Abstract
Objective
To assess the potential relation between plaque composition and vascular remodelling by using spectral analysis of intravascular ultrasound (IVUS) radiofrequency data.
Methods and results
41 coronary vessels with non‐significant (< 50% diameter stenosis by angiography), ⩽ 20 mm, non‐ostial lesions located in non‐culprit vessels underwent IVUS interrogation. IVUS radiofrequency data obtained with a 30 MHz catheter, were analysed with IVUS virtual histology software. A remodelling index (RI) was calculated and divided into three groups. Lesions with RI ⩾ 1.05 were considered to have positive remodelling and lesions with RI ⩽ 0.95 were considered to have negative remodelling. Lesions with RI ⩾ 1.05 had a significantly larger lipid core than lesions with RI 0.96–1.04 and RI ⩽ 0.95 (22.1 (6.3) v 15.1 (7.6) v 6.6 (6.9), p < 0.0001). A positive correlation between lipid core and RI (r = 0.83, p < 0.0001) and an inverse correlation between fibrous tissue and RI (r = −0.45, p = 0.003) were also significant. All of the positively remodelled lesions were thin cap fibroatheroma or fibroatheromatous lesions, whereas negatively remodelled lesions had a more stable phenotype, with 64% having pathological intimal thickening, 29% being fibrocalcific lesions, and only 7% fibroatheromatous lesions (p < 0.0001).
Conclusions
In this study, in vivo plaque composition and morphology assessed by spectral analysis of IVUS radiofrequency data were related to coronary artery remodelling.
Keywords: ultrasonography, remodelling, atherosclerosis, plaque characterisation
Glagov et al1 described vascular remodelling as a compensatory enlargement of the coronary arteries in response to an increase in plaque area. This concept has further evolved into a dynamic theory whereby vessels may also shrink in response to plaque growth.2 This remodelling modality has been related to a more stable phenotype and clinical presentation,3,4,5,6 whereas several studies showed an increase in inflammatory marker concentrations, larger lipid cores, and pronounced medial thinning in positively remodelled vessels.4,5,7
Recently, retrospective pathological studies have identified morphological and compositional features characteristic of plaque rupture.8,9 This has led to a new classification of coronary lesions that more comprehensively illustrates plaque progression.9
Grey scale intravascular ultrasound (IVUS) is of limited value for identification of specific plaque components.10 However, spectral analysis of IVUS radiofrequency data (IVUS virtual histology (VH)) has the potential to provide detailed quantitative information on plaque composition and has been validated in explanted human coronary segments.11
In this study, we sought to evaluate in vivo the relation between plaque composition and coronary artery remodelling by using ultrasound radiofrequency data analysis. In addition, we classified lesions with respect to their morphology and evaluated the potential relation between lesion type and coronary remodelling.9
METHODS
Patients
Forty one consecutive patients were retrospectively selected after screening a 54 patient database where non‐culprit, angiographically non‐obstructive (<50%), ⩽ 20 mm, non‐ostial lesions were investigated with IVUS. Patients were excluded if they had diffusely diseased vessels or lacked a lesion occluding ⩾ 40% of the cross sectional area (CSA). Lesions located in proximal and mid segments of a coronary artery were included in the study.
Major exclusion criteria were coronary anatomy that precluded safe IVUS examination of a suitable region of interest. Informed, written consent was obtained from all the patients.
IVUS‐VH acquisition and analysis
Details regarding the validation of the technique on explanted human coronary segments have previously been reported.11 Briefly, IVUS‐VH uses spectral analysis of IVUS radiofrequency data to construct tissue maps that classify plaque into four major components. In preliminary in vitro studies, four histological plaque components (fibrous, fibrolipidic, lipid core, and calcified) were correlated with a specific spectrum of the radiofrequency signal.11 These plaque components were assigned colour codes. Calcified, fibrous, fibrolipidic, and lipid core regions were labelled white, green, greenish yellow, and red, respectively.
IVUS‐VH data were acquired after intracoronary administration of nitrates by means of a continuous pullback (0.5 mm/s) with a commercially available mechanical sector scanner (Ultracross 2.9 French, 30 MHz catheter; Boston Scientific, Santa Clara, California, USA) by a dedicated IVUS‐VH console (Volcano Therapeutics, Rancho Cordova, California, USA). The IVUS‐VH data were stored on a CD ROM and sent to the imaging core laboratory for offline analysis. IVUS B mode images were reconstructed from the radiofrequency data by customised software (IVUSLab, Volcano Therapeutics). Subsequently, contours of both the lumen and the media–adventitia interface were detected manually. To account for catheter to catheter variability the acquired radiofrequency data were normalised by a technique known as “blind deconvolution”. Blind deconvolution is an iterative algorithm that deconvolves the catheter transfer function from the backscatter, thus enabling automated data normalisation.12,13 Compositional data of the minimum lumen area (MLA) were expressed as percentage of the plaque CSA corresponding to each plaque component.
The MLA site and a reference site ⩽ 10 mm proximal to the lesion were selected. There were no major side branches between the MLA and reference sites.
Remodelling was assessed by means of the remodelling index (RI), expressed as the external elastic membrane CSA (MLA site) divided by the reference external elastic membrane CSA as previously described.6,14,15.
We defined positive remodelling as RI ⩾ 1.05 and negative remodelling as RI ⩽ 0.95. Values in between were considered neutral (no remodelling). Percentage stenosis of the MLA site was defined as:
vesselareaMLA − lumenareaMLA/vesselareaMLA × 100.
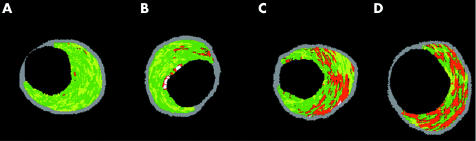
In accordance with previously reported data, we classified lesions as pathological intimal thickening (mainly fibrotic–fibrolipidic tissue, with the lipid core constituting 0% to ⩽ = 3% of the CSA), fibrocalcific lesions (featuring mainly fibrotic plaques, with some calcification and a lipid core occupying between 3–10% of the CSA), fibrous cap atheroma (lipid rich (> 10% CSA) plaques with overlying fibrous tissue), and thin cap fibroatheroma (lipid‐rich (> 10% CSA) plaques with no overlying fibrous tissue). Figure 1 depicts examples of this classification. To classify lesions, these criteria had to be met in the MLA site plus the immediate distal and proximal cross sections. Since the axial resolution of this technique is between 100–150 μm, we assumed that the absence of fibrous tissue overlying a lipid core suggested a cap thickness of below 100–150 μm.16
Figure 1 Minimum lumen area (MLA) sites depicting the progression of atherosclerotic disease. The plaque components were assigned colour codes. Calcified, fibrous, fibrolipidic, and lipid core regions were labelled white, green, greenish yellow, and red, respectively. MLA sites feature (A) pathological intimal thickening and (B) fibrocalcific, (C) fibroatheromatous, and (D) thin cap fibroatheromatous lesions.
Statistical analysis
Discrete variables are presented as counts and percentages. Continuous variables are presented as mean (SD). We looked for correlations between the RI and both plaque components and percentage stenosis MLA by using Pearson correlation coefficients. Differences in means between groups were analysed by a two sided t test or by one way analysis of variance. We compared frequencies by means of the χ2 test. A probability value of p < 0.05 indicated significance. Data were statistically analysed with SPSS software version 11.5 (SPSS Inc, Chicago, Illinois, USA).
RESULTS
Table 1 shows patient characteristics. Mean age was 55.9 (10.9). Most patients were men (83%) with a low prevalence of diabetes (7.3%). The study vessel was the right coronary artery in 19 patients (46.3%), the left anterior descending in 16 patients (39.0%), and the left circumflex in six patients (14.6%).
Table 1 Baseline characteristics (n = 41).
| Age (years) | 55.9 (10.9) |
| Men | 19 (83%) |
| Diabetes | 3 (7.3%) |
| Hypertension* | 12 (29.3%) |
| Current smoking | 8 (19.5%) |
| Previous smoking | 15 (36.6%) |
| Hypercholesterolaemia† | 32 (78%) |
| Family history of coronary disease | 19 (46.3%) |
| Previous myocardial infarction | 6 (14.6%) |
| Artery | |
| Right coronary | 19 (46.3%) |
| Left anterior descending | 16 (39%) |
| Left circumflex | 6 (14.6%) |
| Clinical presentation | |
| No angina‡ | 11 (26.8%) |
| Stable angina | 14 (34.1%) |
| Unstable angina | 6 (14.6%) |
| Myocardial infarction | 10 (24.4%) |
Data are mean (SD) or number (%).
*Blood pressure ⩾160/95 mm Hg or treatment for hypertension; †total cholesterol >5.57 mmol/l or treatment for hypercholesterolemia; ‡these patients were studied at scheduled follow up angiography.
Lesions with positive remodelling had significantly larger lipid core percentages than lesions with no remodelling or negative remodelling (22.1 (6.3)% v 15.1 (7.6)% v 6.6 (6.9)%, respectively, p < 0.0001). Negative remodelling lesions tended to have larger fibrous tissue percentages than lesions with no remodelling and positive remodelling (68.6 (13.7)% v 62.9 (9.5)% v 58.1 (12.9)%, p = 0.13). Table 2 shows these results.
Table 2 Geometrical and compositional data of the minimum lumen area (MLA) site.
| Remodelling index | p Value | |||
|---|---|---|---|---|
| ⩽0.95 | 0.96–1.04 | ⩾1.05 | ||
| Number | 29 (70.7%) | 3 (7.3%) | 9 (22%) | |
| Stenosis (%) | 63.1 (7.5) | 69.1 (8.6) | 59.9 (9.9) | 0.24 |
| Calcific CSA (%) | 1.38 (2.7) | 2.07 (3.2) | 1.67 (1.6) | 0.88 |
| Fibrous CSA (%) | 68.6 (13.7) | 62.9 (9.5) | 58.1 (12.9) | 0.13 |
| Fibrolipidic CSA (%) | 23.5 (9.9) | 19.9 (6.9) | 18.1 (12.6) | 0.39 |
| Lipid core CSA (%) | 6.6 (6.9) | 15.1 (7.6) | 22.1 (6.3) | <0.0001 |
Data are mean (SD).
Percentage stenosis of the MLA site is calculated as vesselareaMLA − lumenareaMLA/vesselareaMLA ×100. Remodelling index (RI) is defined as MLA of the external elastic membrane (EEM) cross sectional area (CSA)/reference EEM CSA.
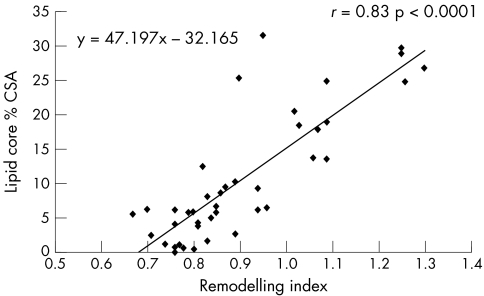
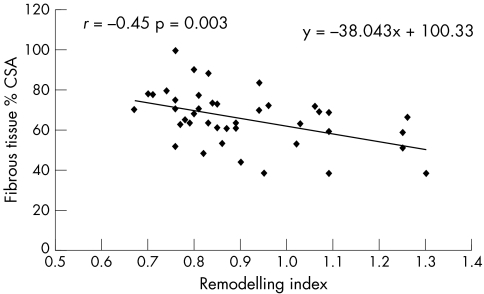
Table 3 presents Pearson correlation coefficients between the RI and both plaque components and percentage stenosis MLA. The positive correlation between the lipid core and the RI was significant (r = 0.83, p < 0.0001) (fig 2). Moreover, fibrous tissue was inversely correlated with the RI (r = −0.45, p = 0.003) (fig 3). Lastly, the percentage stenosis of the MLA and the RI were non‐significantly inversely related (r = −0.27, p = 0.09).
Table 3 Relations between remodelling index (RI), percentage stenosis of the MLA, and plaque composition of the MLA site.
| RI | p Value | |
|---|---|---|
| Lipid core CSA (%) | 0.83 | <0.0001 |
| Fibrous CSA (%) | −0.45 | 0.003 |
| Percentage stenosis MLA | −0.27 | 0.09 |
| Calcific CSA (%) | 0.12 | 0.47 |
| Fibrolipidic CSA (%) | −0.17 | 0.28 |
Data are Pearson correlation coefficients.
Figure 2 Linear regression plot showing positive correlation between lipid core and remodelling. CSA, cross sectional area. Remodelling index is defined as MLA of the external elastic membrane (EEM) CSA/reference EEM CSA.
Figure 3 Linear regression plot showing an inverse relation between fibrous tissue and remodelling.
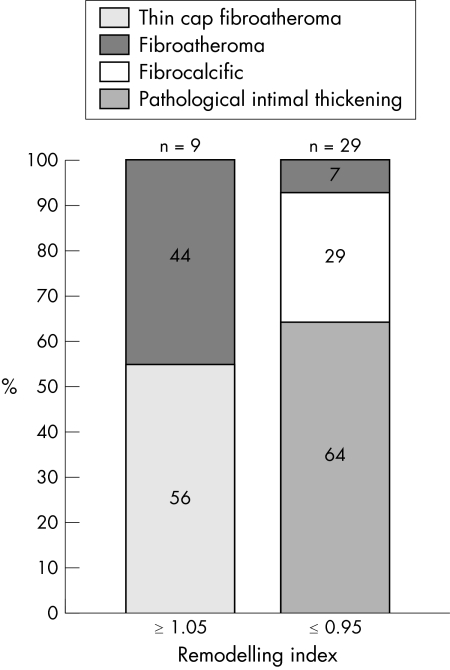
With regard to lesion type, thin cap fibroatheroma and fibroatheromatous lesions comprised 100% of the positively remodelled lesions, whereas negative remodelling lesions had a more stable phenotype: 64% had pathological intimal thickening, 29% were fibrocalcific, and only 7% were fibroatheromatous lesions (p < 0.0001) (fig 4).
Figure 4 Bar graphs illustrating the lesion type frequencies according to remodelling modality. All of the high risk plaques had positive remodelling (56% were thin cap fibroatheroma and 44% fibroatheromatous lesions). Negatively remodelled lesions had a more stable phenotype, with 93% being low risk lesions and only 7% fibroatheromatous lesions.
DISCUSSION
Recently, the relation between vascular remodelling and plaque composition was assessed by IVUS.17,18,19,20 This catheter based diagnostic tool provides an accurate tomographic view of the coronary arteries and in vitro validation studies have shown a high correlation with histological samples.21,22,23 Nevertheless, accurate plaque characterisation with visual interpretation of grey scale IVUS, particularly of lipid rich plaques, remains unresolved.22 On the contrary, spectral analysis of IVUS radiofrequency data (IVUS‐VH) has the potential to provide detailed quantitative information on plaque composition and has been validated in studies of explanted human coronary segments.11
The results of the present study confirm in vivo the relation between plaque composition and coronary remodelling. Lipid core size was significantly larger in positively remodelled coronary lesions than in those with vessel shrinkage. Furthermore, the fibrotic burden of the plaque was significantly and inversely correlated with the RI.
Lastly, positively remodelled lesions had a higher risk phenotype, with 56% of them being classified as thin cap fibroatheroma, the lesion type most likely to rupture.24 On the contrary, negative remodelling was associated with a more stable phenotype: 64% had pathological intimal thickening and no evidence of thin cap fibroatheroma. Fibrocalcific lesions, a potential hallmark of the end stage of atheromatous plaque rupture or erosion with healing and calcification, were found in 29% of negatively remodelled lesions.9
Overall, these findings support the importance of the histological composition of atherosclerotic plaque as a major contributor to its fate as described by Davies et al,8 who showed that plaques with a large lipid core harbour a higher risk of rupture and subsequent thrombosis. The lipid core is a source of metalloproteinases, a group of proteolytic enzymes that have an important function in vascular remodelling mechanisms and whose most common locations are foam cell accumulation areas and shoulder regions.25,26
Conversely, negatively remodelled vessels consisted predominantly of fibrotic plaques. In addition, in line with previously reported data, negatively remodelled lesions had a higher degree of stenosis.2,17,27 The findings of this study are consistent with previous pathological findings in patients after sudden death.5 However, such postmortem studies do not have implications in the natural history of high risk plaques and thus in the clinical outcome of patients. On the contrary, we strongly believe that the identification of these high risk plaques in vivo may provide more insights into the prognosis and natural history of such lesions and into the effect of conventional and emerging anti‐atherosclerotic pharmacological interventions.
Limitations
Since this was a cross sectional study and atherosclerosis is usually a diffuse disease, finding a fully non‐diseased reference site is not guaranteed. Therefore, we cannot rule out the early presence of remodelling in the reference site. In addition, this was a pilot study that needs further confirmation in a larger population. Moreover, classifying lesion types by this technique lacks the accuracy of histopathological classification, since resolution is inferior. Nevertheless, a significant relation was found by using this arbitrary classification. Although histopathological classification remains the ideal, spectral analysis of IVUS radiofrequency data has the potential to provide real time accurate information regarding tissue characterisation and plaque morphology.
Conclusions
In this small clinical study, in vivo plaque composition and morphology assessed by spectral analysis of IVUS radiofrequency data were related to coronary artery remodelling, supporting the role of plaque composition in the mechanisms of vessel remodelling. Lipid core size was significantly larger in positively remodelled coronary lesions than in those with vessel shrinkage. Furthermore, the fibrotic burden of the plaque was significantly and inversely correlated with the RI. The findings of this study are consistent with previous pathological findings. However, postmortem studies do not have the potential to provide prospective information about the natural history of high risk plaques. On the contrary, we strongly believe that the identification of these high risk plaques in vivo may provide more insights into the prognosis and natural history of such lesions and into the effect of conventional and emerging anti‐atherosclerotic pharmacological interventions.
Abbreviations
CSA - cross sectional area
IVUS - intravascular ultrasound
MLA - minimum lumen area
RI - remodelling index
VH - virtual histology
Footnotes
No author has any conflict of interest.
All authors have approved the final manuscript, which has not been published and is not under consideration elsewhere.
References
- 1.Glagov S, Weisenberg E, Zarins C K.et al Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 19873161371–1375. [DOI] [PubMed] [Google Scholar]
- 2.Pasterkamp G, Wensing P J, Post M J.et al Paradoxical arterial wall shrinkage may contribute to luminal narrowing of human atherosclerotic femoral arteries. Circulation 1995911444–1449. [DOI] [PubMed] [Google Scholar]
- 3.Smits P C, Pasterkamp G, Quarles van Ufford M A.et al Coronary artery disease: arterial remodelling and clinical presentation. Heart 199982461–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasterkamp G, Schoneveld A H, van der Wal A C.et al Relation of arterial geometry to luminal narrowing and histologic markers for plaque vulnerability: the remodeling paradox. J Am Coll Cardiol 199832655–662. [DOI] [PubMed] [Google Scholar]
- 5.Varnava A M, Mills P G, Davies M J. Relationship between coronary artery remodeling and plaque vulnerability. Circulation 2002105939–943. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura M, Nishikawa H, Mukai S.et al Impact of coronary artery remodeling on clinical presentation of coronary artery disease: an intravascular ultrasound study. J Am Coll Cardiol 20013763–69. [DOI] [PubMed] [Google Scholar]
- 7.Burke A P, Kolodgie F D, Farb A.et al Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation 2002105297–303. [DOI] [PubMed] [Google Scholar]
- 8.Davies M J, Richardson P D, Woolf N.et al Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J 199369377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Virmani R, Kolodgie F D, Burke A P.et al Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000201262–1275. [DOI] [PubMed] [Google Scholar]
- 10.Peters R J, Kok W E, Havenith M G.et al Histopathologic validation of intracoronary ultrasound imaging. J Am Soc Echocardiogr 19947230–241. [DOI] [PubMed] [Google Scholar]
- 11.Nair A, Kuban B D, Tuzcu E M.et al Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation 20021062200–2206. [DOI] [PubMed] [Google Scholar]
- 12.Kåresen K. Deconvolution of sparse spike trains by iterated window maximization. IEEE Trans Signal Process 1997451173–1183. [Google Scholar]
- 13.Karesen K F, Bolviken E. Blind deconvolution of ultrasonic traces accounting for pulse variance. IEEE Trans Ultrason Ferroelectr Freq Control 199946564–573. [DOI] [PubMed] [Google Scholar]
- 14.Schoenhagen P, Ziada K M, Kapadia S R.et al Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: an intravascular ultrasound study. Circulation 2000101598–603. [DOI] [PubMed] [Google Scholar]
- 15.Gussenhoven E J, Geselschap J H, van Lankeren W.et al Remodeling of atherosclerotic coronary arteries assessed with intravascular ultrasound in vitro. Am J Cardiol 199779699–702. [DOI] [PubMed] [Google Scholar]
- 16.Nair A, Calvetti D, Vince D G. Regularized autoregressive analysis of intravascular ultrasound data: improvement in spatial accuracy of plaque tissue maps. IEEE Trans Ultrason, Ferroelectr Freq Control 200451420–431. [DOI] [PubMed] [Google Scholar]
- 17.Mintz G S, Kent K M, Pichard A D.et al Contribution of inadequate arterial remodeling to the development of focal coronary artery stenoses: an intravascular ultrasound study. Circulation 1997951791–1798. [DOI] [PubMed] [Google Scholar]
- 18.Tauth J, Pinnow E, Sullebarger J T.et al Predictors of coronary arterial remodeling patterns in patients with myocardial ischemia. Am J Cardiol 1997801352–1355. [DOI] [PubMed] [Google Scholar]
- 19.Sabate M, Kay I P, de Feyter P J.et al Remodeling of atherosclerotic coronary arteries varies in relation to location and composition of plaque. Am J Cardiol 199984135–140. [DOI] [PubMed] [Google Scholar]
- 20.Fuessl R T, Kranenberg E, Kiausch U.et al Vascular remodeling in atherosclerotic coronary arteries is affected by plaque composition. Coron Artery Dis 20011291–97. [DOI] [PubMed] [Google Scholar]
- 21.Tobis J M, Mallery J A, Gessert J.et al Intravascular ultrasound cross‐sectional arterial imaging before and after balloon angioplasty in vitro. Circulation 198980873–882. [DOI] [PubMed] [Google Scholar]
- 22.Potkin B N, Bartorelli A L, Gessert J M.et al Coronary artery imaging with intravascular high‐frequency ultrasound. Circulation 1990811575–1585. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura R A, Edwards W D, Warnes C A.et al Intravascular ultrasound imaging: in vitro validation and pathologic correlation. J Am Coll Cardiol 199016145–154. [DOI] [PubMed] [Google Scholar]
- 24.Farb A, Tang A L, Burke A P.et al Sudden coronary death: frequency of active coronary lesions, inactive coronary lesions, and myocardial infarction. Circulation 1995921701–1709. [DOI] [PubMed] [Google Scholar]
- 25.Galis Z S, Khatri J J. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res 200290251–262. [PubMed] [Google Scholar]
- 26.Galis Z S, Sukhova G K, Lark M W.et al Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 1994942493–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasterkamp G, Wensing P J, Hillen B.et al Impact of local atherosclerotic remodeling on the calculation of percent luminal narrowing. Am J Cardiol 199779402–405. [DOI] [PubMed] [Google Scholar]