Abstract
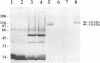
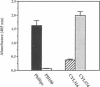
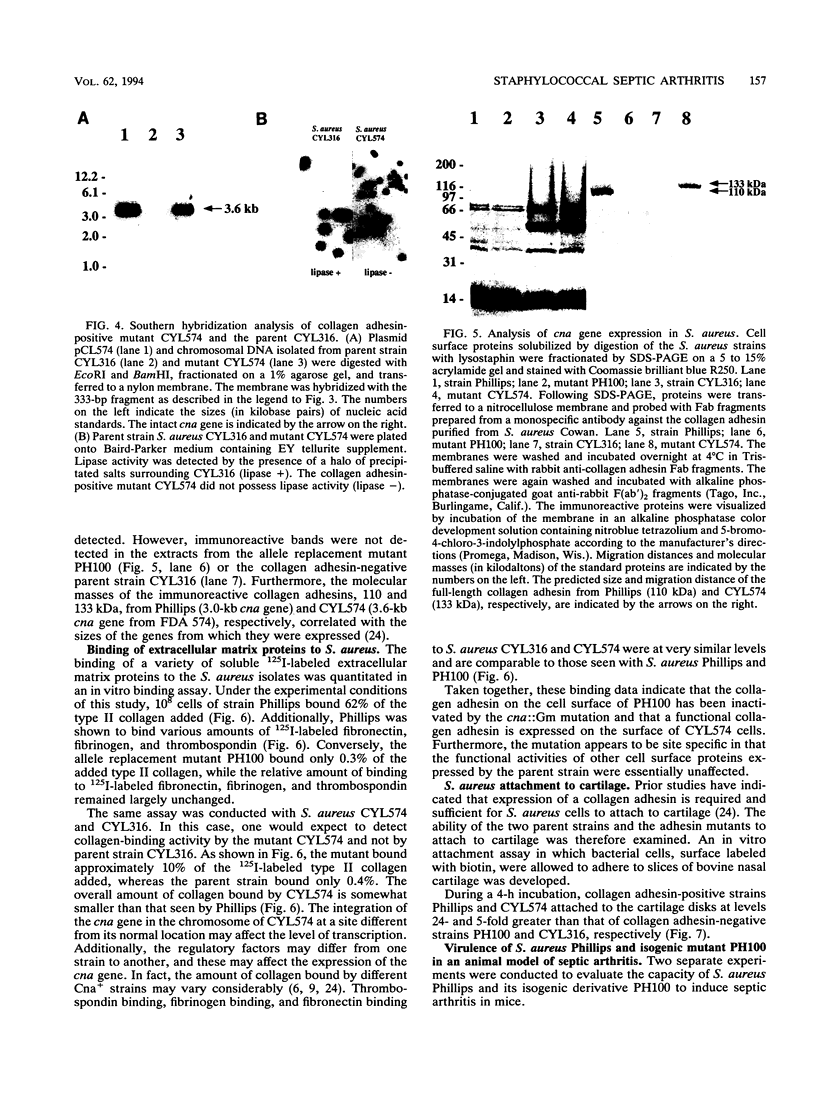
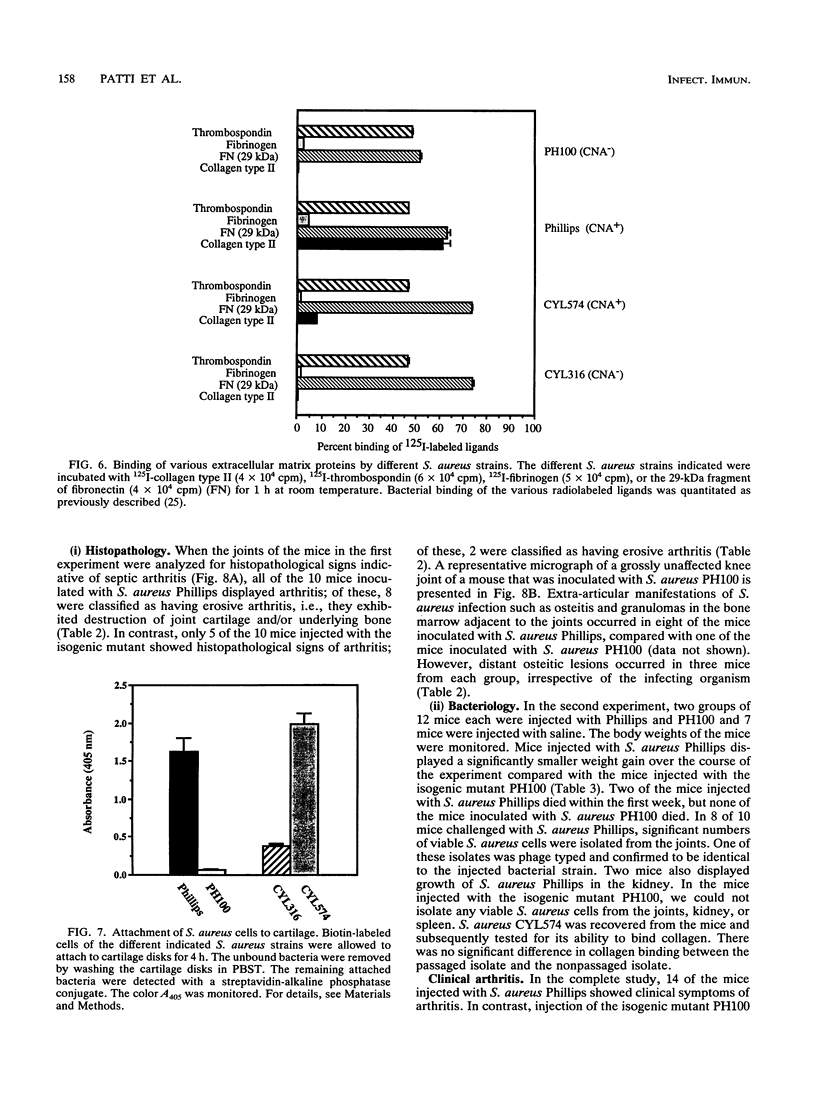
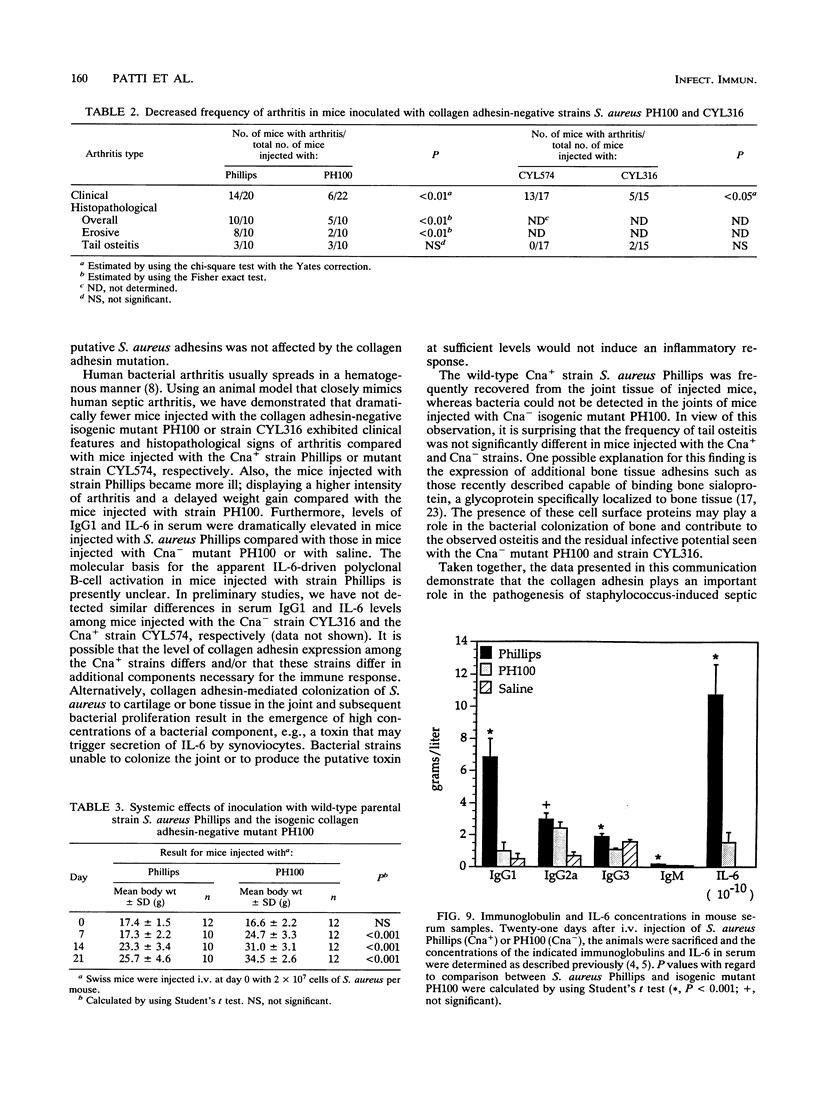
The importance of a collagen-binding adhesin in the pathogenesis of septic arthritis has been examined by comparing the virulence of two sets of Staphylococcus aureus mutants in an animal model. Collagen adhesin-negative mutant PH100 was constructed by replacing the chromosomal collagen adhesin gene (cna) in a clinical strain, Phillips, with an inactivated copy of the gene. Collagen adhesin-positive mutant S. aureus CYL574 was generated by introducing the cna gene into CYL316, a strain that normally lacks the cna gene. Biochemical, immunological, and functional analyses of the generated mutants and their respective parent strains showed that binding of 125I-labeled collagen, expression of an immunoreactive collagen adhesin, and bacterial adherence to cartilage were directly correlated with the presence of a functional cna gene. Greater than 70% of the mice injected with the Cna+ strains developed clinical signs of arthritis, whereas less than 27% of the animals injected with Cna- strains showed symptoms of disease. Furthermore, mice injected with the Cna+ strain Phillips had remarkably elevated levels of immunoglobulin G1 and interleukin-6 compared with mice injected with the Cna- mutant PH100. Taken together, these results demonstrate that collagen adhesin plays an important role in the pathogenesis of septic arthritis induced by S. aureus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelnour A., Tarkowski A. Polyclonal B-cell activation by an arthritogenic Staphylococcus aureus strain: contribution of T-cells and monokines. Cell Immunol. 1993 Apr 1;147(2):279–293. doi: 10.1006/cimm.1993.1069. [DOI] [PubMed] [Google Scholar]
- Augustin J., Götz F. Transformation of Staphylococcus epidermidis and other staphylococcal species with plasmid DNA by electroporation. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):203–207. doi: 10.1016/0378-1097(90)90283-v. [DOI] [PubMed] [Google Scholar]
- Borck K., Beggs J. D., Brammar W. J., Hopkins A. S., Murray N. E. The construction in vitro of transducing derivatives of phage lambda. Mol Gen Genet. 1976 Jul 23;146(2):199–207. doi: 10.1007/BF00268089. [DOI] [PubMed] [Google Scholar]
- Bremell T., Abdelnour A., Tarkowski A. Histopathological and serological progression of experimental Staphylococcus aureus arthritis. Infect Immun. 1992 Jul;60(7):2976–2985. doi: 10.1128/iai.60.7.2976-2985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremell T., Lange S., Yacoub A., Rydén C., Tarkowski A. Experimental Staphylococcus aureus arthritis in mice. Infect Immun. 1991 Aug;59(8):2615–2623. doi: 10.1128/iai.59.8.2615-2623.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton T. B., Rissing J. P., Horner J. A., Plowman K. M., Scott D. F., Sprinkle T. J., Best G. K. Binding of a Staphylococcus aureus bone pathogen to type I collagen. Microb Pathog. 1990 Jun;8(6):441–448. doi: 10.1016/0882-4010(90)90031-k. [DOI] [PubMed] [Google Scholar]
- Goldenberg D. L., Reed J. I. Bacterial arthritis. N Engl J Med. 1985 Mar 21;312(12):764–771. doi: 10.1056/NEJM198503213121206. [DOI] [PubMed] [Google Scholar]
- Holderbaum D., Spech T., Ehrhart L. A., Keys T., Hall G. S. Collagen binding in clinical isolates of Staphylococcus aureus. J Clin Microbiol. 1987 Dec;25(12):2258–2261. doi: 10.1128/jcm.25.12.2258-2261.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiswirth B. N., Löfdahl S., Betley M. J., O'Reilly M., Schlievert P. M., Bergdoll M. S., Novick R. P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983 Oct 20;305(5936):709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- Lantz M. S., Switalski L. M., Kornman K. S., Hök M. Bacteroides intermedius binds fibrinogen. J Bacteriol. 1985 Aug;163(2):623–628. doi: 10.1128/jb.163.2.623-628.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Y., Buranen S. L., Ye Z. H. Construction of single-copy integration vectors for Staphylococcus aureus. Gene. 1991 Jul 15;103(1):101–105. doi: 10.1016/0378-1119(91)90399-v. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Iandolo J. J. Integration of staphylococcal phage L54a occurs by site-specific recombination: structural analysis of the attachment sites. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5474–5478. doi: 10.1073/pnas.83.15.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchansky J. B., Benson A. K., Atherly A. G. Construction, transfer and properties of a novel temperature-sensitive integrable plasmid for genomic analysis of Staphylococcus aureus. Mol Microbiol. 1989 Jan;3(1):65–78. doi: 10.1111/j.1365-2958.1989.tb00105.x. [DOI] [PubMed] [Google Scholar]
- McGavin M. H., Krajewska-Pietrasik D., Rydén C., Hök M. Identification of a Staphylococcus aureus extracellular matrix-binding protein with broad specificity. Infect Immun. 1993 Jun;61(6):2479–2485. doi: 10.1128/iai.61.6.2479-2485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavin M. J., Raucci G., Gurusiddappa S., Hök M. Fibronectin binding determinants of the Staphylococcus aureus fibronectin receptor. J Biol Chem. 1991 May 5;266(13):8343–8347. [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Schultz-Cherry S., Hök M. Transforming growth factor-beta complexes with thrombospondin. Mol Biol Cell. 1992 Feb;3(2):181–188. doi: 10.1091/mbc.3.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly M., de Azavedo J. C., Kennedy S., Foster T. J. Inactivation of the alpha-haemolysin gene of Staphylococcus aureus 8325-4 by site-directed mutagenesis and studies on the expression of its haemolysins. Microb Pathog. 1986 Apr;1(2):125–138. doi: 10.1016/0882-4010(86)90015-x. [DOI] [PubMed] [Google Scholar]
- Patti J. M., Jonsson H., Guss B., Switalski L. M., Wiberg K., Lindberg M., Hök M. Molecular characterization and expression of a gene encoding a Staphylococcus aureus collagen adhesin. J Biol Chem. 1992 Mar 5;267(7):4766–4772. [PubMed] [Google Scholar]
- Rydén C., Yacoub A. I., Maxe I., Heinegård D., Oldberg A., Franzén A., Ljungh A., Rubin K. Specific binding of bone sialoprotein to Staphylococcus aureus isolated from patients with osteomyelitis. Eur J Biochem. 1989 Sep 15;184(2):331–336. doi: 10.1111/j.1432-1033.1989.tb15023.x. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Patti J. M., Butcher W., Gristina A. G., Speziale P., Hök M. A collagen receptor on Staphylococcus aureus strains isolated from patients with septic arthritis mediates adhesion to cartilage. Mol Microbiol. 1993 Jan;7(1):99–107. doi: 10.1111/j.1365-2958.1993.tb01101.x. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Speziale P., Hök M. Isolation and characterization of a putative collagen receptor from Staphylococcus aureus strain Cowan 1. J Biol Chem. 1989 Dec 15;264(35):21080–21086. [PubMed] [Google Scholar]
- Voytek A., Gristina A. G., Barth E., Myrvik Q., Switalski L., Hook M., Speziale P. Staphylococcal adhesion to collagen in intra-articular sepsis. Biomaterials. 1988 Jan;9(1):107–110. doi: 10.1016/0142-9612(88)90080-4. [DOI] [PubMed] [Google Scholar]
- Waldvogel F. A., Papageorgiou P. S. Osteomyelitis: the past decade. N Engl J Med. 1980 Aug 14;303(7):360–370. doi: 10.1056/NEJM198008143030703. [DOI] [PubMed] [Google Scholar]