Abstract
Objective
To develop a technique for volumetric analysis of real time three dimensional echocardiography (RT3DE) data aimed at quantifying left ventricular (LV) mass and to validate the technique against magnetic resonance (MR) assumed as the reference standard.
Design
RT3DE, which has recently become widely available, provides dynamic pyramidal data structures that encompass the entire heart and allows four dimensional assessment of cardiac anatomy and function. However, analysis techniques for the quantification of LV mass from RT3DE data are fundamentally two dimensional, rely on geometric modelling, and do not fully exploit the volumetric information contained in RT3DE datasets. Twenty one patients underwent two dimensional echocardiography (2DE), RT3DE, and cardiac MR. LV mass was measured from 2DE and MR images by conventional techniques. RT3DE data were analysed to semiautomatically detect endocardial and epicardial LV surfaces by the level set approach. From the detected surfaces, LV mass was computed directly in the three dimensional space as voxel counts.
Results
RT3DE measurement was feasible in 19 of 21 patients and resulted in higher correlation with MR (r = 0.96) than did 2DE (r = 0.79). RT3DE measurements also had a significantly smaller bias (−2.1 g) and tighter limits of agreement (2SD = ±23 g) with MR than did the 2DE values (bias (2SD) −34.9 (50) g). Additionally, interobserver variability of RT3DE (12.5%) was significantly lower than that of 2DE (24.1%).
Conclusions
Direct three dimensional model independent LV mass measurement from RT3DE images is feasible in the clinical setting and provides fast and accurate assessment of LV mass, superior to the two dimensional analysis techniques.
Keywords: real time three dimensional echocardiography, semiautomated surface detection, left ventricular mass, cardiac magnetic resonance imaging
Left ventricular (LV) mass is an important clinical variable in patient management, since this parameter is an independent and strong predictor of morbid cardiac events and death.1,2 Conventional LV mass measurements derived from manual tracing of two dimensional echocardiography (2DE) images are subjective3 and time consuming to calculate, and they rely on geometric assumptions of uniform chamber size and shape.4 Importantly, LV mass measurements are highly dependent on the ability to obtain non‐foreshortened long axis images from apical acoustic windows,5 which is in many patients compromised by limited access to the apex through the intercostal spaces.
We have recently shown that this limitation can be overcome by extracting anatomically correct,6 non‐foreshortened, orthogonal two dimensional apical views from transthoracic real time three dimensional echocardiography (RT3DE) data. From these views, biplane LV volumes are calculated by manually tracing endocardial and epicardial contours. This approach was used to test a fast and simple, widely available technique to asses LV mass and to evaluate the effects of foreshortening as the source of underestimation of LV mass by conventional 2DE analysis.
Although our previous results showed that LV mass can be measured from RT3DE datasets more accurately than with the conventional 2DE approach,6 the data analysis procedure we used is still two dimensional and thus fraught with the limitations inherent in this method. Firstly, it relies on the subjective selection of non‐foreshortened two dimensional apical views and manual tracing of endocardial and epicardial boundaries; and secondly, LV mass computations are derived from model based biplane volumes, which may be inaccurate.
RT3DE datasets are dynamic pyramidal data structures that encompass the entire heart and are obtained from a single acoustic window, allowing four dimensional assessment of cardiac anatomy and function.7,8 Our current study was designed to test the hypothesis that detection of LV endocardial and epicardial surfaces in three dimensional space may allow even more accurate direct measurements of LV mass, without the need for subjective plane selection and geometrical modelling. Accordingly, our goal was to develop and validate a new semiautomated method, based on level set models,9,10,11 to measure LV mass based on rapid detection of LV endocardial and epicardial surfaces. To achieve this goal, we evaluated the accuracy and interobserver variability of LV mass measurements based on this newly developed technique by using cardiac magnetic resonance (MR) measurements as the reference for comparison.
METHODS
In the current investigation, we studied the same group of patients reported previously.6 Patients referred for cardiac MR imaging were enrolled in the study if they had adequate transthoracic two dimensional acoustic windows (apical four and two chambers) that allowed adequate endocardial visualisation without contrast enhancement. Exclusion criteria were dyspnoea precluding a 12 second breath hold, atrial fibrillation, pacemaker or defibrillator implantation, claustrophobia, and other well known contraindications to MR. On the basis of these criteria, 21 patients (mean (SD) age 48 (16) years; 13 men, eight women) were recruited: seven patients with suspected coronary artery disease, seven with dilated cardiomyopathy, two after a myocardial infarction, three with aortic disease, one with a right atrial mass, and one with mitral valve regurgitation. After institutional review board committee approval, written informed consent was obtained from all patients. Echocardiographic imaging, including 2DE and RT3DE data acquisition, was performed on the same day as the MR study.
MR imaging
Cardiac MR images were obtained with a 1.5 T scanner (General Electric) with a phased array cardiac coil. ECG gated localising spin echo sequences were used to identify the long axis of the heart. Steady state free precession (fast imaging employing steady‐state acquisition or FIESTA) dynamic gradient echo cine loops were obtained during 12 second breath holds with a temporal resolution of 20 frames/cardiac cycle. For all patients, 6–10 short axis cine loops were obtained from the atrioventricular ring to the apex (9 mm slice thickness, no gaps).
Two dimensional echocardiography
Transthoracic 2DE harmonic images were recorded with a commercial ultrasound scanner (SONOS 7500, Philips Medical Systems) equipped with a broad band transducer (S3, 2−4 MHz). Echocardiographic images were recorded from the apical window, with the patient in the left lateral decubitus position. Five consecutive apical four and two chamber loops were acquired during a breath hold while taking care to avoid foreshortening during acquisition. Loops were stored digitally for offline review and analysis (EnConcert, Philips Medical Systems).
Real time three dimensional echocardiography
Transthoracic RT3DE imaging was performed in the same setting with a fully sampled matrix array transducer (X 4) in the harmonic three dimensional mode. Care was taken to include the entire LV cavity within the pyramidal three dimensional scan volume. RT3DE datasets were then acquired in the wide angled acquisition mode, wherein four wedge shaped subvolumes (93° × 21°) were acquired over four cardiac cycles during a breath hold with ECG gating. Acquisition of each subvolume was triggered to the R wave of every other heart beat to allow sufficient time for the probe to be recalibrated and each subvolume to be stored.
Analysis of MR images
Cardiac MR images were analysed with commercial software (MASS Analysis, General Electric). Consecutive slices from the LV base, defined as the highest slice in which the LV outflow tract was not visible down to the lowest slice in which the LV cavity was visible, were selected for analysis. In every slice, LV endocardial contours were traced semiautomatically frame by frame, with the papillary muscles included in the LV cavity, and manually corrected when necessary to optimise boundary position. Then LV volumes were computed throughout the cardiac cycle by a disk–area summation method (modified Simpson's rule). The end diastolic frame was then identified as the frame with the maximum LV volume reached during the cardiac cycle and was used to trace the LV epicardial contour. The end diastolic volume (EDV) included in the epicardium (EDVep) was computed with the same disk–area summation method and was used to calculate the LV mass as (EDVep − EDV) times the mass density constant (1.05 g/cm3). The MR data served as the reference standard for comparisons with 2DE and RT3DE echocardiographic data. All tracings were analysed by an investigator experienced in cardiac MR analysis who had no knowledge of the echocardiographic measurements.
Analysis of 2DE images
The 2DE loops were analysed off line (Enconcert, Philips Medical Systems). For both apical four and two chamber views, from the five acquired loops, the one with the best image quality and least apical foreshortening was selected for analysis. The end diastolic frame was selected as the image with the largest LV cavity area. Endocardial and epicardial contours were manually traced while including the papillary muscles in the LV cavity and used to calculate end diastolic endocardial and epicardial volumes by the biplane Simpson's formula.4,12 The difference between epicardial and endocardial volumes was computed for each view, averaged for both views, and multiplied by the mass density constant (1.05 g/cm3) to calculate a biplane estimate of LV mass.
Analysis of RT3DE images
The RT3DE datasets were analysed by custom software,11 which allows semiautomated LV surface detection based on the level set approach.9 This method uses an implicit representation of curves in the form of a partial differential equation to track boundaries, without geometrical assumptions or a priori shape knowledge.10
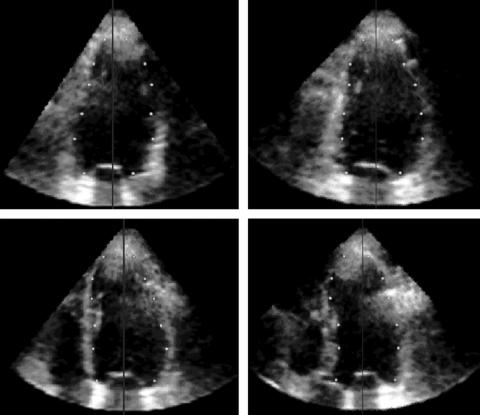
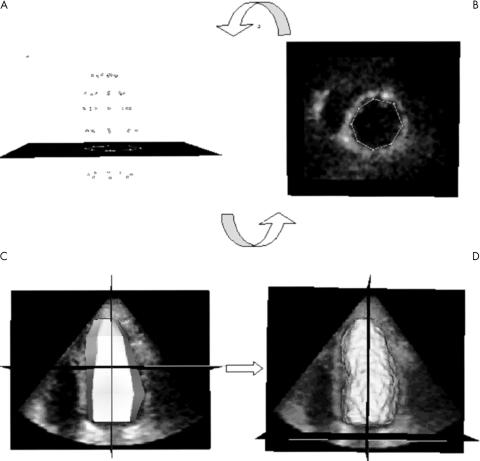
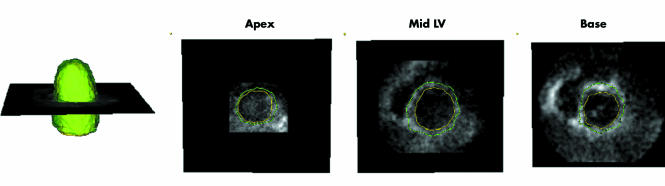
Initially, the dynamic dataset was displayed in the three dimensional space. Then a manual initialisation procedure of the LV endocardial position was required. The end diastolic frame, visually determined by the operator as the largest LV cavity in the cardiac cycle, was selected for analysis. To detect the LV endocardium (fig 1), the operator manually defined the long axis of the left ventricle in the three dimensional dataset; then two points were manually selected in each of four evenly 45° rotated long axis planes, one on each side of the endocardial interface. This procedure was repeated at six to eight different short axis planes, from apex to base. To be consistent with cardiac MR tracings, papillary muscles were included in the LV cavity initialisation. Once the points were manually initialised on the four evenly 45° rotated long axis planes, the position of the points was verified in multiple short axis views from base to apex, thus resolving possible ambiguities. During this phase, the operator had the opportunity to adjust the position of the points, if needed (fig 2A, B).
Figure 1 Left ventricular (LV) endocardial surface position is initialised in four 45° rotated apical planes. After the long axis of the left ventricle (vertical line) was selected, two points were manually selected in each plane at each of six different levels (from apex to base, white dots).
Figure 2 (A, B) Point verification and correction and (C, D) endocardial surface detection. The initialised points (A) are shown in each short axis plane (B) for verification and correction if required. The endocardial surface (C) obtained by joining these points is the initial condition for the three dimensional endocardial detection algorithm, which results in the calculated LV endocardial surface (D).
All selected points were then joined to define a surface (fig 2C), being the initial condition for the level set partial differential equation, which guided the evolution of this surface within the volumetric dataset towards the endocardial position.
After a fully automated iterative process, the final endocardial surface was detected (fig 2D), from which the EDV was measured by counting voxels confined within the surface.
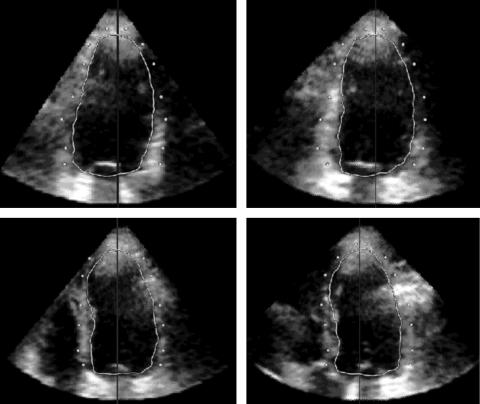
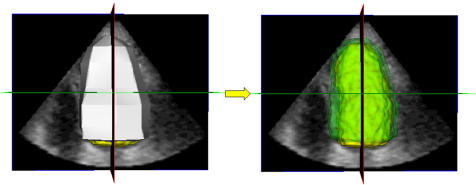
To detect the epicardial surface (fig 3), the same initialisation procedure was repeated on the same frame. The epicardial points were then selected on the same four long axis planes with the cross section of the detected LV endocardial surface, which was superimposed to guide the initialisation. The initial epicardial surface was then calculated by joining the selected points and, after an iterative process, the final epicardial surface was obtained (fig 4). EDVep was measured from voxel counts and LV mass was then obtained as 1.05 × (EDVep − EDV).
Figure 3 LV epicardial surface initialisation procedure shown in the format of fig 1. The cross section of the detected endocardial surface was displayed to guide the selection of epicardial points (white dots).
Figure 4 Initial LV epicardial surface obtained by joining the selected points (left) and the final calculated LV epicardial surface (right) shown in semitransparent green with the endocardial surface shown in yellow.
To visually verify the correctness of the detected endocardial and epicardial surfaces, both surfaces were superimposed on the volumetric data (fig 5), which allowed cross sectioning in any arbitrary plane and corrections in the initial points when necessary.
Figure 5 Example of the detected LV endocardial and epicardial surfaces (left) and corresponding short axis cross sections from apex to base (right panels). This display allowed visual verification of the position of the calculated endocardial and epicardial surfaces in multiple planes.
Interobserver variability
To determine the interobserver variability in the measurement of LV mass with 2DE and RT3DE, three independent observers blinded to the results obtained with MR and the other echocardiographic technique analysed the images on separate days. Interobserver variability for 2DE and RT3DE was calculated as the SD of the mean of three observers and expressed as the percentage of the mean.
Statistical analysis
Values were expressed as mean (SD). 2DE and RT3DE measurements obtained by each observer were compared with MR values by paired t test. Linear regression was analysed, and Pearson's correlation coefficient and SEE were computed. In addition, agreement between 2DE and RT3DE with the MR reference standard was evaluated by Bland‐Altman analysis. The t test was applied to verify the significance of the bias (paired t test versus null values).
RESULTS
RT3DE echocardiography was feasible in all patients with the exception of two patients with dilated cardiomyopathy, whose hearts did not completely fit into the pyramidal scan volume. In the remaining 19 patients, MR values for EDV ranged from 79–390 ml (172 (74) ml) and LV mass measurements ranged from 57–222 g, with a mean of 126 (39) g.
The time required to analyse a single RT3DE dataset, including endocardial and epicardial surface initialisation, correction, and computation of LV mass, was 190 (60) seconds. Table 1 presents the mean (SD) LV mass obtained by each of the three observers from RT3DE and 2DE data sets. Whereas mean values of LV mass did not differ significantly between MR and RT3DE, with 2DE all three observers consistently and significantly (p = 0.001) underestimated LV mass (average error 28 (19)%) compared with MR.
Table 1 LV mass measured in 19 patients by three observers by manual tracing of two dimensional echocardiography (2DE) and semiautomated surface detection applied to real time three dimensional echocardiography (RT3DE).
| Observer | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| RT3DE (g) | 119 (41) | 127 (37) | 129 (35) |
| 2DE (g) | 99 (37)* | 70 (21)* | 95 (26)* |
Data are mean (SD). All values were compared with a magnetic resonance reference of 126 (39) g.
*p<0.05, paired t test versus magnetic resonance.
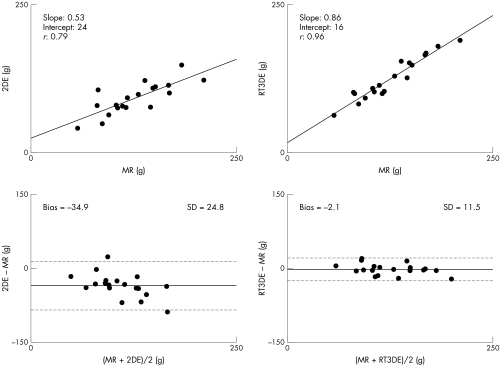
The combined measurements of LV mass for the three observers by biplane 2DE resulted in a correlation of r = 0.79 (SEE = 20 g, p < 0.001) with MR values (fig 6, top left). Bland‐Altman analysis (fig 6, bottom left) confirmed the underestimation by 2DE by showing a bias (p = 0.001) of −34.9 g (28% of the mean) with relatively wide 95% limits of agreement at ±50 g (±40% of the mean MR value).
Figure 6 Comparison of LV mass measured by two dimensional echocardiography (2DE) (left) and real time three dimensional echocardiography (RT3DE) (right) versus magnetic resonance (MR) in 19 patients. In linear regression (top) and Bland‐Altman (bottom) analyses, each dot represents the mean of the three observers. Horizontal dashed lines represent the 95% limits of agreement.
The combined semiautomated measurements of LV mass by the three observers by the RT3DE technique resulted in a correlation of r = 0.96 (SEE = 10.5 g, p < 0.001) with MR values (fig 6, top right). Bland‐Altman analysis (fig 6, bottom right) showed no significant underestimation by RT3DE, as reflected by a minimum bias of −2.1 g (2% of the mean) and narrow 95% limits of agreement of ±23 g (±18% of the mean MR value).
The interobserver variability of LV mass derived from manual tracing of the 2DE images was 24 (12)% (ranging in individual patients from 4–50%). The interobserver variability of RT3DE data and the semiautomated surface detection algorithm was significantly lower (12 (8)%, p = 0.001 v 2DE, range 1–26%).
DISCUSSION
Although M mode and 2DE have been traditionally used for the quantification of LV mass,13 their dependence on geometric modelling have limited their use. In fact, both the M mode and two dimensional area–length formulas for calculating LV mass assume that the left ventricle has a fixed geometric shape, which may result in errors, particularly in patients with wall motion abnormalities. Moreover, the inadvertent use of oblique cuts in M mode echocardiography and foreshortened apical views with 2DE can potentially result in additional inaccuracies.6,14,15,16,17
Three dimensional echocardiography, as a basis for offline three dimensional reconstruction from multiple planes, has been shown to reliably quantify LV mass in both animal experiments18,19,20,21 and human studies.16,22,23 The enhanced accuracy with reduced interobserver variability has been obtained with a variety of methods for data acquisition, including the gated rotational approach18,24 and freehand scanning with locator devices.21,25,26 Despite the obvious advantages of using a three dimensional imaging approach to quantify LV mass, these methods have not been successfully incorporated into clinical practice. This is probably due to the cumbersome and lengthy data acquisition coupled with the extensive time requirements for data analysis.
The recent introduction of the fully sampled matrix array transducer for near real time three dimensional imaging and rendering of the left ventricle has solved the majority of the problems inherent in three dimensional acquisition. However, in all previous studies,24,27,28,29,30 analysis of RT3DE datasets was essentially two dimensional, since it relied on computation of the LV endocardial or epicardial surface by interpolating manually traced or semiautomatically detected contours on multiple two dimensional planes extracted from the three dimensional datasets. This two dimensional analysis can potentially introduce errors due to its inability to detect asymmetries or changes in LV shape between individually traced planes. In our recent paper,6 we proved that even two dimensional analysis of RT3DE data by extraction of biplane views improves the accuracy of LV mass measurements compared with the conventional 2DE method.
In the present study, we hypothesised that true three dimensional analysis, free of both geometric modelling and the need to subjectively select and manually trace non‐foreshortened two dimensional apical views, could further improve the accuracy of echocardiographic evaluation of LV mass. To test this hypothesis, we developed a novel volumetric analysis technique for direct LV mass measurement from endocardial and epicardial surfaces detected from RT3DE data. This technique was validated against the MR reference standard in the same group of patients who took part in our previous study, with the same datasets to obtain reference measurements.6
In contrast to previously presented approaches for RT3DE data analysis,24,27,28,29,30 which were based on detection of LV contours on multiple two dimensional planes and their interpolation to generate the LV surface, our three dimensional technique directly detects the LV surface in three dimensional space from all the voxel information contained in the volumetric dataset to guide the evolution of the surface, starting from the manually initialised cloud of points. In contrast to another recently described three dimensional analysis technique applied to RT3DE data,31 the level set image segmentation approach that we used to detect LV endocardial and epicardial surfaces is completely independent of any a priori information about the shape characteristics of the left ventricle and its deformation throughout the cardiac cycle.9 Therefore, this novel approach allows the detection of a variety of ventricular endocardial and epicardial shapes.
Our results showed that the LV mass obtained from the RT3DE data generated by this analysis technique resulted in higher level of agreement with MR values than did conventional 2DE measurements. 2DE manual tracings consistently underestimated LV mass compared with MR, resulting in larger biases and wider limits of agreement than with RT3DE. The interobserver variability of the 2DE LV mass measurements was twice that of the RT3DE technique, indicating that the semiautomated surface detection procedure applied to RT3DE datasets provides more reproducible measurements than does the conventional 2DE technique. This interobserver variability was achieved despite allowing every reader, during the initialisation procedure, to modify the image contrast and luminance by linear stretching of the histogram to optimise point selection. Since the algorithm uses all the information contained in the original three dimensional dataset, once the LV surface was initialised these changes had no effect on the determination of the final LV endocardial or epicardial surface. However, compared with other imaging techniques, the interobserver variability of LV mass with RT3DE is still relatively high at 12.5%. The reasons for these results are multiple: firstly, visualisation of the epicardium is not optimal; secondly, initialisation points in different planes are manually selected; and thirdly, the basal segments of the heart are usually not as well visualised as the proximal ones, thus increasing the subjectivity of the analysis.
Although the manual initialisation step might have introduced some variability in the results, the initialisation process required manual selection of points at only six to eight different short axis levels in four rotated long axis planes, which constitutes an improvement over the conventional techniques. Also, visualisation of the selected points both in the long axis and in multiple short axis views allowed operators to verify and correct their position before automated endocardial and epicardial surface detection.
Moreover, the results of this study confirmed our hypothesis that true three dimensional analysis provides higher accuracy in LV mass measurements (r = 0.96) than does biplane calculation from anatomically correct apical views extracted from RT3DE data (r = 0.90 (6)). Importantly, Bland‐Altman analysis showed tighter limits of agreement (2SD = ±23 g) with the MR reference than with the latter technique (2SD = ±34 g).6 Compared with other previous studies based on manual or semiautomated tracing of multiple two dimensional LV contours for computation of LV mass in humans,19,30 our method resulted in similar or even higher correlations, with no significant underestimation of LV mass and comparable interobserver variability.
The availability of an accurate, fast, and easy to use quantification tool for LV mass computation from RT3DE images, without the need for time consuming and cumbersome volume reconstruction, may lead to the use of this technique in clinical practice.
Although three dimensional echocardiography with a fully sampled matrix array transducer is often referred to as a real time technique, it requires separate acquisition of subvolumes over eight consecutive beats. This may limit the applicability of this method to patients with severe dyspnoea, atrial fibrillation, and cardiac arrhythmias. Moreover, LV mass cannot be measured in patients with severely dilated hearts, which do not completely fit into the pyramidal scan volume. Patients with severe dilated cardiomyopathy, ventricular aneurysms, or ventricles with heavy trabeculations need to be studied further to define better the applicability of this technique to this population. However, we excluded these patients, since our goal was to test and validate the newly developed three dimensional analysis technique rather than to establish the applicability of RT3DE imaging to a wide range of clinical scenarios.
Another limitation is the limited image quality, which sometimes precludes the accurate delineation of endocardial and epicardial borders with RT3DE. The use of contrast enhancement may potentially solve this problem. However, the potential increased accuracy of LV measurements with contrast enhanced RT3DE still has to be determined.
In summary, despite these limitations, this study indicates that our three dimensional analysis based on semiautomated detection of the LV endocardial and epicardial surface applied to RT3DE images allows rapid and accurate measurement of LV mass, superior to conventional 2DE measurement and two dimensional analysis techniques applied to RT3DE data.
Abbreviations
2DE - two dimensional echocardiography
EDV - end diastolic volume
EDVep - epicardial end diastolic volume
LV - left ventricular
MR - magnetic resonance
RT3DE - real time three dimensional echocardiography
Footnotes
The study was supported by the “Marco Polo” grant from University of Bologna, Italy (C C) and a grant in aid from the American Heart Association (V M)
References
- 1.Levy D, Garrison R J, Kannel W B.et al Prognostic implications of echocardiographically determined left‐ventricular mass in the Framingham heart study: reply. N Engl J Med 19903231706–1707. [DOI] [PubMed] [Google Scholar]
- 2.Ghali J K, Liao Y, Simmons B.et al The prognostic role of the left ventricular hypertrophy in patients with or without coronary artery disease. Ann Intern Med 1992117831–836. [DOI] [PubMed] [Google Scholar]
- 3.Byrd B F, Wahr D, Wang Y S.et al Left ventricular mass and volume mass ratio determined by two‐dimensional echocardiography in normal adults. J Am Coll Cardiol 198561021–1025. [DOI] [PubMed] [Google Scholar]
- 4.Wyatt H L, Heng M K, Meerbaum S.et al Cross‐sectional echocardiography. 1. Analysis of mathematic models for quantifying mass of the left ventricle in dogs. Circulation 1979601104–1113. [DOI] [PubMed] [Google Scholar]
- 5.Schiller N B, Shah P M, Crawford M.et al Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two‐Dimensional Echocardiograms. J Am Soc Echocardiogr 19892358–367. [DOI] [PubMed] [Google Scholar]
- 6.Mor‐Avi V, Sugeng L, Weinert L.et al Fast measurement of left ventricular mass with real‐time three‐dimensional echocardiography: comparison with magnetic resonance imaging. Circulation 20041101814–1818. [DOI] [PubMed] [Google Scholar]
- 7.Sugeng L, Weinert L, Lang R M. Left ventricular assessment using real time three dimensional echocardiography. Heart 20038929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugeng L, Weinert L, Thiele K.et al Real‐time three‐dimensional echocardiography using a novel matrix array transducer. Echocardiography 200320623–635. [DOI] [PubMed] [Google Scholar]
- 9.Osher S, Sethian J A. Fronts propagating with curvature‐dependent speed: algorithms based on Hamilton‐Jacobi formulations. J Comput Phys 19887912–49. [Google Scholar]
- 10.Malladi R, Sethian J A, Vemuri B C. Shape modelling with front propagation: a level set approach. IEEE Trans Pattern Anal Mach Intell 199517158–175. [Google Scholar]
- 11.Corsi C, Saracino G, Sarti A.et al Left ventricular volume estimation for real‐time three‐dimensional echocardiography. IEEE Trans Med Imaging 2002211202–1208. [DOI] [PubMed] [Google Scholar]
- 12.Helak J W, Reichek N. Quantitation of human left ventricular mass and volume by two‐dimensional echocardiography: in‐vitro anatomic validation. Circulation 1981631398–1407. [DOI] [PubMed] [Google Scholar]
- 13.Devereux R B, Reichek N. Echocardiographic determination of left ventricular mass in man: anatomic validation of method. Circulation 197755613–618. [DOI] [PubMed] [Google Scholar]
- 14.Myerson S G, Montgomery H E, World M J.et al Left ventricular mass: reliability of M‐mode and 2‐dimensional echocardiographic formulas. Hypertension 200240673–678. [DOI] [PubMed] [Google Scholar]
- 15.Missouris C G, Forbat S M, Singer D R J.et al Echocardiography overestimates left ventricular mass: a comparative study with magnetic resonance imaging in patients with hypertension. J Hypertens 1996141005–1010. [PubMed] [Google Scholar]
- 16.Kuhl H P, Bucker A, Franke A.et al Transesophageal 3‐dimensional echocardiography: in‐vivo determination of left ventricular mass in comparison with magnetic resonance imaging. J Am Soc Echocardiogr 200013205–215. [PubMed] [Google Scholar]
- 17.Kuhl H P, Hanrath P, Franke A. M‐mode echocardiography overestimates left ventricular mass in patients with normal left ventricular shape: a comparative study using three‐dimensional echocardiography. Eur J Echocardiogr 20034312–319. [DOI] [PubMed] [Google Scholar]
- 18.Teupe C, Takeuchi M, Yao J F.et al Determination of left ventricular mass by three‐dimensional echocardiography: in vitro validation of a novel quantification method using multiple equi‐angular rotational planes for rapid measurements. Int J Cardiovasc Imaging 200218161–167. [DOI] [PubMed] [Google Scholar]
- 19.Gopal A S, Keller A M, Shen Z Q.et al 3‐Dimensional echocardiography: in‐vitro and in‐vivo validation of left ventricular mass and comparison with conventional echocardiographic methods. J Am Coll Cardiol 199424504–513. [DOI] [PubMed] [Google Scholar]
- 20.Sapin P M, Gopal A S, Clarke G B.et al Three‐dimensional echocardiography compared to two‐dimensional echocardiography for measurement of left ventricular mass anatomic validation in an open chest canine model. Am J Hypertens 19969467–474. [DOI] [PubMed] [Google Scholar]
- 21.Leotta D F, Munt B, Bolson E L.et al Quantitative three‐dimensional echocardiography by rapid imaging from multiple transthoracic windows: in vitro validation and initial in vivo studies. J Am Soc Echocardiogr 199710830–839. [DOI] [PubMed] [Google Scholar]
- 22.Sapin P M, Schroder K M, Gopal A S.et al Comparison of 2‐dimensional and 3‐dimensional echocardiography with cineventriculography for measurement of left‐ventricular volume in patients. J Am Coll Cardiol 1994241054–1063. [DOI] [PubMed] [Google Scholar]
- 23.Kuhl H P, Franke A, Merx M.et al Quantification of left ventricular function and mass using transesophageal three‐dimensional echocardiography: validation of a method that uses long‐axis cutplanes. Eur J Echocardiogr 20001213–221. [DOI] [PubMed] [Google Scholar]
- 24.Kuhl H P, Franke A, Janssens U.et al Three‐dimensional echocardiographic determination of left ventricular volumes and function by multiplane transesophageal transducer: dynamic in vitro validation and in vivo comparison with angiography and thermodilution. J Am Soc Echocardiogr 1998111113–1124. [DOI] [PubMed] [Google Scholar]
- 25.Chuang M L, Beaudin R A, Riley M F.et al Three‐dimensional echocardiographic measurement of left ventricular mass: comparison with magnetic resonance imaging and two‐dimensional echocardiographic determinations in man. Int J Cardiac Imaging 200016347–357. [DOI] [PubMed] [Google Scholar]
- 26.Gopal A S, Schnellbaecher M J, Shen Z Q.et al Freehand three‐dimensional echocardiography for measurement of left ventricular mass: in vivo anatomic validation using explanted human hearts. J Am Coll Cardiol 199730802–810. [DOI] [PubMed] [Google Scholar]
- 27.Kuhl H P, Schreckenberg M, Rulands D.et al High‐resolution transthoracic real‐time three‐dimensional echocardiography: quantitation of cardiac volumes and function using semi‐automatic border detection and comparison with cardiac magnetic resonance imaging. J Am Coll Cardiol 2004432083–2090. [DOI] [PubMed] [Google Scholar]
- 28.Qin J X, Jones M, Shiota T.et al Validation of real‐time three‐dimensional echocardiography for quantifying left ventricular volumes in the presence of a left ventricular aneurysm: in vitro and in vivo studies. J Am Coll Cardiol 200036900–907. [DOI] [PubMed] [Google Scholar]
- 29.Qin J J, Jones M, Shiota T.et al New digital measurement method for left ventricular volume using real‐time three‐dimensional echocardiography: comparison with electromagnetic flow method and magnetic resonance imaging. Eur J Echocardiogr 2000196–104. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins C, Bricknell K, Hanekom L.et al Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real‐time three‐dimensional echocardiography. J Am Coll Cardiol 200444878–886. [DOI] [PubMed] [Google Scholar]
- 31.Gerard O, Billon A C, Rouet J M.et al Efficient model‐based quantification of left ventricular function in 3‐D echocardiography. IEEE Trans Med Imaging 2002211059–1068. [DOI] [PubMed] [Google Scholar]