Takotsubo cardiomyopathy (TTC) consists of an acute onset of transient akinesia of the apical and mid portions of the left ventricle, without significant coronary artery stenosis. TTC is often accompanied by chest pain, dynamic reversible ST‐T segment abnormalities, and increased cardiac enzymes disproportionate to the extent of akinesia.1
Until now, it was believed that wall motion abnormalities (WMA) in this syndrome invariably affect the left ventricular (LV) apex. However, we present a syndrome mimicking classic TTC without involvement of the LV apex.
METHODS
We retrospectively evaluated consecutive patients admitted with an acute coronary syndrome between January 2004 and December 2004. Patients who met the following criteria were selected: firstly, reversible akinesia/dyskinesia beyond a single major coronary artery vascular distribution on left ventriculography sparing the LV apex; secondly, no coronary artery diameter stenosis > 50% on angiography; thirdly, increased cardiac enzymes; and lastly, available results of a gadolinium enhanced cardiovascular magnetic resonance (CMR) scan. Laboratory tests, serial ECGs, and echocardiography were performed according to standard protocol for management of acute coronary syndromes at our institution.
RESULTS
Four patients (three women) were identified (table 1). A triggering stressor was identifiable in three patients. All patients had ST segment abnormalities (table 1). All ST segment abnormalities returned to normal on day 3 except for the negative T waves in patient 4, which persisted during hospitalisation but had disappeared on a follow up ECG six months later. Mean time delay between onset of symptoms and left heart catheterisation was 8.6 hours (range 3.5–13). Regional LV akinesia was observed in all patients (table 1, figs 1 and 2). Coronary angiography was unremarkable in three patients and showed mild arteriosclerosis with no significant stenosis in one patient (patient 4). Echocardiography showed complete resolution of WMA in patient 3 (day 16) and patient 4 (day 2), mild basal‐septal hypokinesia in patient 1 (day 5), and moderate hypokinesia of the posterior and lateral segments in patient 4 (day 5). WMA in patient 4 had disappeared on a follow up echocardiogram six months later. Mean time delay between presentation and CMR was 10 days (range 2–14 days). WMA resolved or improved significantly in all patients. Areas of delayed hyperenhancement were not detected in any patient. One patients had mildly increased inflammatory markers. None of the patients recalled flu‐like symptoms within eight weeks before admission or noted fatigue or malaise on admission.
Table 1 Patients' characteristics.
| Characteristic | Patient number | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Age (years) | 55 | 51 | 41 | 77 |
| Sex | Female | Female | Female | Male |
| Chest pain | No | Yes | Yes | Yes |
| ECG abnormalities (leads) | ||||
| ST elevation | aVR, aVL | V2, V3 | No | No |
| ST depression | II, III, aVF, V3–V6 | No | V2, V3, V4 | V5, V6 |
| T wave inversion | aVR, aVL, V2 | No | No | II, III, aVF, V5, V6 |
| QT prolongation | Max QTc = 511 ms | No | Max QTc = 493 ms | No |
| Peak creatine kinase (U/l) (normal range 0–145) | 550 | 260 | 275 | 154 |
| Peak troponin I (μg/l) (normal range 0–0.4) | 21.2 | 1.85 | 2.63 | 1.55 |
| C reactive protein (mg/l) (normal range 0–5) | 1 | 1 | 7 | 2 |
| Leucocyte count (×109/l) (normal range 3.6–11.0) | 10.5 | 8.2 | 12.6 | 11.0 |
| Left ventricular WMA | Basal | Anterolateral | Basal | Posterobasal |
| Mid‐portion | Diaphragm | Mid‐portion | Posterolateral | |
| Triggering factor | Colonoscopy | Stressful job | Neck pain | Unknown |
Max, maximum; WMA, wall motion abnormalities.
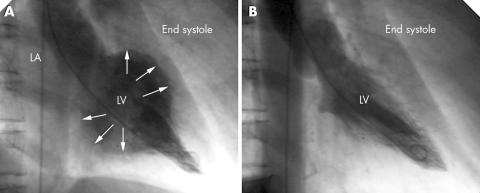
Figure 1 (A) Initial left ventriculogram of patient 1 showing akinesia of the left ventricular basal and mid‐portions and severe mitral regurgitation and hyperkinesias of the apex (arrows indicate inner border of the left ventricle). (B) Repeat ventriculography 12 days later showing resolution of the abnormalities. LA, left atrium; LV, left ventricle.
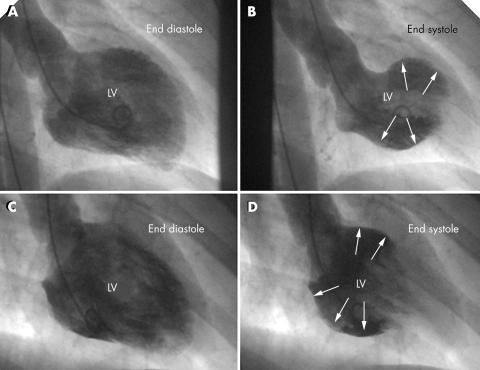
Figure 2 (A, B) Initial left ventriculogram of patient 2 showing anterolateral and diaphragmal akinesia (arrows). (C, D) Initial left ventriculogram of patient 3 showing involvement of the LV basal and mid‐portions (arrows).
DISCUSSION
Until now, it was believed that in TTC the LV apex is invariably affected, a belief that has been attributed to anatomical differences in sympathetic innervation of the heart.2 However, in our retrospective analysis, we were able to identify patients with a clinical syndrome resembling TTC who did not have LV apex involvement. All patients presented with transient ST segment abnormalities suggestive of myocardial ischaemia, reversible WMA beyond a single major coronary artery vascular distribution, and mild increase of cardiac enzymes. Similar to findings in classic TTC, our findings were that most patients were women and a triggering event was detectable in three of four cases. However, both WMA and ST segment abnormalities in our patients resolved more rapidly than in classic TTC. Although direct comparison between our patients and those with classic TTC in other series is not possible, the extent of affected myocardium seems to have been less in our patients than in classic TTC. This may be due to a less severe initial insult and may explain the differences in the time course of recovery.
The pathophysiological mechanisms underlying TTC remain obscure. The distribution of WMA in our patients clearly argues against the hypothesis of LV outflow tract obstruction. The affected area in patients 1 and 3 (LV base) also argues against the hypothesis of multiple coronary vasospasms. In both scenarios one would expect a more apical myocardial involvement.
None of our patients had clinical symptoms suggestive of myocarditis and only one patient had mildly increased inflammatory markers. Delayed hyperenhancement on gadolinium enhanced CMR, which is seen in up to 88% of patients with myocarditis,3 was absent in all patients. Thus, focal myocarditis appears unlikely to be the underlying mechanism.
As previously described, the pattern of myocardial dysfunction, limited release of cardiac enzymes, and recovery within a short time is reminiscent of myocardial stunning, which may be caused by increased local noradrenaline (norepinephrine) release.1,4 Anatomical differences in sympathetic innervation of the heart then possibly explain the large variety of affected LV segments. Interestingly, stunning‐like involvement of the LV base has also been described in pheochromocytoma.5
In conclusion, our results suggest that an acute reversible heart injury syndrome exists, which seems to be a variant form of TTC.
Footnotes
This work was done without any financial support. The authors have no financial interest in this article
References
- 1.Desmet W J, Adriaenssens B F, Dens J A. Apical ballooning of the left ventricle: first series in white patients. Heart 2003891027–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owa M, Aizawa K, Urasawa N.et al Emotional stress‐induced ‘ampulla cardiomyopathy': discrepancy between the metabolic and sympathetic innervation imaging performed during the recovery course. Jpn Circ J 200165349–352. [DOI] [PubMed] [Google Scholar]
- 3.Mahrholdt H, Goedecke C, Wagner A.et al Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation 20041091250–1258. [DOI] [PubMed] [Google Scholar]
- 4.Kono T, Morita H, Kuroiwa T.et al Left ventricular wall motion abnormalities in patients with subarachnoid hemorrhage: neurogenic stunned myocardium. J Am Coll Cardiol 199424636–640. [DOI] [PubMed] [Google Scholar]
- 5.Yamanaka O, Yasumasa F, Nakamura T.et al “Myocardial stunning”‐like phenomenon during a crisis of pheochromocytoma. Jpn Circ J 199458737–742. [DOI] [PubMed] [Google Scholar]