Abstract
Objective
To determine whether dietary supplementation with α linolenic acid (ALA) can modify established and emerging cardiovascular risk markers.
Design
Systematic review and meta‐analysis of randomised controlled trials identified by a search of Medline, Embase, Cochrane Controlled Trials Register (CENTRAL), and the metaRegister of Controlled Trials (mRCT).
Patients
All human studies were reviewed.
Main outcome measures
Changes in concentrations of total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, very low density lipoprotein (VLDL) cholesterol, triglyceride, fibrinogen, and fasting plasma glucose, and changes in body mass index, weight, and systolic and diastolic blood pressure.
Results
14 studies with minimum treatment duration of four weeks were reviewed. ALA had a significant effect on three of the 32 outcomes examined in these studies. Concentrations of fibrinogen (0.17 μmol/l, 95% confidence interval (CI) −0.30 to −0.04, p = 0.01) and fasting plasma glucose (0.20 mmol/l, 95% CI −0.30 to −0.10, p < 0.01) were reduced. There was a small but clinically unimportant decrease in HDL (0.01 mmol/l, 95% CI −0.02 to 0.00, p < 0.01). Treatment with ALA did not significantly modify total cholesterol, triglycerides, weight, body mass index, LDL, diastolic blood pressure, systolic blood pressure, VLDL, and apolipoprotein B.
Conclusions
Although ALA supplementation may cause small decreases in fibrinogen concentrations and fasting plasma glucose, most cardiovascular risk markers do not appear to be affected. Further trials are needed, but dietary supplementation with ALA to reduce cardiovascular disease cannot be recommended.
Keywords: α linolenic acid, cardiovascular prevention, systematic review
The cardiovascular benefits of fish oil are now well established,1, but it is unclear whether α linolenic acid (ALA) confers similar benefits. ALA is a plant ω‐3 fatty acid, precursor of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), the two main ω‐3 polyunsaturated fatty acids found in fish oils.2 However, unlike fish oils, ALA is inexpensive to produce and is more palatable than cod liver oil.
Clinical trials of dietary supplementation such as the Lyon diet heart study, in which ALA was a component, suggest that ALA may confer cardiovascular benefits.3 This has led to calls for trials specifically evaluating the effect of substituting oils containing ALA.
We therefore systematically reviewed randomised controlled trials to investigate the impact of ALA on cardiovascular risk markers.
METHODS
We searched Medline, Embase, and the Cochrane Controlled Trials Register (CENTRAL) databases for published studies and the metaRegister of Controlled Trials (mRCT) for unpublished studies by using the search terms linolenic acid, plant oils, flax, linseed, canola, rapeseed, perilla, juglans, pumpkin, and purslane with a standard search filter to identify randomised controlled trials. We identified additional studies by searching references cited in identified primary studies. We restricted our search to studies of humans and included articles in languages other than English.
Studies were included if they had a control or comparison arm and had either a randomised crossover design (with a washout interval of ⩾ 4 weeks) or a parallel group design (with ⩾ 4 weeks of intervention). The units of measurement were converted to the common unit suggested by SI notation. Where crossover studies provided independent data for each intervention period we used data for only the first intervention period. Criteria for assessment of trial quality were the method of randomisation, blinding or objective measurements, loss to follow up, and systematic difference in care between intervention groups. When there was more than one control or ALA comparison group, we pooled results of the similar groups. We analysed subgroups stratified by the ALA dose used and type of control, and by comparing included and excluded trials.
Statistical analysis
Outcomes were changes in total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, very low density lipoprotein (VLDL) cholesterol, triglyceride, fibrinogen, fasting plasma glucose, body mass index, weight, and systolic and diastolic blood pressures. All data were analysed with Review Manager (version 4.2.3; Update Software, Oxford, UK).
For each trial we calculated the changes in the means between the beginning and the end of each intervention and estimated the standard deviation of the treatment effect. If the standard deviations of change were not provided, we derived them from the 95% confidence intervals or the standard error.
We used a fixed effect meta‐analysis model to calculate overall results. When a significant heterogeneity was observed, we used a random effect model instead. Heterogeneity was determined by χ2 (p < 0.10).
RESULTS
From 2566 references identified (1800 in Medline, 1955 in Embase, and 931 in CENTRAL) we reviewed 46 published clinical studies and one unpublished trial reporting the effects of ALA on cardiovascular risk markers. We excluded 31 of the 47 studies because they were not placebo controlled, provided insufficient information, or had a treatment period of < 4 weeks. We identified 28 outcomes in 16 published papers, reporting 14 studies. Twelve studies, involving 744 subjects, had outcomes in common and were included in the quantitative meta‐analysis. Table 1 shows selected characteristics of the included trials.4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19
Table 1 Randomised controlled trials assessing the effect of ALA on established cardiovascular risk factors and emerging risk markers.
| Author | Treatment | Type of intervention | Country | No of subjects | Participants (condition, sex, age group*) | Length of treatment |
|---|---|---|---|---|---|---|
| Arjmandi et al16† | Flaxseed, sunflower seed | Breads, muffins | USA | 38 | Hypercholesterolaemic, postmenopausal women, 56.3 years | 6 weeks |
| Bemelmans et al4 | ALA, LA | Margarine | Netherlands | 265 | Cardiovascular risks, men and women, 55 years | 104 weeks |
| Finnegan et al5 | LA, fish oil, ALA | Margarine, capsules | UK | 150 | Moderately hyperlipidaemic, men and women, 53 years | 6 months |
| Finnegan et al15 | LA, fish oil, ALA | Margarine, capsules | UK | 150 | Moderately hyperlipidaemic, men and women, 53.3 years | 6 months |
| Junker et al10 | Olive oil, sunflower oil, rapeseed oil | Margarine, bread | Germany | 69 | Healthy, men and women, 24–27 years | 4 weeks |
| Karvonen et al11 | Camelina oil, olive oil, rapeseed oil | Oil | Finland | 68 | Hypercholesterolaemic, men and women, 50–53 years | 6 weeks |
| Kestin et al6 | Fish oil, linseed, safflower oil | Emulsion | Australia | 33 | Hypercholesterolaemic, men and women, 45.9 years | 6 weeks |
| Kratz et al12 | Olive oil, sunflower oil, rapeseed oil | Margarine, breads | Germany | 58 | Healthy, men and women, 26 years | 4 weeks |
| Kratz et al19 | Olive oil, sunflower oil, rapeseed oil | Margarine, breads | Germany | 48 | Healthy, men and women, 25.4 years | 4 weeks |
| Meshcheriakova et al13 | Fish oil, linseed oil, sunflower oil | Diet | Russia | 120 | NIDDM, 54–56 years | 4 weeks |
| Pang et al8 | ALA, LA | Muffins, diet | Australia | 29 | Healthy, men, 24–25 years | 6 weeks |
| Rallidis et al7 | Linseed oil, safflower oil | Oil | Greece | 76 | Dyslipidaemic, men, 51 years | 12 weeks |
| Södergren et al14† | Rapeseed oil, saturated oil (butter, olive oil) | Fat products | Finland | 19 | Hyperlipidaemic, men and women, 50 years | 4 weeks |
| St Onge et al9* | Olive oil, functional oil | Meals | Canada | 28 | Overweight, men, 26–61 years | 4 weeks |
| Thies et al17 | Placebo, ALA, GLA, ARA, DHA, fish oil | Oil capsules | UK | 46 | Healthy, men and women, 61–66 years | 12 weeks |
| Wensing et al18 | Oleic acid, ALA, EPA+DHA | Shortening | Netherlands | 38 | Healthy, men and women, >60 years | 6 weeks |
*Age data are means, except in St Onge, where age is given as an interval; †crossover studies.
ALA, α linolenic acid; ARA, arachidonic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; GLA, γ linolenic acid; LA, linolenic acid; NIDDM, non‐insulin dependent diabetes mellitus.
Body weight and blood pressure
Six studies measured body weight and three4,5,6 reported systolic and diastolic blood pressures. Three reported the body mass index4,6,7 and three5,8,9 reported weight. Comparisons between ALA and control groups were not significant (p > 0.05) for either body weight or blood pressure (table 2).
Table 2 Summary of the effect of the ALA acid on outcomes.
| Outcome | References | No of trials | No of subjects | Effect size (95% CI) | p Value† | χ2‡ |
|---|---|---|---|---|---|---|
| Total cholesterol (mmol/l) | 4–14 | 11 | 790 | −0.01 (−0.08 to 0.06)* | 0.75 | 19.64 |
| High density lipoprotein (mmol/l) | 4–10, 12, 14, 16 | 10 | 661 | −0.01 (−0.02 to −0.00) | <0.01 | 5.28 |
| Low density lipoprotein (mmol/l) | 4–10, 12, 14, 16 | 10 | 680 | 0.03 (−0.04 to 0.10)* | 0.42 | 16.38 |
| Triglycerides (mmol/l) | 4–9, 13, 14 | 9 | 629 | 0.01 (−0.11 to 0.14)* | 0.83 | 23.36 |
| Weight (kg) | 5, 8, 9 | 3 | 166 | −0.18 (−0.72 to 0.36) | 0.52 | 0.79 |
| Body mass index (kg/m2) | 4, 6, 7 | 3 | 335 | −0.04 (−0.11 to 0.03) | 0.28 | 1.59 |
| Systolic blood pressure (mm Hg) | 4–6 | 3 | 348 | −0.72 (−2.01 to 0.58) | 0.28 | 1.92 |
| Diastolic blood pressure (mm Hg) | 4–6 | 3 | 348 | −0.17 (−0.82 to 0.48) | 0.61 | 0.11 |
| Fibrinogen (μmol/l) | 4, 10, 15 | 3 | 382 | −0.17 (−0.30 to −0.04) | 0.01 | 3.32 |
| Fasting plasma glucose (mmol/l) | 14, 15 | 2 | 127 | −0.20 (−0.30 to −0.10) | < 0.01 | 0.11 |
| VLDL cholesterol (mmol/l) | 6, 14 | 2 | 60 | −0.02 (−0.08 to 0.03) | 0.37 | 0.49 |
| Apolipoprotein B (mmol/l) | 14, 15 | 2 | 127 | −0.03 (−0.11 to 0.04) | 0.43 | 2.03 |
*Random effects mode; †p value for effect size; ‡χ2 statistic for heterogeneity between trials.
CI, confidence interval; VLDL, very low density lipoprotein.
Cholesterol and triglycerides
Eleven studies4,5,6,7,8,9,10,11,12,13,14 with a total of 790 subjects reported changes in total cholesterol and eight studies4,5,6,7,8,9,13,14 reporting triglyceride concentrations enrolled a total of 629 subjects. As significant heterogeneity was evident (p < 0.05) a random effect model was used. The pooled mean differences were −0.01 mmol/l for total cholesterol and 0.01 mmol/l for triglycerides (table 2). HDL and LDL cholesterol4,5,6,7,8,9,10,12,14,16 were studied in a total of 661 and 680 patients, respectively. We also identified two studies that measured VLDL cholesterol.6,14 Heterogeneity between the studies for both HDL cholesterol and VLDL cholesterol were not significant (p > 0.10), although heterogeneity was significant (χ2 = 16.38, p = 0.06) for LDL cholesterol. The pooled mean difference in HDL concentrations was −0.01 mmol/l (p < 0.01). The effect sizes of LDL and VLDL cholesterol were not significant (table 2).
Glucose and fibrinogen
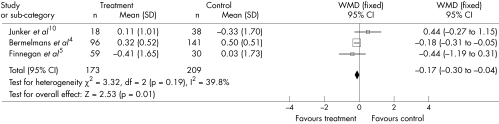
We identified two studies assessing the effect of ALA on fasting plasma glucose.14,15 A fixed effects analysis showed a significant (p < 0.01) reduction in the mean difference of 0.20 mmol/l (−0.30, −0.10 mmol/l). The effect of ALA on fibrinogen was examined in three studies4,10,15 with 382 subjects. The pooled mean difference in fibrinogen was a significant (p = 0.01) decrease of 0.17 μmol/l (−0.30, −0.04 µmol/l) (fig 1). Heterogeneity between the studies was not significant for both outcomes (p > 0.10).
Figure 1 Size effect of α linolenic acid compared with placebo on fibrinogen concentration. CI, confidence interval; WMD, weighted mean difference.
Emerging cardiovascular risk markers
Several changes in plasma markers were reported in only one of the identified studies. The following markers of inflammation were identified in only one study: tumour necrosis factor α, interleukin 6, C reactive protein, cell adhesion molecule 1, vascular cell adhesion molecule 1,7,17 and thrombogenic factors such as factor VII, factor XII, von Willebrand factor, thromboxane, Imax, platelet aggregation velocity, plasminogen activator inhibitor 1, tissue plasminogen activator, and D dimer.4,5,10,14,18
Apolipoproteins A and A IV, fatty acids, apolipoprotein B, and Lp(a) lipoprotein were also reported.5,14,19 Although the effect of some markers was significant over time, only vascular cell adhesion molecule 117 was significantly different between treatments.
Subgroup analyses did not show significant differences when analysed by type of placebo or by the dose used of ALA (above and below 5 g/day). Funnel plots for selected outcomes did not provide evidence of publication bias in favour of trials with positive outcomes.
DISCUSSION
This systematic review provides the most reliable assessment yet of whether ALA is associated with established and emerging risk markers for coronary heart disease. Our systematic review indicates that ALA significantly affects fibrinogen and fasting plasma glucose concentrations, decreasing fibrinogen concentrations by 0.17 μmol/l and fasting glucose by 0.20 mmol/l. No other statistically or clinically significant findings were evident in the quantitatively evaluated cardiovascular risk markers.
A limitation of the meta‐analysis was that most trials were small; they did not describe the method of randomisation and not all of them were blinded. For some potential risk markers we were unable to identify two or more studies to allow pooling. We were unable to obtain data from unpublished studies and did not attempt to obtain patient level data. Although we did not adjust statistically for multiple comparisons, the two clinically important differences we observed were highly significant. The subgroup analysis showed no significant difference by either type or dose of placebo; the small dose of olive oil used as a placebo (table 1) is therefore unlikely to have masked any important differences.
On the basis of estimates from a meta‐analysis of observational studies, a 2.9 μmol/l reduction in fibrinogen concentration would lead to a relative risk reduction of 80% in coronary heart disease.20 Therefore, a reduction of 0.17 μmol/l attributable to ALA would be expected to lead to a reduction of 6% in coronary heart disease. This is a much smaller reduction than that observed in the Lyon diet heart study, in which patients were randomly assigned to a Mediterranean diet and margarine high in ALA. Fibrinogen is therefore unlikely to mediate a clinically important effect of ALA on cardiovascular risk.
ALA is a metabolic precursor of DHA and EPA and any risk reduction may be mediated through conversion to this fatty acid. However, the metabolic overall conversion rate is low2 and varies between the sexes, being higher in women.21 Our review suggests that the impact of ALA on decreased cardiovascular risk is unlikely to be mediated through conversion to DHA or EPA, since we noted no changes consistent with increased concentrations of these fatty acids.
Although supplementation with ALA may lead to a small decrease in fibrinogen concentrations and fasting plasma glucose, most established cardiovascular risk factors or emerging risk markers do not appear to be affected. Further trials are needed but on the basis of this meta‐analysis, dietary supplementation with ALA to reduce cardiovascular disease cannot be recommended.
ACKNOWLEDGEMENTS
The authors have no conflicts of interest to declare. EW was supported by Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). AF is supported by an NHS R&D career scientist award.
Abbreviations
ALA - α linolenic acid
CENTRAL - Cochrane Controlled Trials Register
DHA - docosahexaenoic acid
EPA - eicosapentaenoic acid
HDL - high density lipoprotein
LDL - low density lipoprotein
mRCT - metaRegister of Controlled Trials
VLDL - very low density lipoprotein
Footnotes
Ethical approval was not required for this study.
References
- 1.Bucher H C, Hengstler P, Schindler C.et al N‐3 polyunsaturated fatty acids in coronary heart disease: a meta‐analysis of randomized controlled trials. Am J Med 2002112298–304. [DOI] [PubMed] [Google Scholar]
- 2.Gerster H. Can adults adequately convert alpha‐linolenic acid (18:3n‐3) to eicosapentaenoic acid (20:5n‐3) and docosahexaenoic acid (22:6n‐3)? Int J Vitam Nutr Res 199868159–173. [PubMed] [Google Scholar]
- 3.De Lorgeril M, Salen P, Martin J L.et al Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon diet heart study. Circulation 199999779–785. [DOI] [PubMed] [Google Scholar]
- 4.Bemelmans W J, Broer J, Feskens E J.et al Effect of an increased intake of alpha‐linolenic acid and group nutritional education on cardiovascular risk factors: the Mediterranean alpha‐linolenic enriched Groningen dietary intervention (MARGARIN) study. Am J Clin Nutr 200275221–227. [DOI] [PubMed] [Google Scholar]
- 5.Finnegan Y E, Minihane A M, Leigh Firbank E C.et al Plant‐ and marine‐derived n‐3 polyunsaturated fatty acids have differential effects on fasting and postprandial blood lipid concentrations and on the susceptibility of LDL to oxidative modification in moderately hyperlipidemic subjects. Am J Clin Nutr 200377783–795. [DOI] [PubMed] [Google Scholar]
- 6.Kestin M, Clifton P, Belling G B.et al n‐3 fatty acids of marine origin lower systolic blood pressure and triglycerides but raise LDL cholesterol compared with n‐3 and n‐6 fatty acids from plants. Am J Clin Nutr 1990511028–1034. [DOI] [PubMed] [Google Scholar]
- 7.Rallidis L S, Paschos G, Liakos G K.et al Dietary alpha‐linolenic acid decreases C‐reactive protein, serum amyloid A and interleukin‐6 in dyslipidaemic patients. Atherosclerosis 2003167237–242. [DOI] [PubMed] [Google Scholar]
- 8.Pang D, Allman Farinelli M A, Wong T.et al Replacement of linoleic acid with alpha‐linolenic acid does not alter blood lipids in normolipidaemic men. Br J Nutr 199880163–167. [PubMed] [Google Scholar]
- 9.St Onge M P, Lamarche B, Mauger J F.et al Consumption of a functional oil rich in phytosterols and medium‐chain triglyceride oil improves plasma lipid profiles in men. J Nutr 20031331815–1820. [DOI] [PubMed] [Google Scholar]
- 10.Junker R, Kratz M, Neufeld M.et al Effects of diets containing olive oil, sunflower oil, or rapeseed oil on the hemostatic system. Thromb Haemost 200185280–286. [PubMed] [Google Scholar]
- 11.Karvonen H M, Aro A, Tapola N S.et al Effect of alpha‐linolenic acid‐rich Camelina sativa oil on serum fatty acid composition and serum lipids in hypercholesterolemic subjects. Metabolism 2002511253–1260. [DOI] [PubMed] [Google Scholar]
- 12.Kratz M, Cullen P, Kannenberg F.et al Effects of dietary fatty acids on the composition and oxidizability of low‐density lipoprotein. Eur J Clin Nutr 20025672–81. [DOI] [PubMed] [Google Scholar]
- 13.Meshcheriakova V A, Plotnikova O A, Sharafetdinov K.et al [Comparative study of effects of diet therapy including eiconol or linseed oil on several parameters of lipid metabolism in patients with type 2 diabetes mellitus]. Vopr Pitan 20017028–31. [PubMed] [Google Scholar]
- 14.Sodergren E, Gustafsson I B, Basu S.et al A diet containing rapeseed oil‐based fats does not increase lipid peroxidation in humans when compared to a diet rich in saturated fatty acids. Eur J Clin Nutr 200155922–931. [DOI] [PubMed] [Google Scholar]
- 15.Finnegan Y E, Howarth D, Minihane A M.et al Plant and marine derived (n‐3) polyunsaturated fatty acids do not affect blood coagulation and fibrinolytic factors in moderately hyperlipidemic humans. J Nutr 20031332210–2213. [DOI] [PubMed] [Google Scholar]
- 16.Arjmandi B H, Khan D A, Juma S.et al Whole flaxseed consumption lowers serum LDL‐cholesterol and lipoprotein(a) concentrations in postmenopausal women. Nutr Res 1998181203–1214. [Google Scholar]
- 17.Thies F, Miles E A, Nebe von Caron G.et al Influence of dietary supplementation with long‐chain n‐3 or n‐6 polyunsaturated fatty acids on blood inflammatory cell populations and functions and on plasma soluble adhesion molecules in healthy adults. Lipids 2001361183–1193. [DOI] [PubMed] [Google Scholar]
- 18.Wensing A G, Mensink R P, Hornstra G. Effects of dietary n‐3 polyunsaturated fatty acids from plant and marine origin on platelet aggregation in healthy elderly subjects. Br J Nutr 199982183–191. [PubMed] [Google Scholar]
- 19.Kratz M, Wahrburg U, von Eckardstein A.et al Dietary mono‐ and polyunsaturated fatty acids similarly increase plasma apolipoprotein A‐IV concentrations in healthy men and women. J Nutr 20031331821–1825. [DOI] [PubMed] [Google Scholar]
- 20.Danesh J, Collins R, Appleby P.et al Association of fibrinogen, C‐reactive protein, albumin, or leukocyte count with coronary heart disease: meta‐analyses of prospective studies. JAMA 19982791477–1482. [DOI] [PubMed] [Google Scholar]
- 21.Burdge G C, Wootton S A. Conversion of alpha‐linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr 200288411–420. [DOI] [PubMed] [Google Scholar]