For the majority of surgeons, mitral valve repair has become the method of choice for surgical correction of mitral regurgitation. However, the feasibility of repair depends not only on the pathology but also on the experience of the surgeon and/or team. Success rates may reach almost 100% in the most common form of isolated prolapse of the middle scallop of the posterior leaflet. Nevertheless, mitral repair is still subject to a pronounced, sometimes painful, learning curve. The durability of the repair also depends on the type of pathology and on many other factors, including the technique used.
Since its inception in the 1970s, the techniques of mitral valve repair have been the subject of many modifications and improvements which have made it a more predictable and reproducible method. One area where much improvement was made was ischaemic regurgitation, which was initially considered refractory to repair and for which there is now a growing experience and much improved results. Also, advancements have occurred in the treatment of regurgitation related to cardiomyopathy, previously considered a contraindication for repair. By contrast, repair for rheumatic disease still carries the worst results, although the characteristics of the usually underdeveloped and young population may still make it preferable to mitral valve replacement.
In this work, we analyse the state of the art of mitral valve repair and its results according to our experience and in the light of recent reports.
MITRAL VALVE REPAIR: ACCEPTED ALTERNATIVE TO PROSTHETIC REPLACEMENT
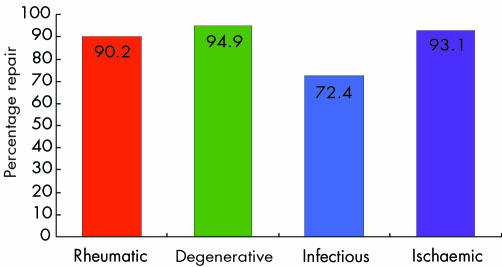
After an initial period of distrust, in the late 1970s and early 1980s, when it was developed mainly through the efforts of Carpentier,1 Duran,2 and others, mitral valve repair has since become a generally accepted alternative to prosthetic replacement for surgical treatment of virtually all forms of mitral valve disease; indeed, most surgeons now recognise its superiority, in terms of both early and late results. The feasibility of mitral valvoplasty varies with the different types of pathology (fig 1) and from centre to centre, with global values that range from < 10% to nearly 90%. In many centres the use of this method is still the exception rather than the norm.
Figure 1 Feasibility of mitral valvoplasty according to pathology, in more than 2500 patients in Coimbra, Portugal, during the past 16 years
The advantages of mitral valvoplasty parallel the disadvantages of prosthetic valve replacement. Valvoplasty does not require lifelong anticoagulation, compared with mechanical prostheses, especially in patients who have no other reason for anticoagulation, such as the presence of atrial fibrillation. It is also essential to keep in mind that the incidence of thromboembolic complications of mechanical prostheses and of degradation of bioprostheses, another important complication of valve replacement, is more frequent in the mitral than in the aortic position.
ANATOMY AND PHYSIOLOGY OF THE MITRAL VALVE
The normal function of the mitral valve results from synchronised movement of all its components: the annulus, the leaflets, the commissures, the chordae tendineae, and the papillary muscles. Together, these structures are known as the mitral apparatus and their architecture is designed to cause a very low level of mechanical stress during the ventricular systole.
The anterior portion of the annulus is part of the fibrous skeleton of the heart, between the two fibrous bodies, and is to be considered non‐distensible, whereas its posterior portion, which accounts for about two thirds of the perimeter of the orifice, is a less well defined structure and can be distended by disease. Recently uncovered evidence, however, appears to suggest that even the anterior annulus is prone to dilatation.3,4 The normal annulus is saddle shaped and roughly round during diastole, and has a kidney‐like shape during systole.
The anterior leaflet, or aortic, is larger than the posterior, or mural, which is narrower but has a longer attachment to the annulus. Each leaflet has a rough zone that comes into apposition with that of its counterpart; the combined surface area of both leaflets is more than twice the area of the mitral orifice, allowing for a large area of leaflet coaptation during the ventricular systole, effectively decreasing mechanical stress. Moreover, the area of the mitral orifice during systole is reduced by 25–40%, mainly as a result of a dynamic reduction of the size of the posterior annulus that decreases the anteroposterior diameter of the valve orifice and increases the area of leaflet apposition. To help this action, contraction of the papillary muscles, synchronous with left ventricular systole, ensures adequate tethering of the mitral valve and places the orientation of the coaptation zones of the leaflets in an almost vertical direction for an effective and unstressed closure.
Experimental changes in the geometry of one of the parts of the mitral complex resulted in a significant increase of the mechanical stress over the whole valve structure. Likewise, dilatation of the mitral ring in a way that would not be enough to produce mitral regurgitation, by reducing but not eliminating leaflet apposition, causes an increase in the stress in the fibrous trigones and leaflets, which is double that in the normal valves. This may initiate a vicious circle which may lead to regurgitation. This form of regurgitation, characteristic of dilated and of some ischaemic cardiomyopathies, has recently been dubbed functional regurgitation. Reducing stress in the valve components by restoring the normal geometry of the valvar mechanism appears to be essential for long term durability of mitral valve repair.
Papillary muscle blood supply is also of clinical interest, as the posterior muscle is exclusively fed by branches of the posterior descending artery, originating from either the right or the circumflex arteries, depending on which is the dominant system; thus, it is more prone to ischaemia and necrosis. The anterior papillary muscle, on the other hand, receives its blood supply from two sources—from the septal branches of the left anterior descending artery, and from the branches of the circumflex artery. Thus, it is less prone to ischaemia and rupture caused by coronary artery occlusion than is the posterior papillary muscle.
TECHNIQUES OF MITRAL VALVE REPAIR
General considerations
Modern mitral valve repair was initiated in the 1970s by the pioneer work of surgeons like Carpentier1 and Duran.2 Rheumatic mitral stenosis had been successfully treated for a long time, firstly by closed commissurotomy and later by open heart surgery, but previous attempts to treat mitral regurgitation, although with some initial success, had a high rate of failure on follow up. With his work, Carpentier showed the way to sustained success by describing the principles and the techniques that bear his name and are still regarded today as fundamental for a good repair.5
Successful repair is based on a proper understanding of the anatomic and functional alterations of the diseased valve and Carpentier's functional classification is based on an analysis of leaflet motion (table 1).
Table 1 Functional classification of mitral valve regurgitation.
| • Type I : normal leaflet motion |
| • Type II : excessive leaflet motion (one or both leaflets prolapse) |
| • Type III : restricted leaflet motion |
| - IIIa: opening is restricted |
| - IIIb: closure is restricted |
A prolapse is considered to be present when the free edge of one or both leaflets overrides the plan of the valve orifice (annulus) during systole. It must be distinguished from billowing of the leaflets where there is a protrusion of the body of the leaflet, which has excess tissue, into the left atrium during systole while maintaining apposition of the free edges below the plan of the orifice.
These types of mitral valve regurgitation express themselves differently according to pathology. Type I is present when there is annular dilatation, as in cardiomyopathy, or with leaflet perforation, as in endocarditis. Type II is caused by elongation or rupture of chordae tendineae, as often occurs in degenerative disease, or of the papillary muscles, as occurs in ischaemic disease. Type IIIa occurs when there is commissural fusion and leaflet or chordal thickening, as seen in rheumatic disease. In type IIIb there is chordal retraction (rheumatic) or papillary muscle retraction (ischaemic) or displacement (ischaemic or functional cardiomyopathy).
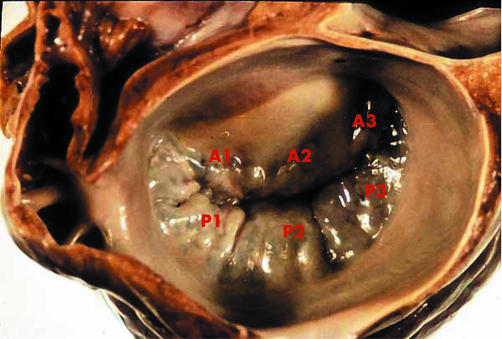
Identification of the malfunction of each of the components of the mitral valve apparatus is essential for a successful repair. To this purpose, preoperative or intraoperative echocardiographic examination, preferably transoesophageal, is of paramount importance to give the surgeon an idea about the feasibility of repair and a roadmap of the lesions to be corrected. For proper identification of the location of lesions in the leaflets, a segmental classification has been created (fig 2) where the three scallops of the posterior leaflet are designated as P1 (close to the anterolateral commissure), P2 (the middle scallop), and P3 (close to the posteromedial commissure). The corresponding segments of the anterior leaflet are called A1, A2, and A3.
Figure 2 Segmental anatomy of the mitral valve leaflets. See text for explanation of labelling.
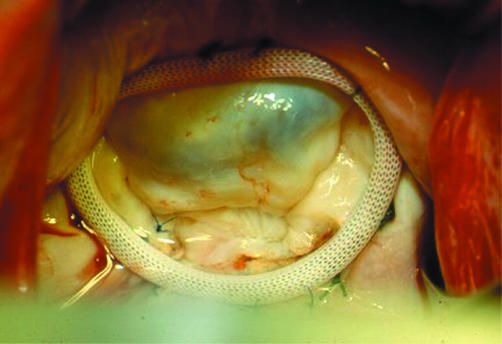
The aim of mitral repair is not only to obtain a valve that no longer leaks, but also to achieve anatomical restoration of all components of the mitral apparatus, so that the mechanical stress on the valve, whose tissue is abnormal, is reduced to a minimum. To this end, standard mitral valve repair is based on two main groups of technical steps: the first, to correct any abnormal motion of the leaflets and chordal apparatus; and the second, to remodel and stabilise the annulus by implantation of a ring (fig 3).
Figure 3 Introperative photograph taken immediately after repair of mitral regurgitation caused by isolated posterior leaflet prolapse. The valve is being tested by intraventricular injection of saline and is fully competent with good apposition of the leaflets.
Prolapse of the posterior leaflet
Isolated prolapse of the posterior leaflet, with a greater or lesser degree of dilatation of the annulus, is the most frequent lesion leading to mitral regurgitation and accounts for up to 60% of the cases in most surgical series. The standard technique for repair in these cases is quadrangular resection of the prolapsing segment followed by re‐approximation and suture of the resection margins. This resection may include up to 50% of the length of the posterior leaflet.
If the posterior leaflet is very wide and redundant, systolic anterior motion (SAM) can result, causing left ventricular outflow obstruction. These cases should be treated by a sliding plasty of the posterior leaflet which consists of detaching the leaflet from the annulus on both sides of the quadrangular resection and resuturing it closer to the opposite segment, taking wider bites on the leaflet tissue; this not only decreases its surface area but also redistributes the stress in the suture lines and annulus.
Prolapse of the anterior leaflet
This situation is usually the result of elongation or rupture of the anterior leaflet chordae, and the repair is technically more demanding than that of posterior leaflet prolapse. Unlike the posterior leaflet, resection is usually not feasible because there is not enough tissue for reconstitution of the leaflet. Carpentier5 described two techniques that were effective to restore normal motion of the leaflet. The first is chordal shortening, in which a trench is created within a papillary muscle and the excess chordal length buried into the trench by a suture passing through the muscle. This technique is difficult to master and is characterised by a high incidence of failure on long term follow up, probably caused by continued elongation.
The second technique is chordal transfer, where a normal free edge chorda of the posterior leaflet or a secondary chorda of the anterior leaflet is detached and re‐sutured to the free edge of the prolapsed segment of the anterior leaflet. Good long term results have been reported, but it has the disadvantage of having to manipulate the posterior leaflet and of a longer operating time. It can also be very challenging when there are ruptured chordae to both anterior and posterior leaflets, because of the lack of reference for the correct chordal length. On the other hand, although the transferred chordae may have the correct length, they are thin and fragile and are also prone to rupture, resulting in failure of the repair.
For these reasons, the idea of replacing ruptured or elongated chordae by artificial chordae was around for a long time but was largely unsuccessful because of a lack of suitable materials; it became clinically reliable when polytetrafluoroethylene (PTFE) sutures were used as neochordae. This material is highly durable and is rapidly embedded by fibroblasts of the endocardium, becoming non‐thrombogenic while maintaining flexibility.
David and co‐workers,6 among others, were pioneers of the use of PTFE, which has since been gradually expanding. It is a simple technique, consisting of a double armed 5/0 or 4/0 PTFE suture passed through the body of a papillary muscle and then through the free edge of the prolapsing leaflet to which it is tied. The only difficulty with this technique is finding the correct length, as this type of material makes very slippery knots and is effectively overcome by experience. Its advantages are that PTFE neochordae can be used in all cases independent of the condition of the posterior leaflet, extensive prolapse can be treated as several sutures can be used, and chordae that are thin and fragile but not yet ruptured can be reinforced. Its use has been advocated even in posterior leaflet prolapse, instead of quadrangular resection.7
A new technique for the treatment of leaflet prolapse was proposed by Alfieri's group in 1995 and raised considerable interest because of its technical simplicity and ease of use. In this procedure, the prolapsing portion of the anterior leaflet is sutured to the matching edge of the posterior leaflet with a monofilament suture.8 This technique, also known as the edge‐to‐edge or double orifice repair, is much simpler to do than the more sophisticated and proven techniques, but it does not yet have the test of time and concerns have been raised about the changes that the altered blood flow pattern may cause to the leaflets.
Bileaflet prolapse
Bileaflet prolapse and Barlow's syndrome are complex lesions caused by severe myxomatous degeneration and characterised by billowing and/or prolapse of both leaflets which are usually uniformly thickened but pliable, with a great excess of leaflet tissue. The annulus is almost always notably dilated, and the chordae are often elongated and prone to rupture.
In these cases, repair is usually very demanding, as it requires intervention on both leaflets and respective chordae and there are no references for normality. Multiple chordal substitution, sometimes associated with posterior leaflet resection, is used most frequently but ultimately the Alfieri technique may have its most important indication here.9
Restricted leaflet motion
Restricted leaflet motion is seen in rheumatic disease and is almost always caused by a mixture of incomplete opening and incomplete closure of the leaflets, because of commissural fusion, leaflet thickening and a greater or lesser degree of chordal thickening and retraction. The posterior mitral annulus is also frequently enlarged. This is a type of lesion considered less suitable for repair, but if the anterior leaflet is still pliable and mobile, commissurotomy with the incision dividing the fused chordae under the commissures and then longitudinally splitting the papillary muscles, can restore ample movement to the anterior leaflet with good opening of the valve. Resection of secondary chordae of the posterior leaflet may permit some additional mobility of this structure and better coaptation.5
Restricted closure is seen in ischaemic disease and in dilated cardiomyopathy. It is the result of leaflet tethering caused by papillary muscle dysfunction of ischaemic aetiology, and also by the abnormal spherical shape of the dilated ventricle in cardiomyopathy, causing lateral and apical displacement of the papillary muscles. This has been shown recently in the studies by Yu and colleagues10 who demonstrated the complex mechanism of this type of regurgitation, which results from the interaction of an increased anteroposterior diameter of the annulus, increased distance between the two papillary muscles, and also the distance between the annulus and the base of the papillary muscles.
Because it is usually not possible to restore the anatomy to normal in these cases, the technical solution has been the use of a notably undersized ring annuloplasty, as discussed below, but edge‐to‐edge repair has also been advocated.
Ring annuloplasty
Since its introduction by Carpentier in the early 1970s, ring annuloplasty has been an integral part of mitral valve repair. The objective is to fix the annulus, reshaping it to the typical systolic kidney‐like format, to reduce its area and to avoid further dilatation. The classic Carpentier ring is rigid and is sized by matching appropriate sizers to the size of the anterior leaflet.5 Recognising that the annulus is a complex structure that moves in a non‐planar fashion during the cardiac cycle, Duran2 later proposed a totally flexible ring that does not significantly impair the natural motion of the mitral annulus, allowing it to acquire its physiologic saddle‐like shape during systole, hence preserving better the function of the basal segments of the left ventricle. Another possible advantage of the flexible ring is a decrease in the tension on the suture lines. Carpentier subsequently introduced a modified version of the classic ring, the Physio ring,11 which is selectively rigid in its anterior portion and flexible in the posterior.
Because it is thought that the pathological dilatation of the mitral annulus is non‐homogeneous and is mainly limited to its posterior segment, Cooley and later Cosgrove, among others, proposed a partial ring that supports and is sutured only to the posterior annulus, avoiding the anterior segment where the neighbouring aortic valve cusps are at risk.12 Currently, there is a choice between many different annuloplasty rings but they can all be classified, basically, as rigid or flexible and total or partial.
David and colleagues13 reported better left ventricular function when using a flexible versus a rigid ring, but that advantage was present only during the first few months after surgery and a recent study from Miller's group showed that the Carpentier Physio ring and the flexible Duran ring have the same immobilising effect on the posterior leaflet.14 On the other hand, Hueb and co‐workers3 have recently shown that the intertrigonal distance of the anterior annulus can increase significantly in dilated cardiomyopathy, as has also been shown by Tibayan and colleagues4 in ischaemic patients. These data strongly support the choice of a complete annuloplasty ring. The few long term studies with different types of rings, such as the report on 1072 patients from the Cleveland Clinic, strongly support the need for an annuloplasty, but did not show differences between the different types of rings.15
Our own experience appears to indicate that, at least in rheumatic patients, the use of a rigid or semi‐rigid complete ring is essential, because in this pathology the annulus is not only dilated and deformed but has also lost the capability to contract during systole. Thus, it needs reshaping, which can only be achieved with pre‐shaped rings.
RESULTS OF MITRAL VALVE REPAIR
From what was discussed above, it is quite evident that the feasibility of mitral valvoplasty varies with the different pathologies (fig 1). With experienced surgical teams, success rates can reach almost 100% in cases of isolated posterior leaflet prolapse, but it is far lower in young patients with rheumatic disease. In other pathologies, it depends on the experience of the surgeon and on the characteristics of the population. Although chronic ischaemic mitral regurgitation was, in the past, considered unfavourable for repair, recent reports give account of high rates of success.
Degenerative mitral valve disease is the most common cause of mitral regurgitation in developed countries, and mitral valve repair is associated with very good short and long term outcomes in these cases. Perioperative mortality is less than 2% and the longest follow up, reported by Braunberger from Carpentier's group,16 of a series of 162 patients operated on between 1970 and 1984, showed a 20 year survival of 48%, which is similar to that of the general population with the same age distribution. Freedom from mitral valve reoperation was 94% at 10 years and 92% at 20 years, and 85% of the patients had none or trivial mitral regurgitation. Another important series of 488 patients from David and colleagues17 had a hospital mortality of 1%, and the 15 year survival was 61%. In this group, 15 year freedom from significant mitral regurgitation was 85%. The largest group of patients was reported by Gillinov and co‐workers,15 from the Cleveland Clinic, with a 0.3% surgical mortality and a 10 year freedom from reoperation of 93%.
These results show the low operative risk associated with the procedure and the stability of repair in the long term, which makes this operation the gold standard of surgical treatment for degenerative mitral regurgitation. With a good evaluation of the valve by echocardiography, identifying the pathology to be corrected, and the newly available techniques of chordal replacement, 95% of the valves can be repaired, as pointed out by Gillinov and David and their co‐workers.15,17
The results in other types of pathology are less favourable, but for the most part superior to those observed with valve replacement. Some deserve especial discussion.
Functional mitral regurgitation
It is now well known that the presence of mitral regurgitation after myocardial infarction significantly decreases long term survival, and surgery has been advised when regurgitation is grade 2 or greater.18 As the leaflets are usually normal but with restricted mobility (Carpentier type III b), the technique most frequently advocated is over‐reduction of the mitral annulus to promote adequate coaptation of the restricted leaflets. Surgical mortality ranges from 5–10%, which is satisfactory for this kind of patient; however, five year survival is only about 50%, and 30% of the patients have significant recurrent mitral regurgitation, as was shown by Tahta and colleagues19 and, more recently, by McGee colleagues20 from the Cleveland Clinic in a very large series of 585 patients.
However, Calafiore and co‐workers21 reported a better five year survival with repair (75%) than with a prosthesis (66%), similar to the experience of Grossi and colleagues.22 Finally, Bax and colleagues,23 using a stringent downsizing of the mitral annulus with a Carpentier Physio ring, had an operative mortality of 5.6%; at two year follow up all patients had no or minimal (grade 1) mitral regurgitation.
Ischaemic mitral regurgitation is difficult to repair because of the several mechanisms involved, and ring annuloplasty only solves the problem at the annular level by forcefully bringing the leaflets together. The issue of leaflet tethering by malalignment of the papillary muscles, which results from progressive left ventricle dilation, is not solved by the surgery and can cause late reappearance of regurgitation. To address this problem, new solutions have to be proposed, such as the Coapsys annuloplasty system (Myocor), in which artificial chords are implanted across the left ventricular cavity and supported by synthetic buttons over the epicardium, decreasing the radius of this cavity and the distance between the papillary muscles, and consequently decreasing also the tethering effect on the mitral leaflets. These and other devices are currently under investigation. Very recently, Baxter‐Edwards, the makers of the Carpentier ring, launched a new ring (GeoForm), still in the experimental phase, with a much narrower anteroposterior diameter and a camel hump shape that suspends the postero‐basal portion of the annulus and ventricular wall, which is intended to compensate the abnormal shape and movement of this section of the ischaemic/infarcted myocardium.
Rheumatic mitral regurgitation
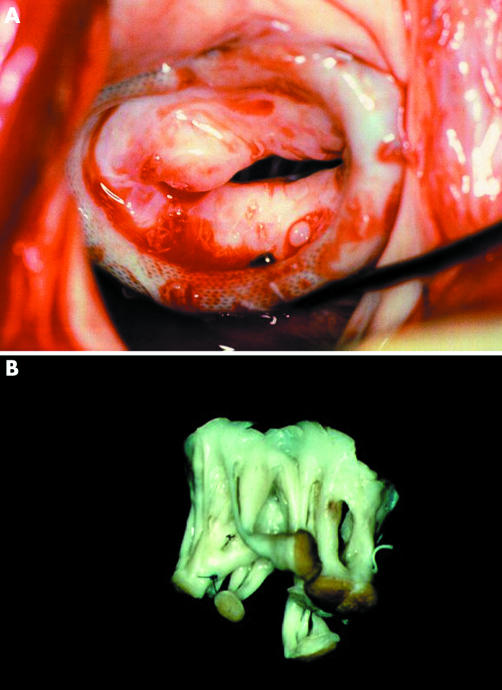
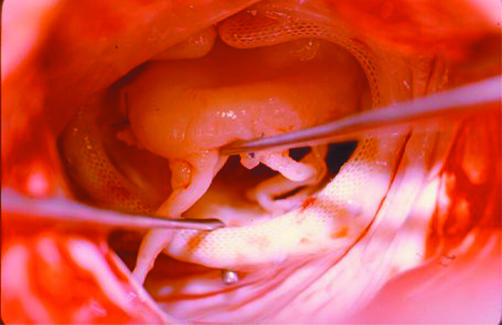
Rheumatic disease causes progressive fibrotic thickening of the mitral leaflets and scarring of chordal structures and, when it causes significant regurgitation, the appropriateness of valve repair has been disputed. In young populations the possibility of repair may be high but the long term durability of repair is low because of progress of the rheumatic disease. Nevertheless, it appears that repair has advantages over replacement in these patients. In our own experience,24 mitral valve repair in a South African population with a mean age of 22 years and with half of the patients being younger than 20 years, led to good medium term results, both in terms of survival and of freedom from reoperations. These results have recently been confirmed by other surgeons in other underdeveloped population groups.25,26 Recurrence of regurgitation is due to ongoing rheumatic activity or progression of the scarring (fig 4).
Figure 4 Intraoperative photograph during reoperations for recurrent mitral regurgitation, four years after valvoplasty in a young patient. Continued rheumatic activity caused thickening and retraction of the leaflets (A). The subvalvar apparatus was grossly fibrotic and distorted (B).
In an older population, when the repair is possible, long term results are good and stable, as was shown in the important series of Chauvaud and colleagues27 that included 951 patients having mitral repair for regurgitation with a 2% hospital mortality and a 10 and 20 year survival of 89% and 82%, respectively. Freedom from reoperation was 82% and 55% in the same time periods, and the main cause for reoperation was progressive fibrosis of the mitral valve.
Also Yan and co‐workers28 had a 10 year freedom from reoperation of 72%. Furthermore, in their experience, survival and freedom from thromboembolic events was better than with valve replacement (88% v 73% and 92% v 71%, respectively). Although it is evident that in this aetiology mitral repair has long term results that are not as good as those achieved in degenerative disease, it avoids the risks associated with anticoagulation, especially in a population which is characteristically non‐compliant with all types of medication. On the other hand, reoperations on repaired valves are low risk procedures.
Repair of mitral valve regurgitation: key points
The present approach to mitral valve repair is not only to correct the regurgitation but also to restore valve architecture to as near normal as possible, in order to decrease mechanical stress on the valve apparatus
Isolated posterior leaflet prolapse is the most common; its repair is technically simple and well standardised, with excellent long term results
Anterior leaflet repair is more demanding but the use of artificial chordae has made it feasible in the majority of cases
Long term studies with different types of ring strongly support the need for an annuloplasty, but did not show differences between different types of rings
In rheumatic regurgitation, the feasibility of repair is lower, but when it is possible the long term results are good and better than with valve replacement, especially in older patients
CONCLUSION
Mitral valve repair aims at achieving absence of residual regurgitation and a valve architecture as close to normal as possible, to obtain the lowest possible mechanical stress on the valve structures, which we believe will give the best chance for a stable repair in the long term. The evolution of a repaired mitral valve depends on the prior physiopathology, whether stenosis or regurgitation, and on the techniques used for repair—usually multiple, sometimes very complex, and frequently utilising foreign materials, such as artificial chordae, prosthetic rings, etc (fig 5).
Figure 5 Chordal rupture occurred in this patient two years after initial repair. Another conservative procedure was possible using chordal replacement with PTFEE sutures.
For all these reasons, a thorough knowledge of the anatomy and physiology of the mitral valve is essential when planning and performing mitral valvoplasty, for which a good preoperative and intraoperative transoesophageal echocardiogram is an essential part. The best feasibility and long term results are achieved in degenerative aetiology, specifically in the flail posterior leaflet, which is by far the most frequent situation. In all other cases the results are less good, yet generally better than those of valve replacement.
Finally, the physiology of the repaired valve is significantly dependent on the geometry of the left heart cavities, especially the ventricle. Reverse remodelling of the left ventricle after mitral valvoplasty is essential for the stability and durability of the repair. A dilated or dilating left ventricle predisposes to recurrence of mitral regurgitation because of malalignment of the papillary muscles. This is, currently, the most challenging problem surgeons are faced with.
Footnotes
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article
References
- 1.Carpentier A, Deloche A, Dauptain J.et al A new reconstructive operation for correction of mitral and tricuspid insufficiency. J Thorac Cardiovasc Surg 1971611–13.A landmark publication, especially if one considers that cardiac/valve surgery was still in its infancy at this time. [PubMed] [Google Scholar]
- 2.Duran C M, Pomar J L, Cucchiara G. A flexible ring for atrioventricular heart valve reconstruction. J Cardiovasc Surg (Torino) 197819417–420. [PubMed] [Google Scholar]
- 3.Hueb A C, Jatene F B, Moreira L F.et al Ventricular remodeling and mitral valve modifications in dilated cardiomyopathy: new insights from anatomic study. J Thorac Cardiovasc Surg 20021241216–1224.This was the first report of pathological dilatation of the anterior mitral annulus, until then believed to be non‐extensible because it is part of the fibrous skeleton of the heart. [DOI] [PubMed] [Google Scholar]
- 4.Tibayan F A, Rodriguez F, Langer F.et al Annular remodeling in chronic ischemic mitral regurgitation: ring selection implications. Ann Thorac Surg 2003761549–1554. [DOI] [PubMed] [Google Scholar]
- 5.Carpentier A. Cardiac valve surgery–the “French correction”. J Thorac Cardiovasc Surg 198386323–337.Twelve years after the original paper, Carpentier presents, for the first time, the excellent long term results of mitral valve repair. [PubMed] [Google Scholar]
- 6.David T E. Replacement of chordae tendinae with expanded polytetrafluoroethylene sutures. J Card Surg 19944286–290. [DOI] [PubMed] [Google Scholar]
- 7.David T E, Omran A, Armstrong S.et al Long‐term results of mitral valve repair for myxomatous disease with and without chordal replacement with polytetrafluoroethylene sutures. J Thorac Cardiovasc Surg 19981151279–85 discussion 12856.Since this report, expanded polytetrafluoroethylene (PTFE) sutures have been used extensively for replacement/reinforcement of chordae tendineae, in preference to Carpentier's shortening techniques [DOI] [PubMed] [Google Scholar]
- 8.Maisano F, Torraca L, Oypizzi M.et al The edge‐to‐edge technique : a simplified method to correct mitral insufficiency. Eur J Cardiothorac Surg 199813240–245. [DOI] [PubMed] [Google Scholar]
- 9.Alfieri O, Maisano F, De Boniset al The double orifice technique in mitral valve repair: a simple solution for complex problems. J Thorac Cardiovasc Surg 2001122674–681.This is the first report of results with the Alfieri technique in the hands of its creator. [DOI] [PubMed] [Google Scholar]
- 10.Yu H Y, Su M Y, Liao T Y. Functional mitral regurgitation in chronic ischemic coronary artery disease: analysis of geometric alterations of mitral apparatus with magnetic resonance imaging. J Thorac Cardiovasc Surg 2004128543–551. [DOI] [PubMed] [Google Scholar]
- 11.Carpentier A F, Lessana A, Relland J Y.et al The “physio‐ring”: an advanced concept in mitral valve annuloplasty. Ann Thorac Surg 1995601177–85 discussion 11856. [DOI] [PubMed] [Google Scholar]
- 12.Cosgrove D M, Arcidi J M, Rodriguez l.et al Initial experience with the Cosgrove‐Edwards annuloplasty system. Ann Thorac Surg 199560499–503. [DOI] [PubMed] [Google Scholar]
- 13.David T E, Komeda M, Pollick C.et al Mitral valve annulopasty: the effect of the type on left ventricular function. Ann Thorac Surg 198947524–527.In this much cited paper, but not always correctly, the authors discuss the relative performances of rigid (Carpentier) and flexible (Duran) annuloplasty rings. Early advantages of flexible rings had disappeared after six months. [DOI] [PubMed] [Google Scholar]
- 14.Green G R, Dazum P, Glassom J R.et al Restricted posterior leaflet motion after mitral ring annuloplasty. Ann Thorac Surg 1999682100–2106. [DOI] [PubMed] [Google Scholar]
- 15.Gillinov A M, Cosgrove D M, Blackstone E H.et al Durability of mitral valve repair for degenerative disease. J Thorac Cardiovasc Surg 1998116734–743. [DOI] [PubMed] [Google Scholar]
- 16.Braunberger E, Deloche A, Berrebi A.et al Very long‐term results (more than 20 years) of valve repair with Carpentier's techniques in nonrheumatic mitral valve insufficiency. Circulation 2001104(suppl I)I8–11.This paper from the Cleveland Clinic group strongly supports the need for an annuloplasty, but did not show differences between the different types of rings. [PubMed] [Google Scholar]
- 17.David T E, Ivanov J, Armstrong S.et al Late outcomes of mitral valve repair for floppy valves: implications for asymptomatic patients. J Thorac Cardiovasc Surg 20031251143–1152. [DOI] [PubMed] [Google Scholar]
- 18.Paparella D, Mickleborough L L, Carson S.et al Mild to moderate mitral regurgitation in patients undergoing coronary bypass grafting: effect on operative mortality and long‐term significance. Ann Thorac Surg 2003761094–1100.This important paper draws attention to the advantages of mitral valve repair during coronary revascularisation, even for less than severe mitral regurgitation. [DOI] [PubMed] [Google Scholar]
- 19.Tahta S A, Oury J H, Maxwell J M.et al Outcome after mitral valve repair for functional ischemic mitral regurgitation. J Heart Valve Dis 20021111–18 discussion 1819. [PubMed] [Google Scholar]
- 20.McGee E C, Gillinov A M, Blackstone E H.et al Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2004128916–924. [DOI] [PubMed] [Google Scholar]
- 21.Calafiore A M, Di Mauro M, Gallina S.et al Mitral valve surgery for chronic ischemic mitral regurgitation. Ann Thorac Surg 2004771989–1997. [DOI] [PubMed] [Google Scholar]
- 22.Grossi E A, Goldberg J D, La Pietra A.et al Ischemic mitral valve reconstruction and replacement: comparison of long‐term survival and complications. J Thorac Cardiovasc Surg 20011221107–1124. [DOI] [PubMed] [Google Scholar]
- 23.Bax J J, Braun J, Somer S T.et al Restrictive annuloplasty and coronary revascularization in ischemic mitral regurgitation results in reverse left ventricular remodeling. Circulation 2004110(suppl II)II103–II108.Using a stringent downsizing of the mitral annulus, these authors achieved excellent medium term results with no or minimal mitral regurgitation. This is now a well accepted practice in ischaemic mitral regurgitation. [DOI] [PubMed] [Google Scholar]
- 24.Antunes M J, Magalhães M P, Colsen P R.et al Valvuloplasty for rheumatic mitral valve disease. A surgical challenge. J Thorac Cardiovasc Surg 19879444–56.One of the first sizeable series of rheumatic patients, showing good long term results in the rheumatic mitral valve. [PubMed] [Google Scholar]
- 25.Pomerantzeff P M, Brandão C M, Faber C M.et al Mitral valve repair in rheumatic patients. Heart Surg Forum 20003273–276. [PubMed] [Google Scholar]
- 26.Choudhary S K, Talwar S, Dubey B.et al Mitral valve repair in a predominantly rheumatic population. Long‐term results. Tex Heart Inst J 2001288–15. [PMC free article] [PubMed] [Google Scholar]
- 27.Chauvaud S, Fuzellier J F, Berrebi A.et al Long‐term (29 years) results of reconstructive surgery in rheumatic mitral valve insufficiency. Circulation 2001104(suppl I)I12–I15. [DOI] [PubMed] [Google Scholar]
- 28.Yau T M, El‐Ghoneimi Y A, Armstrong S.et al Mitral valve repair and replacement for rheumatic disease. J Thorac Cardiovasc Surg 200011953–60. [DOI] [PubMed] [Google Scholar]