Abstract
The fatty acid composition of phospholipids and the contents of docosahexaenoic acid (DHA)-containing diacyl phosphatidylcholine and diacyl phosphatidylethanolamine molecular species were determined from brains of five fresh-water fish species from a boreal region adapted to 5°C, five fresh-water fish species from a temperate region acclimated to 5°C, five fresh-water fish species from a temperate region acclimated to 20°C, and three fresh water fish species from a subtropic region adapted to 25–26°C, as well as six mammalian species and seven bird species. There was little difference in DHA levels of fish brains from the different thermal environments; mammalian and bird brain phospholipids contained a few percentage points less DHA than those of the fish investigated. Molecular species of 22:6/22:6, 22:6/20:5, 22:6/20:4, 16:0/22:6, 18:0/22:6, and 18:1/22:6 were identified from all brain probes, and 16:0/22:6, 18:0/22:6, and 18:1/22:6 were the dominating species. Cold-water fish brains were rich in 18:1/22:6 diacyl phosphatidylethanolamine (and, to a lesser degree, in diacyl phosphatidylcholine), and its level decreased with increasing environmental/body temperature. The ratio of 18:0/22:6 to 16:0/22:6 phosphatidylcholine and phosphatidylethanolamine was inversely related to body temperature. Phospholipid vesicles from brains of cold-acclimated fish were more fluid, as assessed by using a 1,6-diphenyl-1,3,5-hexatriene fluorescent probe, than those from bird brains, but the fluidities were almost equal at the respective body temperatures. It is concluded that the relative amounts of these molecular species and their ratios to each other are the major factors contributing to the maintenance of proper fluidity relationships throughout the evolutionary chain as well as helping to maintain important brain functions such as signal transduction and membrane permeability.
Docosahexaenoic acid (DHA) is an important constituent of brain phospholipids and plays a role in maintaining structural and functional integrity of membranes. DHA is paired in glycerophospholipids mostly with palmitic acid (16:0) and stearic acid (18:0) and, in some cases, with another long-chain polyunsaturated fatty acid such as 22:6 in position sn-1 and accumulates in phosphatidylethanolamines. It has been postulated that optimal neurological development, information processing, the capacity of nerve cells to conduct electrical stimuli (1), and cognitive functions (2–4) depend on an adequate supply of this fatty acid. There is an agreement in the literature that mammalian brain accretes its DHA during specific periods of intrauterine and postnatal life, and after these periods, there is no way to alter its fatty acid composition (5–7). In contrast to mammals, brains of poikilotherms, such as fish, grow continuously after resorption of yolk sac until adulthood is reached. Mourente and coworkers (8, 9) demonstrated that juvenile turbot (Sophtalmus maximus) accumulated DHA during brain development. Similarly, Shields et al. (10) demonstrated that halibut (Hipoglossus hipoglossus) larvae, after their first feeding, accumulated DHA during brain development. However, the level of DHA resisted dietary influences in adult sea bass (Dicentrachus labrax; Pagliarani et al., ref. 11).
In addition to possible dietary influences, poikilotherms must adapt biophysical properties of their brain membranes to fluctuating environmental temperatures. In ensuring constant fluidities at the relevant body temperature, unsaturated fatty acids, like DHA, because of their low melting points, seem to be good candidates. Chang et al. (12), Thillard and van den Bruin (13), Wodtke (14), Roy et al. (15, 16), and Kitajka et al. (17) demonstrated some accumulation of DHA in different brain membranes of Chrassius auratus, Channa punctatus, Cyprinus carpio, and Clarias battrachus during acclimation/adaptation to low environmental temperature. This process was accompanied by increase of fluidity and a reorganization of molecular species composition—first of all of diacyl phosphatidylethanolamines, characterized by an augmentation of molecular species containing a monounsaturated fatty acid (18:1) in the position sn-1 and a polyunsaturated fatty acid (20:4, 22:6) in the position sn-2—in fish acclimated (C. carpio) or adapted (Acerina cernua) to low environmental temperatures compared with those acclimated or adapted to higher temperatures (C. carpio and Cattla cattla; refs. 18 and 19). It was concluded that it was not the gross amounts of long chain polyunsaturates, such as DHA, but their specific pairing with a monounsaturated fatty acid that might be important in controlling biophysical characteristics and thus functional integrity of membranes, including brain. If this hypothesis is correct, a decrease of these molecular species with increasing body temperatures should be observed throughout vertebrate evolution. In this article, molecular species composition of diacyl phosphatidylethanolamines and phosphatidylcholines of brains is compared in relation to body temperature of vertebrates from cold-adapted fish to birds. It is hoped that the data presented can contribute to a better understanding of the role of DHA in the brain.
Materials and Methods
Animals.
Brains of five fish species (Abramis brama, A. cernua, Coregonus alba, Esox lucius, and Rutilus rutilus) from a boreal region (Finland) adapted to 5°C, five fish species (C. carpio, Ctenopharyngodon idella, E. lucius, Hypophthalmichthys mollitrix, and Hypophthalmichthys nobilis) from a temperate region (Hungary) acclimated to 5°C, one marine fish species (Oncorhynchus tshawytscha) adapted to 10°C, five fish species (Clareas clareas, C. carpio, E. lucius, H. mollitrix, and Tilapia tilapia) from a temperate region acclimated to 20–22°C, three subtropic (West Bengal, India) fish species (C. cattla, C. battrachus, and Hylsa ilisa) adapted to 25–26°C, six mammalian species (cat, Felix catus; hare, Lepus europeus; pig, Sus scropha; sheep, Ovis aureus; cow, Bos taurus; and rat, Rattus norvegicus), and seven bird species (gull, Larus ridibundus ridibundus; wild duck, Anas plstyrhynchos; wild goose, Branta canadensis; hen, Gallus domesticus; turtle dove, Streptopelia decoato; pheasant, Phasianus colchicus; and crow, Corvus frugilegus frugilegus) were investigated. The fishes were collected from their natural habitats, except C. clareas and T. tilapia, which were reared artificially at the Institute of Fish Research, Szarvas. Brains were frozen in liquid nitrogen immediately after removal and kept at −70°C until processing.
Extraction and Analysis of Lipids.
Lipids were extracted from pooled brains (five of each) according to the method described by Folch et al. (20). Phospholipids were separated by silicic acid column chromatography by using chloroform to remove neutral lipids and methanol to obtain polar lipids. Polar lipids were fractionated further into polar head groups on TLC according to the method of Fine and Sprecher (21) on Silicagel G plates (Merck) and visualized under UV light after spraying with 0.5% 8-anilino-1-naphthalene sulfonic acid in 50% (vol/vol) methanol. Phosphatidylethanolamine and phosphatidylcholine were removed from the plates and extracted with chloroform:methanol:H2O (50:50:0.1, vol/vol) containing butylated hydroxytoluene (0.01%). Finally, the extracted probes were stored in benzene at −70°C.
Fatty acids from total phospholipids were separated after transesterification in absolute methanol containing 5% (vol/vol) HCl on a Supelcowax (Supelco) capillary column (30 m; i.d. = 0.32 mm) in a Hewlett–Packard model 8890 gas chromatograph.
Molecular species composition of purified phosphatidylcholines and phosphatidylethanolamines was determined according to the method of Takamura et al. (22) after liberation of diacylglycerols by phospholipase C of Bacillus cereus (Sigma), anthroxylation, and separation of diacyl subclasses by TLC. A 25-cm Supelcosyl LC-18 (Supelco) column (i.d. = 2.1 mm) served to segregate the individual molecular species with acetonitrile:isopropanol (80:20, vol/vol) as solvent at a flow rate of 0.2 ml/min. Synthetic phospholipids of Sigma and Avanti Polar Lipids as well as retention data of Bell and Dick (23) served to identify the eluted peaks.
Determination of Membrane Fluidity.
We added 2 μl of 2 mM 1,6-diphenyl-1,3,5-hexatriene (DPH; Molecular Probes) in tetrahydrofurane to phospholipid vesicles (phospholipid:DPH, 1,000:1 vol/vol), which dried on the wall of a glass test tube under high vacuum. Multilamellar vesicles were prepared by rehydrating the lipid film in 3 ml of 20 mM Tris⋅HCl (pH 7.4) with vigorous vortexing. Steady-state anisotropy parameter between 5 and 40°C was measured with a T format fluorescence spectrometer (Quanta Master QM-1, Photon Technology International, Princeton) as Rss = (RV/RH − 1)/RV/RH + 2), where RV and RH represent the ratios of intensities detected in the two emission channels with the excitation polarizer in vertical and horizontal positions, respectively. Excitation and emission wavelengths were 360 and 430 nm, respectively. The temperature of the sample was controlled by a circulating water bath and measured directly in the cuvettes with a platinum electrode. Measurements were done between 5 and 40°C with a heating rate of 0.4°C/min.
Results
Fatty Acid Composition of Brain Phospholipids and Body Temperature.
Table 1 collects the average fatty acid composition of brain phospholipids of vertebrates of different body temperatures. The level of 22:6 was almost identical in all species, varying between 19 and 22% of the total. Environmental or body temperature had no effect on fatty acid compositions as evident from comparison of data obtained for fish from different thermal environments, i.e., subtropic warm- and cold-adapted temperate fresh water fish and fish from a boreal region. Among the vertebrates, the mammalian brains contained less 22:6. Levels of the other major fatty acids like 16:0, 18:0, and 18:1 were also similar. All of the brain probes were characterized by a low level of unsaturated fatty acids of the n-6 family (18:2 and 20:4), and the same was true for some members of n-3 family (18:3, 20:5, and 22:5).
Table 1.
The average fatty acid compositions of phospholipids in brains of vertebrates
| Fatty acid | Percentage of total
|
|||||
|---|---|---|---|---|---|---|
| Cold-blooded animals
|
Warm-blooded animals
|
|||||
| Boreal 5°C | Temperate 5°C | Temperate 22°C | Subtropic 26°C | Mammals 37°C | Birds 41°C | |
| 14:0 | 1.5 ± 0.4 | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.7 ± 0.5 | 2.4 ± 1.0 | 0.5 ± 0.4 |
| DMA16:0 | 1.7 ± 0.7 | 1.6 ± 0.8 | 1.4 ± 0.5 | ND | 3.5 ± 1.6 | 2.9 ± 1.2 |
| 16:0 | 15.9 ± 2.6 | 13.3 ± 2.2 | 15.6 ± 3.1 | 18.2 ± 3.8 | 13.1 ± 4.5 | 15.4 ± 1.2 |
| 16:1n-7 | 3.1 ± 2.2 | 7.1 ± 1.9 | 5.4 ± 2.1 | 19.4 ± 0.7 | 5.4 ± 2.1 | 1.3 ± 0.4 |
| 16:2 | 2.3 ± 1.0 | ND | 0.4 ± 0.3 | 1.0 ± 0.8 | ND | ND |
| DMA18:0 | 2.1 ± 0.5 | 3.2 ± 1.6 | 3.1 ± 1.2 | ND | 3.5 ± 0.6 | 4.6 ± 0.5 |
| DMA18:1 | 2.3 ± 1.0 | 2.9 ± 1.1 | 2.5 ± 1.5 | ND | 2.9 ± 0.9 | 1.5 ± 0.5 |
| 18:0 | 11.8 ± 5.2 | 8.7 ± 1.7 | 11.3 ± 2.8 | 10.4 ± 0.7 | 18.9 ± 2.7 | 18.1 ± 2.7 |
| 18:1n-9 | 20.5 ± 2.6 | 24.6 ± 3.4 | 23.5 ± 4.0 | 20.9 ± 5.6 | 17.6 ± 4.7 | 13.0 ± 2.5 |
| 18:2n-6 | 1.3 ± 0.5 | 0.5 ± 0.2 | 1.1 ± 0.7 | 0.7 ± 0.5 | 1.6 ± 0.8 | 1.3 ± 0.5 |
| 18:3n-3 | ND | 0.4 ± 0.2 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.7 ± 0.2 | tr |
| 20:1n-9 | 1.4 ± 0.3 | 0.8 ± 0.7 | 1.1 ± 0.8 | 0.3 ± 0.1 | ND | ND |
| 20:2n-6 | ND | 0.2 ± 0.1 | 1.0 ± 0.1 | 0.4 ± 0.2 | ND | ND |
| 20:3n-6 | ND | 0.5 ± 0.2 | 0.7 ± 0.5 | 0.6 ± 0.3 | ND | ND |
| 20:4n-6 | 2.5 ± 0.5 | 4.9 ± 1.4 | 4.8 ± 1.6 | 1.0 ± 0.7 | 9.5 ± 4.9 | 9.7 ± 2.7 |
| 20:5n-3 | 2.8 ± 1.8 | 1.8 ± 0.4 | 1.2 ± 0.6 | 1.3 ± 0.8 | 1.3 ± 0.4 | 0.2 ± 0.1 |
| 22:4n-6 | ND | 0.2 ± 0.1 | 0.6 ± 0.4 | 2.0 ± 0.2 | 3.2 ± 1.7 | 2.0 ± 1.0 |
| 22:5n-3 | 2.2 ± 2.1 | 0.7 ± 0.5 | 1.2 ± 0.6 | 1.1 ± 0.9 | 2.3 ± 0.7 | 2.6 ± 0.6 |
| 22:6n-3 | 20.2 ± 3.2 | 21.1 ± 2.5 | 17.7 ± 4.1 | 17.8 ± 3.6 | 13.1 ± 4.7 | 17.5 ± 2.7 |
| 24:1n-9 | 5.8 ± 2.7 | 4.2 ± 1.6 | 5.3 ± 1.9 | 3.2 ± 1.2 | 3.1 ± 0.4 | 3.5 ± 1.7 |
DMA, dimethyl acetate; ND, not detected; tr, trace, less than 0.1%.
DHA-Containing Phospholipid Molecular Species in Brains of Vertebrates.
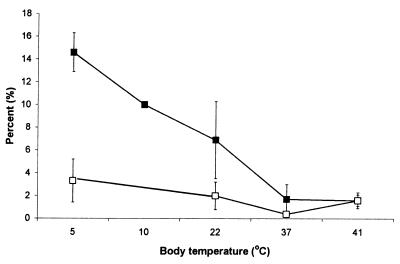
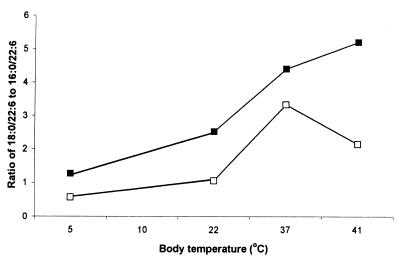
Detailed molecular species data have already been published for C. cattla from subtropic region, C. carpio from the temperate region, and A. cernua from the boreal region (24). In Table 2, the average content of diacyl phosphatidylcholine and phosphatidylethanolamine molecular species, comprising 45–50% of the total, containing DHAs in brains from fish to birds, was measured. The dominating species were 16:0/22:6, 18:0/22:6, and 18:1/22:6, and 22:6/22:6, 20:5/22:6, and 20:4/22:6 were present only in low amounts and were not included in Table 2. The level of 18:1/22:6 phosphatidylethanolamine and, to a lesser degree, of phosphatidylcholine showed a decreasing tendency from boreal fish to warm-blooded animals. Fig. 1 shows the relationship between body temperature and the level of 18:1/22:6 phosphatidylcholine and phosphatidylethanolamine. Data on boreal fish and cold-adapted temperate fish are combined in Fig. 1. It is interesting to note that the value obtained for 18:1/22:6 phosphatidylethanolamine from O. tshawytscha (Fig. 1) adapted to 10°C fits the curve exactly. Phosphatidylethanolamine was more rich in 18:1/22:6 species than was phosphatidylcholine, but the latter also showed decreasing tendency with increasing body temperature. Table 2 also includes the level of 18:0/22:6 phosphatidylcholine and phosphatidylethanolamine. The ratio of 18:0/22:6 to 16:0/22:6, increased with increased body temperature, particularly for phosphatidylethanolamine (Fig. 2).
Table 2.
DHA-containing phospholipid molecular species in brains of vertebrates
| Species group | Percentage of Total
|
|||||
|---|---|---|---|---|---|---|
| Phosphatidylethanolamines
|
Phosphatidylcholines
|
|||||
| 18:1/22:6 | 18:0/22:6 | 16:0/22:6 | 18:1/22:6 | 18:0/22:6 | 16:0/22:6 | |
| Cold-adapted fish* | 14.7 ± 1.7 | 19.8 ± 2.9 | 14.6 ± 2.0 | 3.3 ± 1.9 | 8.2 ± 2.1 | 14.8 ± 5.1 |
| Warm-adapted fish | 6.9 ± 3.4 | 30.8 ± 7.7 | 12.2 ± 1.2 | 2.2 ± 1.21 | 7.2 ± 17.1 | 16.2 ± 6.1 |
| Subtropic fish | 2.4 ± 0.1 | 49.1 ± 5.6 | 18.6 ± 9.0 | 1.8 ± 0.7 | 8.5 ± 6.5 | 27.1 ± 8.1 |
| Mammals | 1.7 ± 1.3 | 29.6 ± 4.2 | 6.7 ± 3.2 | 1.7 ± 1.3 | 29.5 ± 4.2 | 6.7 ± 3.5 |
| Birds | 1.7 ± 0.5 | 39.5 ± 6.2 | 7.6 ± 2.3 | 1.6 ± 0.5 | 12.6 ± 9.4 | 5.8 ± 3.3 |
Combined data of boreal and cold-adapted fish from temperate region.
Figure 1.
Level of 18:1/22:6 phosphatidylcholine and phosphatidylethanolamine in brain phospholipids of vertebrates in relation to body temperature. Filled squares, phosphatidylethanolamine; open squares, phosphatidylcholine.
Figure 2.
The ratio of 18:0/22:6 to 16:0/22:6 phosphatidylcholine and phosphatidylethanolamine in brain phospholipids of different vertebrates in relation to body temperature. Filled squares, phosphatidylethanolamine; open squares, phosphatidylcholine.
Fluidity of Phospholipid Vesicles Obtained from Representative Vertebrate Species and the Relationship Between DHA-Containing Phospholipid Molecular Species and Body Temperature.
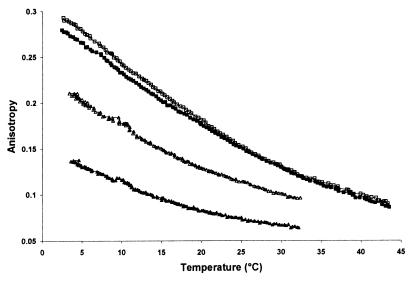
In Fig. 3, the DPH fluorescence anisotropy parameters (Rss) of brain phospholipid vesicles obtained from representative species of a cold- and warm-adapted fish (H. mollitrix) and a representative mammal (rat) and bird (hen) are given. As expected, the most fluid structures were observed with the cold-adapted fish, and the most rigid ones were observed with the bird. Rss vs. temperature curves almost overlapped with rat and bird above 20°C, but below it, vesicles from bird brain were slightly more rigid. It can be taken from Fig. 3 that Rss values were almost identical at the respective body temperature, indicating an almost 100% compensation of the effect of environmental/body temperature on chain ordering of brain phospholipids over the evolutionary scale. Behan-Martin et al. (25), using two different labels (cis- and trans-parinaric acid), reported the same response of brain synaptic vesicles from cold water fish to bird. The difference between their study and the present results is that we used phospholipid vesicles while, in synaptic vesicles, proteins and other membrane components were also present. Our results prove the principal role of phospholipids in controlling the membrane physical state in the course of thermal acclimation/adaptation.
Figure 3.
Temperature dependency of anisotropy parameter (Rss) of DPH embedded in brain phospholipid vesicles of vertebrates of different body temperatures. Filled squares, mammal (pig); open squares, bird (hen); filled triangles, cold-adapted fish (H. mollitrix); open triangles, warm-adapted fish (H. mollitrix).
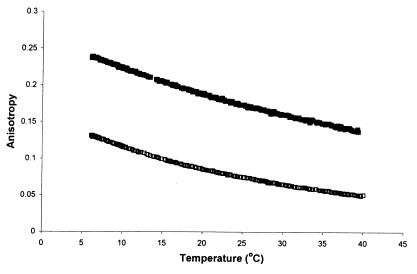
Presumably, the DHA-containing phospholipid molecular species significantly contribute to the observed fluidity relationships presented in Fig. 3. Earlier, we demonstrated that sn-1 monoenic, sn-2 polyenic phospholipid molecular species render artificial membranes more disordered (19). Fig. 4 shows that vesicles formed from 18:0/22:6 phosphatidylcholine are more ordered than those formed from 16:0/22:6 phosphatidylcholine. Thus, decreasing the amount of sn-1 monoenic, sn-2 polyenic phospholipid molecular species as well as increasing the ratio of 18:0/22:6 to 16:0/22:6 from cold-adapted/acclimated fish to bird might be involved in compensation of the effect of temperature on chain ordering over the evolutionary scale.
Figure 4.
Comparison of anisotropy parameter of DPH embedded in synthetic 16:0/22:6 and 18:0/22:6 phosphatidylcholine. Filled squares, 18:0/22:6 phosphatidylcholine; open squares, 16:0/22:6 phosphatidylcholine.
Discussion
Although the number of species involved in this study is limited, we believe that the data obtained adequately represent the fatty acid composition of phospholipids and the molecular species composition of brain diacyl phosphatidylcholines and diacyl phosphatidylethanolamines in the vertebrate kingdom. The data presented in Table 1 suggest that fatty acid composition of brain phospholipids is rather uniform among vertebrates and is characterized by high levels of DHA. Lower levels of DHA in brains of mammals might be explained by the fact that all of them were domesticated several thousand years ago and thus were on an n-3 poor diet and also by the fact that we analyzed total brain tissue (gray and white material) from all species, i.e., it was not possible to separate the two in fish brains. Crawford (26) suggested that the diet of vertebrates became poor in n-3 fatty acids after turning from aquatic to terrestrial life. The same seems to be true for birds: brains of hens contained less 22:6 than did those of the wild duck (16.7% vs. 23%). Ducks feed on wild rice, aquatic plants, and corn in the fields, whereas hens feed mainly on corn rich in 18:2n-6. The fish involved in this study have different feeding habits (feeding on phytoplankton, higher plants, and other animals) and originate from brackish waters and fresh waters, but the level of DHA in their brains is almost identical and is little influenced by body temperature (Table 1). It is striking that wild birds, having the highest body temperature, accumulate as much DHA as fish adapted to low temperatures. In early stages of development, however, DHA content in fish brains, similar to that in mammals, may depend on dietary supply (Shields et al., ref. 10).
Despite similarity in the gross amount of DHA in brains, distribution of this acid in the phospholipid molecule varies according the evolutionary stage of the animal. The present results show that pairing of DHA with other saturated or unsaturated fatty acids might depend on body/environmental temperature. Of the DHA-containing phospholipid molecular species, 16:0/22:6, 18:0/22:6, and 18:1/22:6 exhibited the most characteristic changes throughout the vertebrate evolution. We have shown in this study (Figs. 1 and 2) that the ratios of these three molecular species to each other can be correlated to the evolutionary stage of the species and indeed to their ability to control body temperature.
Accumulation of 18:1/22:6 or, in general, sn-1 monoenic, sn-2 polyenic phosphatidylethanolamine molecular species in cold might be a general phenomenon as demonstrated for the nematode Caenorhabditis elegans (27), for the shrimp Pandulus borealis from the North Atlantic (28), and for marine crustaceans (Monoporeira sp., Gammarus sp., and Mysis sp.) from the Baltic sea (unpublished results). A low level of 18:1/22:6 was found also in the brains of rhesus monkeys (29). A diminishing level of 18:1/22:6 with increasing body temperature can be the result of reduced formation and thus incorporation of 18:1 into phospholipids or reduced in situ desaturation of 18:0/22:6 phosphatidylethanolamine. Although there is no direct experimental evidence for this latter reaction to occur in brains and other tissues, it is interesting to note that the sum of 18:1/22:6 and 18:0/22:6 is almost identical in winter- and summer-acclimated carp (C. carpio; 38.6% vs. 39.6%, respectively) and in summer- and winter-acclimated silver carp (H. nobilis; 32.8% vs. 34.5%, respectively). This value is identical to the one we find with birds from their native environment (35%). It should be noted, however, that sn-1 18:0 is metabolically more stable than sn-16:0 in rat and guinea pig brain (30, 31); 18:1 could not have come from the diet in brains of cold-adapted/acclimated fish, because these fish do not feed in winter. A plausible explanation is that phospholipid deacylation/reacylation in brains of cold-adapted fish is more intensive than in warm-blooded animals. The effect of temperature on this reaction in fish liver has been demonstrated already (32). Whatever the reaction, the present results suggest a reorganization of molecular species composition of phosphatidylethanolamines containing DHA in relation to body temperature during evolution of vertebrates. This reorganization, however, does not affect the gross amount of DHA in brains.
We propose that one of the major functions of these molecular species in brains is to control biophysical properties of membranes according to body temperature. Oleic acid in position sn-1 of phospholipids increases surface area by about 30% relative to that of 18:0/22:6 (33). Michaelson et al. (34) demonstrated that phosphatidylethanolamine contracts the bilayers most probably because of the electrostatic interactions between the positively charged amino groups and the carbonyl oxygen of the neighboring phospholipids and also because of the possible hydrogen bonding of the NH3+ group and the smaller size of the phosphatidylethanolamine head group compared with that of phosphatidylcholine. However, increased surface area might decrease these electrostatic interactions in the polar head group region. In agreement with this possibility, we have shown earlier by using mixtures of either 16:0/18:1 phosphatidylethanolamine and 18:0/22:6 phosphatidylcholine or 18:1/22:6 phosphatidylethanolamine and 18:0/22:6 phosphatidylcholine that 18:1/22:6 phosphatidylcholine or phosphatidylethanolamine, but not 16:0/18:1 phosphatidylethanolamine, increases disorder especially in the upper half of the bilayers (19). The ratio of sn-1 saturated, sn-2 polyunsaturated phosphatidylethanolamines to sn-1 monounsaturated, sn-2 docosahexaenoic phosphatidylethanolamines increases from cold-adapted fish to birds from 2.3 to 25.5 (see Table 2). This ordering effect contributes the increasing ratio from fish to birds of 18:0/22:6 to 16:0/22:6 phosphatidylcholine (Fig. 4). Data presented in Fig. 3 seem to be in accord with this speculation, that is, bilayers of birds are more ordered than those of fish. Thus, biophysical characteristics of membrane phospholipids are under a delicate control of at least three factors: (i) the level of sn-1 monoenic, sn-2 polyenic phosphatidylethanolamines and phosphatidylcholines; (ii) the ratio of sn-1 saturated, sn-2 unsaturated phosphatidylethanolamines to sn-1 monounsaturated, sn-2 polyunsaturated phosphatidylethanolamines; and (iii) the ratio of 18:0/22:6 to 16:0/22:6 phosphatidylcholine. Other membrane components, like proteins, do not seem to modify this picture. Behan-Martin et al. (25) found the same pattern in fluidity of brain synaptic membranes containing all of the components of an intact biomembrane.
Increasing the amount of 18:0/22:6 at the cost of 16:0/22:6 and reducing 18:1/22:6 with increasing body temperature also affect chain ordering, membrane thickness, and in general, membrane architecture. Less packed membranes in poikilothermic animals may facilitate insertion of proteins, opening ionic channels or permeation of solutes at reduced temperatures.
The 18:1/22:6 phosphatidylethanolamine not only increases disorder in membranes but also promotes an increased negative curvature, as indicated by its ability to reduce the bilayer to hexagonal phase transition temperature (35). It is tempting to speculate, but has yet to be proven, that vertebrates, like some prokaryotes (36), maintain a “window” between the bilayer-to-nonbilayer and liquid-crystalline-to-solid-gel transition of their membranes. Presence and proper level of nonbilayer-forming lipids might affect activity of some enzymes (37), the velocity of information processing (38), etc. It has been shown that the rate of fusion of synaptic vesicles with postsynaptic membranes depends on the type of phospholipids present: the greater the amount of “fusogenic” phospholipid in the membrane to be fused, the faster the fusion (39). Thus, a high level of 18:1/22:6 in fish membranes might help to maintain proper fusion and signal transduction rates in animals adapted/acclimated to low environmental temperatures. The same seems to be true for association of soluble G proteins to membranes. We have demonstrated earlier that this process also depends on the ability to form a nonbilayer phase of phosphatidylethanolamines (40). Thus, besides contributing to maintaining proper biophysical properties of brain membranes throughout vertebrate evolution, DHA-containing phosphatidylcholine and phosphatidylethanolamine molecular species may contribute to important brain functions such as signal transduction and information processing.
Acknowledgments
This paper was supported in part by Országos Tudományos Kutatási Alap (Hungary) Grant T022435 (to Z.K.).
Abbreviations
- DHA
docosahexaenoic acid
- DPH
1,6-diphenyl-1,3,5-hexatriene
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.120157297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.120157297
References
- 1.Burdge G C. Biochem Soc Trans. 1998;26:247–252. doi: 10.1042/bst0260246. [DOI] [PubMed] [Google Scholar]
- 2.Carlson S E, Wekman S H, Peeples J M, Wilson W G., III World Rev Nutr Diet. 1994;75:63–69. doi: 10.1159/000423552. [DOI] [PubMed] [Google Scholar]
- 3.Werkman S H, Carlson S E. Lipids. 1996;31:91–97. doi: 10.1007/BF02522417. [DOI] [PubMed] [Google Scholar]
- 4.Yehuda S, Rabinovitz S, Mostofsky D I. Neurochem Res. 1998;23:627–634. doi: 10.1023/a:1022430620205. [DOI] [PubMed] [Google Scholar]
- 5.Clandinin M T, Charpell J E, Leong S, Heim T, Swyer P R, Change G W. Early Hum Dev. 1980;4:131–138. doi: 10.1016/0378-3782(80)90016-x. [DOI] [PubMed] [Google Scholar]
- 6.Clandinin M T, Charpell J E, Leong S, Heim T, Swyer P R, Change G W. Early Hum Dev. 1980;4:363–372. doi: 10.1016/0378-3782(80)90016-x. [DOI] [PubMed] [Google Scholar]
- 7.Connnor W E, Neuringer E, Lin D S. J Lipid Res. 1990;31:237–247. [PubMed] [Google Scholar]
- 8.Mourente G, Tocher D T. Aquaculture. 1992;105:363–371. [Google Scholar]
- 9.Mourente G, Tocher D T, Sargent J R. Lipids. 1991;26:871–877. [Google Scholar]
- 10.Shields R J, Bell J G, Luizi L S, Gara B, Bromage N R, Sargent J R. J Nutr. 1999;129:1186–1194. doi: 10.1093/jn/129.6.1186. [DOI] [PubMed] [Google Scholar]
- 11.Pagliarani A, Pirini M, Trigari G, Ventrella V. Comp Biochem Physiol B Biochem Mol Biol. 1986;83:277–282. doi: 10.1016/0305-0491(86)90366-4. [DOI] [PubMed] [Google Scholar]
- 12.Chang M R C, Roots B I. Neurochem Res. 1985;10:355–375. doi: 10.1007/BF00964605. [DOI] [PubMed] [Google Scholar]
- 13.Thillart G, van den Bruin G. Biochim Biophys Acta. 1981;640:439–447. doi: 10.1016/0005-2736(81)90469-7. [DOI] [PubMed] [Google Scholar]
- 14.Wodtke E. Biochim Biophys Acta. 1978;529:280–291. doi: 10.1016/0005-2760(78)90071-1. [DOI] [PubMed] [Google Scholar]
- 15.Roy R, Ghosh D, Das A B. J Therm Biol. 1992;17:209–215. [Google Scholar]
- 16.Roy R, Dash A B, Ghosh D. Biochim Biophys Acta. 1997;1223:65–74. doi: 10.1016/s0005-2736(96)00176-9. [DOI] [PubMed] [Google Scholar]
- 17.Kitajka K, Buda C, Fodor E, Halver J E, Farkas T. Lipids. 1996;31:1045–1050. doi: 10.1007/BF02522461. [DOI] [PubMed] [Google Scholar]
- 18.Buda C, Dey I, Balogh N, Horváth L I, Maderspach K, Yeo Y K, Farkas T. Proc Natl Acad Sci USA. 1995;91:8234–8238. doi: 10.1073/pnas.91.17.8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fodor E, Jones R H, Buda C, Kitajka K, Dey I, Farkas T. Lipids. 1995;30:1119–1126. doi: 10.1007/BF02536612. [DOI] [PubMed] [Google Scholar]
- 20.Folch J I, Lees M, Sloane-Stanley G H. J Biol Chem. 1957;226:497–500. [PubMed] [Google Scholar]
- 21.Fine J B, Sprecher H. J Lipid Res. 1982;23:660–663. [PubMed] [Google Scholar]
- 22.Takamura M, Kito M. J Biochem. 1991;109:436–439. doi: 10.1093/oxfordjournals.jbchem.a123399. [DOI] [PubMed] [Google Scholar]
- 23.Bell M V, Dick J R. Lipids. 1991;26:565–578. [Google Scholar]
- 24.Buda C, Dey I, Balogh N, Horváth L I, Maderspach K, Yeo Y K, Farkas T. Proc Natl Acad Sci USA. 1995;91:8234–8238. doi: 10.1073/pnas.91.17.8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behan-Martin M K, Jones G R, Bowler W T, Cosins A R. Biochim Biophys Acta. 1993;1151:216–222. doi: 10.1016/0005-2736(93)90106-a. [DOI] [PubMed] [Google Scholar]
- 26.Crawford M A. Nutr Rev. 1992;50:3–11. doi: 10.1111/j.1753-4887.1992.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka T, Ikita K, Ashida T, Motoyama Y, Yamaguchi Y, Satouchi K. Lipids. 1996;31:1173–1178. doi: 10.1007/BF02524292. [DOI] [PubMed] [Google Scholar]
- 28.Farkas T, Dey I, Buda C, Halver J E. Biophys Chem. 1994;50:147–155. doi: 10.1016/0301-4622(94)85027-5. [DOI] [PubMed] [Google Scholar]
- 29.Lin D S, Conner W E, Anderson G J, Neuringer M. J Neurochem. 1992;50:1200–1207. doi: 10.1111/j.1471-4159.1990.tb03125.x. [DOI] [PubMed] [Google Scholar]
- 30.Sambordski R W, Ridgeway N D, Vance D E. J Biol Chem. 1990;265:18322–18329. [PubMed] [Google Scholar]
- 31.Burdge G C, Kelly F J, Postle A D. Biochem J. 1993;290:67–73. doi: 10.1042/bj2900067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farkas T, Roy R. Comp Biochem Physiol. 1989;93:217–222. [Google Scholar]
- 33.Zabelinskii S A, Brovtsyna N B, Chebotareva M, Gorbunova O B, Krivchenko A I. Comp Biochem Physiol B Biochem Mol Biol. 1995;111:127–140. doi: 10.1016/0305-0491(94)00210-l. [DOI] [PubMed] [Google Scholar]
- 34.Michaelson D M, Horwitz A F, Klein M P. Biochemistry. 1974;13:2605–2612. doi: 10.1021/bi00709a021. [DOI] [PubMed] [Google Scholar]
- 35.Giorgione J, Epand R M, Buda C, Farkas T. Proc Natl Acad Sci USA. 1995;92:9767–9770. doi: 10.1073/pnas.92.21.9767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morein G, Anderson A-G, Rilfors L, Lindblom G. J Biol Chem. 1996;271:6801–6809. doi: 10.1074/jbc.271.12.6801. [DOI] [PubMed] [Google Scholar]
- 37.Yang F Y, Hwang F. Chem Phys Lipids. 1996;81:197–202. [Google Scholar]
- 38.Forsyth J S, Willats P, DiModogno M K, Varma S, Colvin M. Biochem Soc Trans. 1998;26:252–257. doi: 10.1042/bst0260252. [DOI] [PubMed] [Google Scholar]
- 39.Glaser P E, Gross R W. Biochemistry. 1994;33:5805–5812. doi: 10.1021/bi00185a019. [DOI] [PubMed] [Google Scholar]
- 40.Escriba P V, Ozaita A, Riibas C, Miralles A, Fodor E, Farkas T, Garcia-Sevilla J A. Proc Natl Acad Sci USA. 1997;94:11375–11380. doi: 10.1073/pnas.94.21.11375. [DOI] [PMC free article] [PubMed] [Google Scholar]