Haemostatic defects or thrombosis associated with secondary erythrocytosis is well known in adult patients with cyanotic congenital heart disease (CCHD).1 One of the most serious complications of this haematological derangement is a cerebrovascular event,2 although a large study of adult patients with CCHD showed no increased risk of stroke.3 Although these patients may not develop apparent stroke, they often have various neurological symptoms, such as headache, dizziness, and tinnitus, in association with blood hyperviscosity.1 To our knowledge, no systematic magnetic resonance imaging (MRI) studies of the brain in this condition have been reported. Investigating the relation between brain MRI findings and blood coagulation abnormalities may be important for adequate management of patients with CCHD.
METHODS
We studied 15 consecutive patients with CCHD aged 18–45 years (median age 24) who had been examined and followed up at the Tsukuba University Hospital for more than 10 years. None of the patients had undergone a radical operation for cardiac defects because of high pulmonary vascular resistance for Fontan circulation in eight patients (three with single ventricle, two with tetralogy of Fallot, two with double outlet right ventricle, and one with corrected transposition of the great arteries and ventricular septal defect (VSD); the latter five patients also had pulmonary atresia, hypoplastic pulmonary arteries, or hypoplastic left ventricle) and because of extremely high pulmonary arterial resistance with a right to left shunt (Eisenmenger's syndrome) in seven (two with VSD, two with VSD and ductus arteriosus, two with atrioventricular septal defect, and one with VSD and transposition of the great arteries). Two patients had undergone Blalock‐Taussig anastomosis but the postoperative courses were uneventful. None of the patients had had cardiopulmonary bypass or catheter intervention. Patients with a history of stroke, procedure related cerebrovascular event, brain abscess, or atrial fibrillation or with a prosthetic valve were excluded from our study. Nine age matched healthy volunteers were also enrolled in this study to provide standard blood parameters but they did not undergo brain MRI studies.
Four patients had intermittently had for years at least one of the hyperviscosity related symptoms: headache, dizziness, irritability, lethargy, double vision, tinnitus, and taste sensation disorder. None of the patients had received antiplatelet or anticoagulant drugs within two weeks before blood sampling. Brain MRI was performed with a 1.5 T superconducting system (Gyroscan, Philips Medical Systems). T1 and T2 weighted images were acquired with spin echo sequences. Each patient with CCHD also underwent magnetic resonance angiography to examine intracranial circulation by use of the three dimensional time of flight technique. Blood samples were collected from the antecubital vein with the use of a lightly fitted tourniquet. The blood sampling volume was corrected for the packed cell volume in each case as described previously.4 Table 1 lists the parameters measured. Detailed methods of blood sampling and various assays have been described previously.4 Oxygen saturation was measured percutaneously at rest. Each parameter was compared between the three groups—that is, patients with abnormal MRI patterns (abnormal MRI group), those without (normal MRI group), and healthy subjects (control group)—by means of a one way analysis of variance followed by Scheffe post hoc comparison.
Table 1 Comparison of blood parameters between patients with and without brain magnetic resonance imaging (MRI) abnormalities and control subjects.
| Abnormal MRI (n = 7) | Normal MRI (n = 8) | Control subjects (n = 9) | p Value | |
|---|---|---|---|---|
| Age (years) | 26.3 (10) | 23.4 (6.5) | 23.1 (5.8) | 0.574 |
| Oxygen saturation (%) | 77.9 (5.8)*** †† | 88.0 (6.6)* | 96.4 (1.3) | <0.001 |
| Packed cell volume (%) | 63.2 (7.6)*** † | 54.3 (7.3)** | 42.4 (2.2) | 0.016 |
| MCV (fl) | 94.6 (9.2) | 96.8 (6.1) | 93.2 (2.9) | 0.971 |
| Platelet count (×109/l) | 173 (44) | 181 (64) | 249 (79) | 0.053 |
| Soluble P selectin (ng/ml) | 105 (38) | 77.7 (36) | 68.7 (24) | 0.100 |
| Prothrombin time (%) | 76.3 (12) | 81.6 (9.8) | 92.2 (15) | 0.118 |
| APTT (s) | 37.1 (4.8) | 38.1 (3.3) | 38.9 (3.3) | 0.560 |
| Fibrinogen (μmol/l) | 9.0 (2.6) | 9.06 (2.9) | 7.79 (1.9) | 0.418 |
| Antithrombin III (%) | 92.1 (19) | 92.3 (11) | 108 (9.6) | 0.306 |
| TAT (ng/ml) | 1.79 (1.2) | 1.18 (0.26) | 1.20 (0.25) | 0.520 |
| Thrombomodulin (ng/ml) | 18.3 (8.6) | 15.7 (5.3) | 23.6 (5.1) | 0.689 |
| Protein C activity (%) | 65.6 (15.9)** † | 88.7 (3.4) | 100 (21) | 0.002 |
| Protein S antigen (%) | 76.0 (12) | 76.3 (20) | 90.7 (15) | 0.199 |
| PAI‐1 antigen (ng/ml) | 6.0 (3.6) | 9.5 (4.5) | 6.3 (3.7) | 0.338 |
Data are mean (SD).
p<0.05 is considered significant (analysis of variance).
Scheffe post hoc test: *p<0.02, **p<0.005, ***p<0.001 v control; †p<0.05, ††p<0.005 v normal MRI.
APTT, activated partial thromboplastin time; MCV, mean corpuscular volume; PAI‐1, plasminogen activator inhibitor 1; TAT, thrombin‐antithrombin complex.
RESULTS
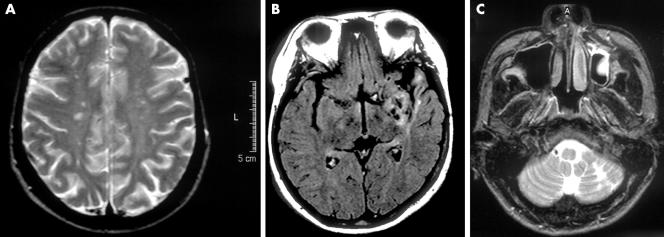
In seven of 15 patients (four with contraindication of Fontan procedure and three with Eisenmenger's syndrome), MRI showed multiple small lesions at various sites of the brain, including the cerebral cortex, corona radiata, centrum semiovale, and cerebellar cortex. These lesions were of low intensity on T1 weighted images and of high intensity on T2 weighted images, indicating chronic cerebral infarction (fig 1). Three of the seven patients also had mild diffuse cortical atrophy. Magnetic resonance angiography showed stenotic lesions of the horizontal portion of the middle cerebral artery and the vertebral artery in a 23 year old man and a stenotic lesion of the vertebral artery in a 45 year old man. In the other eight patients, both MRI and magnetic resonance angiography were normal.
Figure 1 Magnetic resonance images of the brain. (A) T2 weighted image of a 35 year old female patient with Eisenmenger's syndrome, who had no history of stroke but had chronic headache as well as exercise intolerance. Note the multiple small bilateral lesions with high intensity in the centrum semiovale. The packed cell volume was 63%. (B) Fluid attenuated inversion recovery image of a 25 year old female patient with corrected transposition of the great arteries, ventricular septal defect, and hypoplastic pulmonary arteries, who had no history of stroke or any hyperviscosity related symptoms. Note the old infarction and atrophic changes in the left temporal lobe. The packed cell volume was 61%. (C) T2 weighted image of a 23 year old male patient with tetralogy of Fallot and extremely hypoplastic pulmonary arteries, who had chronic headache, dizziness, and taste disorder. Note the old infarction in the left cerebellar hemisphere. The packed cell volume was 74%.
Oxygen saturation, packed cell volume, and protein C activity were significantly different between patients with CCHD with abnormal MRI and with normal MRI and normal subjects. Post hoc comparison showed that these three parameters also differed between the abnormal and normal MRI groups (table 1). Packed cell volume and arterial oxygen saturation in seven patients with abnormal MRI ranged from 60–74% and from 68–83%, respectively. Conversely, none of the patients with a packed cell volume less than 60% or with a saturation over 85% had an abnormal MRI. Patients who had hyperviscosity related symptoms had an abnormal MRI except for one female patient who had had repeated migraine headaches and had a normal MRI. Among the coagulation parameters measured, protein C activity was lower in the group with abnormal MRI than in those without (table 1).
DISCUSSION
The present study showed multiple ischaemic changes in the brain of patients with CCHD even in the absence of a clinically apparent history of stroke. Abnormal MRIs showed multiple chronic infarctions, cortical atrophy, and stenosis of the vertebral or cerebral arteries. This study suggested a tentative packed cell volume of 60% and oxygen saturation of 85% discriminate between patients with and without a brain ischaemic lesion, although the sample size was small because of the strict inclusion criteria—that is, patients with uncorrected CCHD without a history of stroke. Mean corpuscular volume reflects the iron status and low mean corpuscular volume is known to be a risk factor leading to a cerebrovascular event.1,3 However, the volumes were not different in this study (table 1). In addition to the difference in the packed cell volume, plasma protein C activity was lower in the patients with ischaemic lesions than in those without. As we showed previously, some parameters of the coagulation system, including plasma protein C concentration, tend to correlate with the packed cell volume, and consumptive coagulopathy may be a typical mechanism of the unique haematological disorder in patients with CCHD.4 We also showed that the thrombomodulin–protein C–protein S system is suppressed in CCHD due to the consumption of the factors or downregulation of the system.4 In these patients, both thromboembolism and haemostatic defects coexist, just as in disseminated intravascular coagulation induced by, for example, sepsis. Derangement in CCHD, however, progresses much more slowly than in disseminated intravascular coagulation. Interestingly, suppression of the protein C–protein S system has also been implicated in the pathogenesis of cryptogenic stroke in association with paradoxical embolisation through a patent foramen ovale (through right to left shunt as in CCHD).5 Further, brain ischaemic lesions observed in our study may be related to chronic hypoxaemia because resting arterial oxygen saturation in the patients with an abnormal brain MRI was significantly lower than in those without. Chronic profound hypoxaemia might have induced cerebral infarction in cooperation with significant erythrocytosis, although arterial desaturation is closely related to erythrocytosis. Multiple and non‐localised small ischaemic lesions of the brain observed in the present study endorse the abovementioned mechanisms of thrombotic tendency including protein C suppression.
In conclusion, even after excluding patients with clinically apparent stroke, adults with CCHD and secondary erythrocytosis commonly develop subclinical ischaemic changes in the brain demonstrable on MRI, although the timing of onset of these lesions could not be determined. If the results of this study are confirmed in a larger study, the packed cell volume (or resting arterial oxygen saturation) and blood protein C activity may be useful markers of such brain changes and for prediction of susceptibility of patients with CCHD to cerebrovascular events.
Footnotes
No financial support was received for this research.
References
- 1.Perloff J K, Rosove M H, Sietsema K E.et al Cyanotic congenital heart disease: a multisystem disorder. In: Perloff JK, Child JS, eds. Congenital heart disease in adults. 2nd ed. Philadelphia: WB Saunders, 1998199–226.
- 2.Ammash N, Warnes C A. Cerebrovascular events in adult patients with cyanotic congenital heart disease. J Am Coll Cardiol 199628768–772. [DOI] [PubMed] [Google Scholar]
- 3.Perloff J K, Marelli A J, Miner P D. Risk of stroke in adults with cyanotic congenital heart disease. Circulation 1993871954–1959. [DOI] [PubMed] [Google Scholar]
- 4.Horigome H, Murakami T, Isobe T.et al Soluble P‐selectin and thrombomodulin‐protein C‐protein S pathway in cyanotic congenital heart disease with secondary erythrocytosis. Thromb Res 2003112223–227. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi S. Coagulation abnormalities in adults with cryptogenic stroke and patent foramen ovale. J Neurol Sci 1998160158–160. [DOI] [PubMed] [Google Scholar]