [DOI] [PubMed] [Google Scholar]
- 10.Cox J L. Cardiac surgery for arrhythmias. Pacing Clin Electrophysiol 200427266–282.Cox is the pioneer of cardiac surgery to treat AF and in this review covers the history and evolution of the Maze procedure. [DOI] [PubMed] [Google Scholar]
- 11.Khargi K, Hutten B A, Lemke B.et al Surgical treatment of atrial fibrillation; a systematic review. Eur J Cardiothorac Surg 200527258–265.This is a comprehensive review of all the observational studies to date of surgical treatment of AF, comparing “cut and sew” methods with those using newer ablation energy sources to create lines of conduction block. [DOI] [PubMed] [Google Scholar]
- 12.Ernst S, Ouyang F, Lober F.et al Catheter‐induced linear lesions in the left atrium in patients with atrial fibrillation: an electroanatomic study. J Am Coll Cardiol 2003421271–1282.This observational study of 84 patients demonstrated that production of long complete lines of conduction block are more effective in preventing recurrence of AF; however, they are very difficult to achieve and lead to a high rate of complications. [DOI] [PubMed] [Google Scholar]
- 13.Hocini M, Sanders P, Jais P.et al Techniques for curative treatment of atrial fibrillation. J Cardiovasc Electrophysiol 2004151467–1471.This reviews in detail the approach of segmental ostial isolation to disconnect electrically the pulmonary veins. [DOI] [PubMed] [Google Scholar]
- 14.Pappone C, Oreto G, Rosanio S.et al Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation 20011042539–2544.This study was the first large study (251 patients) of wide area circumferential ablation establishing that it was effective and a rival to pulmonary vein isolation. [DOI] [PubMed] [Google Scholar]
- 15.Karch M R, Zrenner B, Deisenhofer I.et al Freedom from atrial tachyarrhythmias after catheter ablation of atrial fibrillation: a randomized comparison between 2 current ablation strategies. Circulation 20051112875–2880. [DOI] [PubMed] [Google Scholar]
- 16.Oral H, Scharf C, Chugh A.et al Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation 20031082355–2360.These two randomised studies have attempted to compare the two major strategies to catheter ablation of AF; however, they have produced contradictory results and the debate continues. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang F, Bansch D, Ernst S.et al Complete isolation of left atrium surrounding the pulmonary veins. New insights from the double‐Lasso technique in paroxysmal atrial fibrillation. Circulation 20041102090–2096. [DOI] [PubMed] [Google Scholar]
- 18.Verma A, Wazni O M, Marrouche N F.et al Pre‐existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: an independent predictor of procedural failure. J Am Coll Cardiol 200545285–292.These two observational studies are the best results published so far for catheter ablation of AF with cure rates of 90% for paroxysmal AF. They both use a technique of electrical isolation of the pulmonary veins inside a wide line of ablation that also encloses the surrounding atrial tissue. [DOI] [PubMed] [Google Scholar]
- 19.Nademanee K, McKenzie J, Kosar E.et al A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol 2004432044–2053.This observational study has taught us a radically different approach to AF ablation that appears to be effective—that is, ablation of fractionated electrograms found in the atria. It is not clear why it is so effective. [DOI] [PubMed] [Google Scholar]
- 20.Wazni O M, Marrouche N F, Martin D O.et al Radiofrequency ablation vs antiarrhythmic drugs as first‐line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 20052932634–2640.Although small (70 patients), to date this is the only randomised study comparing medical treatment and catheter ablation to manage AF. It demonstrated that ablation leads to fewer symptomatic recurrences, less hospitalisation and improved quality of life. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.