Abstract
Objective
To assess prospectively whether preimplantation B‐type natriuretic peptide (BNP) and C reactive protein (CRP) concentrations predict future appropriate therapies from an implantable cardioverter‐defibrillator (ICD).
Design and setting
Prospective cohort study conducted in a tertiary cardiac care centre.
Methods
345 consecutive patients undergoing first time ICD implantation were prospectively studied. Serum BNP and CRP concentrations were obtained the day before ICD implantation. Patients were followed up with device interrogation to assess for appropriate shocks or antitachycardia pacing. Inappropriate therapies were excluded. Mean (SD) follow up was 13 (5) months.
Results
Patients had ischaemic (71%), primary dilated (17%), and valvar or other cardiomyopathies (12%). About half (52%) had ICDs implanted for primary prevention. Sixty three (18%) received appropriate ICD therapies. Serum creatinine, β blocker, statin, and angiotensin converting enzyme inhibitor usage did not differ between therapy and no therapy groups. By univariate comparison, ejection fraction (p = 0.048), not taking amiodarone (p = 0.033), and BNP concentration (p = 0.0003) were risk factors for ICD therapy. However, by Cox regression multivariate analysis, only BNP above the 50th centile was a significant predictor (hazard ratio 2.19, 95% confidence interval 1.07 to 4.71, p = 0.040). Median BNP was 573 ng/l versus 243 ng/l in therapy and no therapy patients, respectively (p = 0.0003). More patients with BNP above the 50th centile (27% v 10%, p = 0.006) received ICD therapies.
Conclusions
A single preimplantation BNP concentration determination is independently predictive of ICD therapies in patients with cardiomyopathies undergoing first time ICD implantation. CRP was not independently predictive of ICD therapies when compared with BNP.
Keywords: natriuretic peptide, implantable cardioverter defibrillator, shock, antitachycardia pacing, C reactive protein
Several clinical trials have now shown the benefit of implantable cardioverter‐defibrillator (ICD) implantation for both primary and secondary prevention of arrhythmic death.1,2,3 However, the ability to better identify subgroups that would most and least benefit from ICD implantation remains of great interest, especially given the high economic cost of ICDs. ICD shocks also cause major discomfort and sometimes debilitating anxiety for patients, so being able to predict shock occurrence would lead to shock avoidance and improvement in patient quality of life. A few studies have identified some risk factors that predict which patients will receive appropriate ICD delivered therapies.4,5,6,7,8,9,10 Clinical risk factors include lower left ventricular ejection fraction (EF), higher New York Heart Association (NYHA) class, and not taking β blockers.8,9 However, the underlying aetiology of cardiomyopathy and whether the device was implanted for primary or secondary prevention may not identify patients at highest risk of arrhythmic events.9,10,11
Serum biomarkers have emerged as an effective and simple way to identify various patient populations at highest risk of adverse cardiac outcomes. In particular, an increased C reactive protein (CRP) concentration is associated with major adverse cardiac events in patients with congestive heart failure (CHF).12 An increased B‐type natriuretic peptide (BNP) concentration not only independently predicts mortality in patients with and without CHF13,14 but also may predict sudden death.15,16 However, whether increased BNP or CRP concentrations predict future arrhythmic events treatable by an ICD is unknown. Therefore, the purpose of our study was to assess whether preimplantation BNP and CRP concentration could predict future appropriate ICD therapies.
METHODS
Patient population
Consecutive patients undergoing first time implantation of either a single or dual chamber ICD at the Cleveland Clinic Foundation between October 2002 and October 2003 were prospectively studied. Patients undergoing ICD implantation at the clinic routinely have blood drawn the day before implant for basic haematological and biochemical analyses. Therefore, obtaining BNP and CRP concentrations did not require a separate visit or blood sampling for the patient. All included patients had (1) presence of structural heart disease, (2) either primary or secondary prevention indications for ICD implantation, and (3) serum biomarker concentrations determined from blood drawn the day before implantation. Exclusion criteria were patients having an existing ICD, implantation of a biventricular ICD, a separate pacing indication, residence too remote to attend follow up in a Cleveland Clinic Health System device clinic, or presence of any of the following conditions within 60 days of implantation: decompensated heart failure, acute coronary syndrome, major surgery, or systemic infection. Baseline demographic data, clinical characteristics, ECG, and an echocardiogram were collected within one month before implantation for included patients. NYHA class was assessed on the day before ICD implantation. All patients gave informed consent for the blood tests and procedures and all data were collected in accordance with institutional ethics review board guidelines at the Cleveland Clinic Foundation.
Serum biomarkers
Patients had their venous blood samples drawn from a forearm vein for both BNP and ultrasensitive CRP concentrations on the day before ICD implantation. All blood samples were drawn in the morning after an overnight fast. CRP concentrations were assayed by immunonephelometry with the Dade Behring BNIII analyser protocol (Dade Behring, Deerfield, Illinois, USA; detection limit 0.175 mg/l, upper measurement limit 24 mg/l, expected reference interval < 0.5–2.0 mg/l, coefficient of variation 7.6%) on non‐EDTA serum separated samples. BNP concentrations were determined according to the manufacturer's specifications with the Biosite Diagnostics assay (Biosite Diagnostics, San Diego, California, USA; detection limit < 5 ng/l, upper measurement limit 5000 ng/l, expected reference interval 5–100 ng/l, coefficient of variation 10.1%) on samples collected in EDTA tubes. Samples were analysed by personnel blinded to the patients' clinical data.
End point and follow up
Patients were followed up after implantation in a Cleveland Clinic Health System device clinic at six weeks, three months, and every six months thereafter (our routine follow up schedule). They were also asked to call the device clinic within 72 hours of an ICD discharge to make an appointment for a visit. At each visit the patient was clinically assessed and the device was interrogated. Occurrence of an ICD shock or antitachycardia pacing was confirmed in all cases by device interrogation. The attending staff electrophysiologist in the clinic then analysed the stored electrograms to determine whether the shock or antitachycardia pacing occurred appropriately for the treatment of either sustained ventricular tachycardia (VT) or ventricular fibrillation (VF). Another electrophysiologist blinded to the first staff member's interpretation also confirmed the appropriateness of the ICD therapy. Both had to agree in their interpretation of the event for the event to be included as an appropriate ICD therapy. Both electrophysiologists were blinded to the patient's BNP and CRP results.
The end point of the study was the occurrence of any appropriate ICD delivered therapy, whether shock or antitachycardia pacing. Inappropriate ICD therapies (caused by supraventricular tachycardia, device malfunction, etc) were not included for analysis. The mean (SD) follow up time for patients was 13.1 (5.2) months.
Statistical analysis
All data are reported as mean (SD) for continuous variables and number of patients (%) for categorical variables unless otherwise indicated. BNP and CRP concentrations are expressed as median (interquartile range). Univariate analyses were carried out by the unpaired, independent samples t test for continuous variables and the χ2 test for categorical variables. However, since the distributions of BNP and CRP concentrations were skewed, these variables were compared by the Mann‐Whitney U test. The incidence of ICD therapy over time was compared between categories of BNP by the Kaplan‐Meier method for time to event curves and the log rank test for comparison. All multivariate analyses by Cox regression analysis with a determination of a hazard ratio and its 95% confidence interval (CI) for each variable in the model. Forward, stepwise regression was initially performed to identify those variables that most affected the hazard ratio of BNP, with a cut off p < 0.10 for entry into the model. Separate forced entry regression analyses were performed to adjust the hazard ratio of BNP for these and other potential confounding variables. Univariate Cox regression with BNP or BNP quartile used as the only variable was also performed to determine an unadjusted hazard ratio. We also divided the population into increasing quartiles with respect to BNP. The hazard ratios of ICD shock were calculated for quartiles two through four compared with the lowest (first) quartile in unadjusted and adjusted analyses. To test for trend, univariate Cox regression was performed by treating quartile as a continuous variable and by using the median value of each quartile. Similar multivariate and quartile analyses were performed for CRP as was described for BNP. A probability value of p < 0.05 was considered significant for all statistical determinations. All data were analysed with SPSS software version 11.0 (SPSS Inc, Chicago, Illinois, USA).
RESULTS
Study patients
Of the 590 patients who underwent ICD surgery at the Cleveland Clinic during the time of this study, 345 met the necessary inclusion criteria and were enrolled in the study. The most common reasons for exclusion were upgrade to or implantation of a biventricular ICD (n = 102, 17.3%), recent cardiac surgery within 60 days of implantation (n = 71, 12%), and remote residence that prevented follow up in one of our device clinics (n = 51, 8.6%). Some patients had more than one reason for exclusion. All enrolled patients underwent the routine follow up. Appropriate ICD therapies were delivered in 63 of the 345 enrolled patients (18.3%), with antitachycardia pacing in 11, antitachycardia pacing with shock(s) in 14, and shock(s) in 38. The mean number of shocks for each patient was 2 (4). Mean time to ICD delivered therapy was 8.2 (4.6) months. No events were excluded because of disagreement between the interpreting electrophysiologists. Table 1 presents baseline characteristics of patients with and without appropriate ICD delivered therapy.
Table 1 Baseline characteristics of patients who did and did not receive therapy from an implantable cardioverter‐defibrillator (ICD).
| Characteristic | ICD therapy (n = 63) | No ICD therapy (n = 282) | p Value |
|---|---|---|---|
| Age (years) | 61 (13) | 64 (13) | 0.22 |
| Men | 54 (86%) | 234 (83%) | 0.72 |
| Primary prevention | 34 (54%) | 147 (52%) | 0.68 |
| History of CHF | 48 (76%) | 226 (80%) | 0.57 |
| CAD | 40 (63%) | 206 (73%) | 0.19 |
| Dilated CM | 14 (22%) | 45 (16%) | 0.14 |
| Valvar/other CM | 9 (14%) | 31 (11%) | 0.29 |
| NYHA class | |||
| I | 15 (24%) | 60 (21%) | 0.12 |
| II | 20 (32%) | 131 (46%) | |
| III | 24 (38%) | 83 (29%) | |
| IV | 4 (6%) | 8 (3%) | |
| LVEF (%) | 23 (11) | 30 (13) | 0.048* |
| β Blocker | 47 (75%) | 197 (70%) | 0.49 |
| Amiodarone | 13 (21%) | 114 (40%) | 0.033* |
| Other antiarrhythmic | 11 (17%) | 28 (10%) | 0.16 |
| ACE inhibitor | 38 (60%) | 183 (65%) | 0.58 |
| Statin | 29 (46%) | 147 (52%) | 0.71 |
| Creatinine (μmol/l) | 124 (71) | 115 (80) | 0.65 |
| BNP (ng/l)† | 573 (205–824) | 243 (103–518) | 0.0003* |
| CRP (mg/l)† | 2.2 (0.35–8.4) | 1.75 (0.47–4.4) | 0.57 |
Data are mean (SD), number (%), or median (interquartile range).
*Significant difference.
ACE, angiotensin converting enzyme; BNP, B‐type natriuretic peptide; CAD, coronary artery disease; CHF, congestive heart failure; CM, cardiomyopathy; CRP, C reactive protein; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
There were no significant differences in the ICD tachycardia therapy programming between the two groups (table 2). The number of tachycardia zones and the cut off values for VT and VF detection were not statistically different. The percentage of patients with antitachycardia pacing programmed in the VT zone(s) was also similar between groups (not significant).
Table 2 Comparison of device settings in patients with and without ICD therapies.
| ICD setting | ICD therapy (n = 63) | No ICD therapy (n = 282) | p Value |
|---|---|---|---|
| Number of tachycardia zones | 2.2 (0.9) | 2.3 (0.9) | 0.89 |
| VF detection setting (beats/min) | 190 (14) | 192 (15) | 0.45 |
| VT detection setting (beats/min) | 164 (29) | 161 (27) | 0.20 |
| Antitachycardia pacing programmed in VT zone(s) | 82.5% | 86.1% | 0.14 |
Data are mean (SD).
VF, ventricular fibrillation; VT, ventricular tachycardia.
Age, sex distribution, history of coronary artery disease (CAD), non‐ischaemic dilated cardiomyopathy (NICM), or CHF did not differ significantly. Interestingly, the proportion of patients with a primary prevention indication for their ICD also did not differ, with 54.0% in the therapy group versus 52.1% in the no therapy group (p = 0.68). Patients in the therapy group had significantly lower EF than did those without therapy (23% v 30%, p = 0.048). A significantly higher number of patients in the no therapy group (40.4%) than in the therapy group (20.6%, p = 0.033) were taking amiodarone. The distribution of NYHA class did not differ significantly between groups (p = 0.12). β Blocker, angiotensin converting enzyme inhibitor, or statin use also did not differ significantly. Serum creatinine was also similar in the two groups.
Serum biomarkers
Table 1 presents serum biomarker concentrations in the two groups. Preimplantation plasma BNP concentration was significantly higher in patients who had an appropriate ICD delivered therapy than in patients who did not have any therapy (median 573 ng/l v 243 ng/l, p = 0.0003). Preimplantation plasma CRP concentration was slightly higher in patients with ICD therapy than in those without, but this difference was not significant (median 2.2 mg/l v 1.7 mg/l, p = 0.57).
Multivariate analysis
Cox regression analysis was performed to obtain adjusted hazard ratios for several clinical variables by forcing them into the model: age, sex, ICD indication, LV function, CAD, NICM, CHF history, advanced NYHA class (III or IV), β blocker use, amiodarone use, plasma BNP concentration, and plasma CRP concentration. Table 3 lists hazard ratios, p values, and their respective 95% CIs. Only a BNP concentration greater than the 50th centile was independently associated with a higher risk of appropriate ICD therapy (hazard ratio 2.19, 95% CI 1.07 to 4.71, p = 0.040). The unadjusted hazard ratio for BNP determined by univariate Cox analysis was 2.61 (95% CI 1.29 to 5.29, p = 0.008). In forward, stepwise Cox regression analysis of the same variables listed in table 3, amiodarone, CAD, EF, and NICM all had p < 0.10 and were therefore identified as potential confounders. However, after adjustment for these four variables in a forced entry model, BNP remained independently predictive (hazard ratio 2.89, p = 0.005). BNP was also adjusted separately for variables significantly associated with high BNP concentrations (namely, age, history of CHF, advanced NYHA class, EF, amiodarone use, and creatinine, identified from table 4); BNP remained independently predictive (hazard ratio 2.11, p = 0.046).
Table 3 Multivariate analysis of predictors of appropriate defibrillator therapy.
| Variable | Hazard ratio | p Value | 95% CI |
|---|---|---|---|
| BNP (upper 50th centile) | 2.19 | 0.040 | 1.07 to 4.71* |
| CAD | 1.82 | 0.21 | 0.71 to 6.54 |
| NYHA III/IV | 1.77 | 0.56 | 0.57 to 2.76 |
| CRP (upper 50th centile) | 1.47 | 0.30 | 0.71 to 3.06 |
| History of CHF | 1.40 | 0.56 | 0.45 to 4.41 |
| ACE inhibitor use | 1.23 | 0.60 | 0.57 to 2.67 |
| Age (per decade) | 1.09 | 0.58 | 0.80 to 1.50 |
| ICD indication (primary) | 0.95 | 0.89 | 0.45 to 2.01 |
| LVEF (per 5%) | 0.85 | 0.11 | 0.70 to 1.04 |
| Dilated CM | 0.69 | 0.59 | 0.17 to 2.76 |
| β Blocker use | 0.64 | 0.28 | 0.28 to 1.44 |
| Sex (male) | 0.45 | 0.18 | 0.14 to 1.44 |
| Amiodarone use | 0.41 | 0.068 | 0.15 to 1.10 |
*Significant difference.
CI, confidence interval.
Table 4 Patient characteristics by BNP centile.
| Characteristic | BNP centile | p Value | |
|---|---|---|---|
| <50th (n = 172) | >50th (n = 173) | ||
| ICD therapy† | 17 (10%) | 46 (27%) | 0.006* |
| Age (years) | 61 (11) | 65 (13) | 0.020* |
| Men | 141 (82%) | 147 (85%) | 0.57 |
| History of CHF | 122 (71%) | 152 (88%) | 0.0012* |
| CAD | 121 (70%) | 125 (72%) | 0.76 |
| Dilated CM | 33 (19%) | 26 (15%) | 0.59 |
| NYHA III/IV | 40 (23%) | 73 (42%) | 0.006* |
| LVEF (%) | 30 (14) | 23 (11) | 0.0010* |
| β Blocker | 121 (70%) | 123 (71%) | 0.99 |
| Amiodarone | 48 (28%) | 79 (46%) | 0.015* |
| ACE inhibitor | 114 (66%) | 107 (62%) | 0.68 |
| Creatinine (μmol/l) | 106 (71) | 124 (80) | 0.032* |
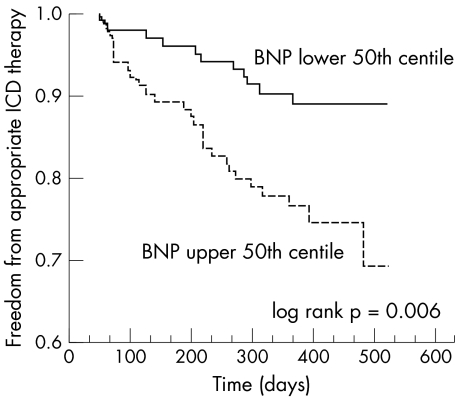
*Significant difference; †comparison of ICD therapy by time to event analysis versus log rank test (fig 1).
Plasma CRP concentration in the upper 50th centile was not independently predictive of ICD therapy when adjusted for the other clinical variables (p = 0.30). When BNP and EF were sequentially removed from the model, CRP still failed to be significantly predictive. Advanced NYHA class (III or IV) was also not independently predictive (p = 0.56). When the model was repeated with individual NYHA classes instead, none of the individual NYHA classes was independently predictive either.
BNP analysis
Occurrence of ICD therapy and clinical characteristics were compared according to plasma BNP concentrations in the upper and lower 50th centiles. Table 4 and fig 1 show the results. The 50th centile BNP concentration was 283 ng/l. As fig 1 shows, patients with a BNP concentration above the 50th centile were much more likely to have an appropriate ICD therapy (log rank p = 0.006). Patients with a BNP concentration above the 50th centile were also older, had lower left ventricular EF, had more advanced NYHA class (III and IV), and were more likely to have a history of CHF and to be taking amiodarone. The two groups did not differ significantly in sex, CAD, NICM, β blocker use, or angiotensin converting enzyme inhibitor use. Serum creatinine concentration was slightly higher in the upper 50th centile group than in the lower 50th centile (124 μmol/l v 106 μmol/l, p = 0.032).
Figure 1 Kaplan‐Meier time to event curves comparing freedom from appropriate implantable cardioverter‐defibrillator (ICD) therapy in patients with B‐type natriuretic peptide (BNP) concentrations in the upper and lower 50th centiles (median BNP 263 ng/l). Patients with serum BNP in the upper 50th centile had a significantly higher incidence of ICD therapy over time than those in the lower 50th centile (log rank p = 0.006).
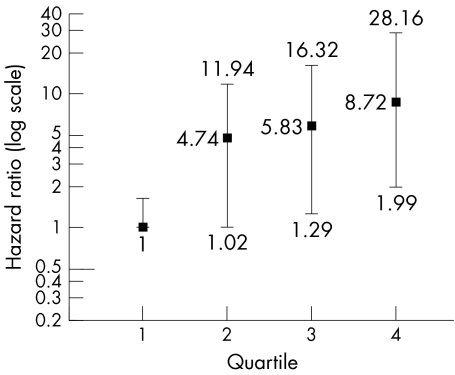
After adjustment for all of the clinical variables included in the multivariate analysis, the hazard ratios of ICD shock for the quartiles of BNP plasma concentrations were compared with those of the lowest quartile. The hazard ratios for quartiles two through four were 4.74, 5.83, and 8.72, respectively, as fig 2 shows (p value for trend 0.006). Median BNP concentrations for quartiles one through four were 60, 192, 450, and 1179 ng/l, respectively. The unadjusted hazard ratio for being in the first quartile (versus not) showed that these patients were much less likely to experience ICD therapy (hazard ratio 0.15, p = 0.009). In contrast, the unadjusted hazard ratio for being in the highest quartile (versus not) was 2.33 (p = 0.01) suggesting a much higher risk of ICD therapy.
Figure 2 Hazard ratios of having an appropriate ICD therapy by increasing quartiles of BNP compared with the lowest quartile after adjustment for age, sex, ICD indication, type of cardiomyopathy, ejection fraction, advanced New York Heart Association class, adjuvant drug treatment, and C reactive protein concentration. The p value for the trend was 0.006.
CRP analysis
CRP was not predictive by univariate Cox analysis (hazard ratio 1.19, 95% CI 0.62 to 2.27, p = 0.60). Quartile analysis for CRP failed to show any significant trend between incidence of ICD therapy and increasing quartile levels. Even when the highest quartile of CRP (> 4.6 mg/l) was used in the multivariate model instead of the upper 50th centile, CRP remained a non‐significant predictor of ICD therapy (p = 0.55).
DISCUSSION
Main findings
The main finding of this study is that a single determination of plasma BNP concentration obtained before ICD implantation is the strongest independent predictor of future appropriate ICD therapies. Patients with BNP concentrations above the 50th centile were at significantly higher risk of ICD therapy. A dose–response relation existed, with risk of ICD therapy increasing with higher BNP concentrations. Importantly, plasma BNP is a stronger predictor than age, EF, NYHA class, primary or secondary indication for ICD, β blocker usage, and amiodarone usage. CRP was not a significant independent predictor either by univariate or by multivariate analysis adjusted for other clinical variables. These are important findings that show the ability of a serum marker to predict future arrhythmic events amenable to ICD therapy.
Predictors of ICD therapies
Several studies have examined risk factors for occurrence of appropriate ICD therapies in ICD patients but the results have not always been consistent. EF seems to be the most reliable predictor of ICD therapies, having been described by several groups.6,7,9,11 Use of amiodarone or β blockers has also been shown to be a negative predictor of ICD shock.5,8 However, clinical variables such as age, type of cardiomyopathy, presenting arrhythmia, and sex have not been reliably identified as independent ICD therapy predictors. Our ICD event rates and patient characteristics are consistent with the literature, suggesting that this is a typical population of ICD patients. Our finding that both amiodarone use and low EF were univariate predictors of ICD therapy, while other variables were not, is also consistent with previous studies.
Recently, NYHA class (particularly class III or above) has been identified as a predictor of ICD shocks, but antitachycardia pacing therapy was not included in the outcome.9 It has also been suggested that patients in NYHA class I–II may have fewer shocks, but this study did not perform multivariate analysis to adjust for EF or other key variables.7 Our study found that NYHA classification was not independently predictive of ICD therapy when adjusted for BNP, EF, and other variables. This is consistent with observations that BNP can predict mortality in patients with CHF independent of functional status17 and can even predict adverse cardiac events in patients without overt heart failure.14,18
To our knowledge, no previous studies have specifically examined the predictive power of BNP or CRP on future ICD therapies. The fact that a single, preimplantation BNP determination was the only predictor of ICD events, even when compared with EF and NYHA class, emphasises the utility of this measurement. Furthermore, NYHA class can be subjective and inconsistent between health care providers.19 BNP offers an objective and quantitative method to assess future ICD therapy risk. We also included a broad range of patients in our study population (primary and secondary prevention, different cardiomyopathies), implying that BNP may be applicable to a wide range of ICD patients.
BNP as a clinical predictor
BNP is released largely from the ventricles in response to increases in intraventricular pressure and myocardial stretch.20 Changes in ventricular pressure and geometry also cause electrophysiological changes that may lead to enhanced arrhythmogenesis, such as enhanced refractoriness, slower conduction, and increased afterdepolarisations.21,22 Eccentric ventricular remodelling (dilatation), in particular, has been shown to be an important predictor of future ICD shock, whereas concentric ventricular hypertrophy may be a negative predictor.23 Therefore, it is reasonable that BNP can predict patients most likely to develop ventricular arrhythmias amenable to ICD therapy.
BNP has already been shown to predict independently an increase in adverse cardiac events and total mortality in various patient populations, including those with CHF,13 acute coronary syndromes,15 and no symptomatic cardiovascular disease.14 In particular, BNP has been found to be the only independent predictor of sudden death in a large number of patients with chronic CHF.16 Recently, another study showed that BNP independently predicted sudden death in mild to moderate CHF, even when compared with assessment of peak oxygen consumption.17 However, neither study specified arrhythmic death as an outcome. Our study shows that BNP can predict future malignant ventricular arrhythmias in a population with cardiomyopathies.
In contrast, CRP was not independently predictive when compared with BNP. Increased CRP concentrations have been shown to predict sudden death in healthy men,24 but many of these events may have been related to acute coronary events. CRP does predict all cause mortality plus hospitalisation in patients with CHF12 but has not been shown specifically to predict lethal arrhythmic events. Perhaps inflammation has less of a role than ventricular stretch and loading in ventricular arrhythmias in patients with CHF.
Clinical implications
The observation that plasma BNP concentration in the upper 50th centile was the major risk factor for future, appropriate ICD therapy has important clinical implications. By identifying those at most risk, a potential opportunity for pre‐emptive measures to avoid ICD discharges may exist. Perhaps more aggressive antitachycardia pacing protocols can be programmed to reduce the need for shock therapy. Since BNP concentrations are directly related to myocardial stretch, our data imply that fluid balance and haemodynamic status may be important factors in causing future arrhythmic events. By maintaining optimal haemodynamic status in ICD patients, it is possible that future ICD therapies can be avoided. However, we cannot conclude whether these measures or others that reduce BNP concentration will prevent future ICD therapies on the basis of these data alone. Lastly, as the indications for ICD therapy expand, concerns are growing about the financial impact that these expanded indications will have on the health care system. Use of serum markers may have a role in helping to select and focus limited resources on those patients who can benefit most from ICDs.
Study limitations
The utility of BNP was not compared with that of invasive testing, such as programmed ventricular stimulation. However, most of our patients did not undergo invasive testing before implantation and most recent trials and guidelines have changed to bypass invasive testing altogether for ICD implantation.3 Data on invasive testing have also shown it to have limited sensitivity in predicting future arrhythmic events.25 Serum markers have the great advantage of being less expensive, less invasive, and more practical for predicting risk in patients who are already undergoing ICD implantation. Although the majority of ICD events in our study were shocks (82%), our end point was both shocks and appropriate antitachycardia pacing. This means we probably included some arrhythmic events composed of slower, more stable VTs as opposed to exclusively analysing rapid VT or VF events that are more often life threatening. However, prediction of slower VTs may be equally relevant given that they too can cause adverse haemodynamic consequences and may encourage programming of more aggressive antitachycardia pacing protocols.
The incidence of ICD therapies may seem lower (18%) in our 13 month series than in other secondary prevention trials. In the AVID (antiarrhythmics versus implantable defibrillators) study, for example, 39% and 26% of patients presenting with VT and VF, respectively, had appropriate shocks after one year.26 However, our population received ICDs for a mix of primary and secondary prevention, and in primary prevention trials such as SCD‐HeFT (sudden cardiac death in heart failure trial), the annual rate of appropriate shock was only 5%.27 Furthermore, the number of patients taking antiarrhythmics and β blockers in our series is almost double that in AVID.1
We also excluded patients implanted with biventricular ICDs and, thus, we cannot assess the potential impact of cardiac resynchronisation therapy on either BNP concentrations or the future risk of appropriate ICD therapies. Furthermore, while postulating the importance of haemodynamic status on occurrence of arrhythmic events in this population may be reasonable, we cannot conclude whether measures that reduce BNP concentration will prevent future ICD therapies on the basis of these data alone.
Lastly, the duration of follow up in this study was limited to just over 13 months, which is short relative to larger ICD clinical trials. The shorter duration of follow up may explain the lower rate of ICD therapies observed in our cohort relative to other studies. Perhaps with a longer follow up and a larger number of events, the predictive power of BNP for ICD therapy may have been determined with more precision. For example, the predictive value of BNP over a longer follow up time is not known. Specifically, with a larger sample and event size, we may have been able to identify specific cut off concentrations of BNP that define increased risk. However, the fact that BNP proved to be a powerful independent predictor despite small numbers of ICD events and a short follow up seems to imply that BNP can be useful for risk stratifying these patients.
Conclusions
A single preimplantation determination of BNP concentration is independently predictive of ICD therapies in patients with cardiomyopathies undergoing first time ICD implantation. CRP was not independently predictive of ICD therapies when compared with BNP.
Abbreviations
AVID - antiarrhythmics versus implantable defibrillators
BNP - B‐type natriuretic peptide
CAD - coronary artery disease
CHF - congestive heart failure
CI - confidence interval
CRP - C reactive protein
EF - ejection fraction
ICD - implantable cardioverter‐defibrillator
NICM - non‐ischaemic dilated cardiomyopathy
NYHA - New York Heart Association
SCD‐HeFT - sudden cardiac death in heart failure trial
VF - ventricular fibrillation
VT - ventricular tachycardia
Footnotes
No competing interests to be declared for this study. No financial support was received for this study. No potential conflicts of interest arise from the publication of this manuscript.
Ethics approval: This study was conducted under the specific ethics guidelines of our institutional review board at the Cleveland Clinic Foundation.
References
- 1.AVID Investigators A comparison of antiarrhythmic‐drug therapy with implantable defibrillators in patients resuscitated from near‐fatal ventricular arrhythmias. The Antiarrhythmics versus implantable defibrillators (AVID) investigators. N Engl J Med 19973371576–1583. [DOI] [PubMed] [Google Scholar]
- 2.Kadish A, Dyer A, Daubert J P.et al Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 20043502151–2158. [DOI] [PubMed] [Google Scholar]
- 3.Moss A J, Zareba W, Hall W J.et al Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002346877–883. [DOI] [PubMed] [Google Scholar]
- 4.Anvari A, Gottsauner‐Wolf M, Turel Z.et al Predictors of outcome in patients with implantable cardioverter defibrillators. Cardiology 199890180–186. [DOI] [PubMed] [Google Scholar]
- 5.Dolack G L. Clinical predictors of implantable cardioverter‐defibrillator shocks (results of the CASCADE trial). Cardiac arrest in Seattle, conventional versus amiodarone drug evaluation. Am J Cardiol 199473237–241. [DOI] [PubMed] [Google Scholar]
- 6.Grimm W, Flores B T, Marchlinski F E. Shock occurrence and survival in 241 patients with implantable cardioverter‐defibrillator therapy. Circulation 1993871880–1888. [DOI] [PubMed] [Google Scholar]
- 7.Levine J H, Mellits E D, Baumgardner R A.et al Predictors of first discharge and subsequent survival in patients with automatic implantable cardioverter‐defibrillators. Circulation 199184558–566. [DOI] [PubMed] [Google Scholar]
- 8.Rankovic V, Karha J, Passman R.et al Predictors of appropriate implantable cardioverter‐defibrillator therapy in patients with idiopathic dilated cardiomyopathy. Am J Cardiol 2002891072–1076. [DOI] [PubMed] [Google Scholar]
- 9.Whang W, Mittleman M A, Rich D Q.et al Heart failure and the risk of shocks in patients with implantable cardioverter defibrillators: results from the triggers of ventricular arrhythmias (TOVA) study. Circulation 20041091386–1391. [DOI] [PubMed] [Google Scholar]
- 10.Reiter M J, Fain E S, Senelly K M.et al Predictors of device activation for ventricular arrhythmias and survival in patients with implantable pacemakers/defibrillators. CADENCE investigators. Pacing Clin Electrophysiol 1994171487–1498. [DOI] [PubMed] [Google Scholar]
- 11.Freedberg N A, Hill J N, Fogel R I.et al Recurrence of symptomatic ventricular arrhythmias in patients with implantable cardioverter defibrillator after the first device therapy: implications for antiarrhythmic therapy and driving restrictions. CARE Group. J Am Coll Cardiol 2001371910–1915. [DOI] [PubMed] [Google Scholar]
- 12.Yin W H, Chen J W, Jen H L.et al Independent prognostic value of elevated high‐sensitivity C‐reactive protein in chronic heart failure. Am Heart J 2004147931–938. [DOI] [PubMed] [Google Scholar]
- 13.Yu C M, Sanderson J E. Plasma brain natriuretic peptide‐‐an independent predictor of cardiovascular mortality in acute heart failure. Eur J Heart Fail 1999159–65. [DOI] [PubMed] [Google Scholar]
- 14.Wang T J, Larson M G, Levy D.et al Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 2004350655–663. [DOI] [PubMed] [Google Scholar]
- 15.Tapanainen J M, Lindgren K S, Makikallio T H.et al Natriuretic peptides as predictors of non‐sudden and sudden cardiac death after acute myocardial infarction in the beta‐blocking era. J Am Coll Cardiol 200443757–763. [DOI] [PubMed] [Google Scholar]
- 16.Berger R, Huelsman M, Strecker K.et al B‐type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation 20021052392–2397. [DOI] [PubMed] [Google Scholar]
- 17.Isnard R, Pousset F, Chafirovskaia O.et al Combination of B‐type natriuretic peptide and peak oxygen consumption improves risk stratification in outpatients with chronic heart failure. Am Heart J 2003146729–735. [DOI] [PubMed] [Google Scholar]
- 18.Wallen T, Landahl S, Hedner T.et al Brain natriuretic peptide predicts mortality in the elderly. Heart 199777264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibelin P. An evaluation of symptom classification systems used for the assessment of patients with heart failure in France. Eur J Heart Fail 20013739–746. [DOI] [PubMed] [Google Scholar]
- 20.Vanderheyden M, Bartunek J, Goethals M. Brain and other natriuretic peptides: molecular aspects. Eur J Heart Fail 20046261–268. [DOI] [PubMed] [Google Scholar]
- 21.Hansen D E, Craig C S, Hondeghem L M. Stretch‐induced arrhythmias in the isolated canine ventricle: evidence for the importance of mechanoelectrical feedback. Circulation 1990811094–1105. [DOI] [PubMed] [Google Scholar]
- 22.Zhu W X, Johnson S B, Brandt R.et al Impact of volume loading and load reduction on ventricular refractoriness and conduction properties in canine congestive heart failure. J Am Coll Cardiol 199730825–833. [DOI] [PubMed] [Google Scholar]
- 23.Dogra V, Oliver R, Lapidus J.et al Apparent protective effect of increased left ventricular wall thickness in an ICD population. J Card Fail 20039412–415. [DOI] [PubMed] [Google Scholar]
- 24.Albert C M, Ma J, Rifai N.et al Prospective study of C‐reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation 20021052595–2599. [DOI] [PubMed] [Google Scholar]
- 25.Bailey J J, Berson A S, Handelsman H.et al Utility of current risk stratification tests for predicting major arrhythmic events after myocardial infarction. J Am Coll Cardiol 2001381902–1911. [DOI] [PubMed] [Google Scholar]
- 26.Klein R C, Raitt M H, Wilkoff B L.et al Analysis of implantable cardioverter defibrillator therapy in the antiarrhythmics versus implantable defibrillators (AVID) trial. J Cardiovasc Electrophysiol 200314940–948. [DOI] [PubMed] [Google Scholar]
- 27.Bardy G H, Lee K L, Mark D B.et al Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med 2005352225–237. [DOI] [PubMed] [Google Scholar]