Abstract
Objective
To determine the accuracy of prenatal and postnatal echocardiography in delineating the degree of cardiac fusion, intracardiac anatomy (ICA), and ventricular function of 23 sets of conjoined twins with thoracic level fusion presenting to a single centre over a 20 year period.
Methods
13 thoracopagus, 5 thoraco‐omphalopagus, and 5 parapagus pairs presenting to the authors' institution between 1985 and 2004 inclusive were assessed. Echocardiographic data were analysed together with operative intervention and outcome. Twins were classified according to the degree of cardiac fusion: separate hearts and pericardium (group A, n = 5), separate hearts and common pericardium (group B, n = 7), fused atria and separate ventricles (group C, n = 2), and fused atria and ventricles (group D, n = 9).
Results
The degree of cardiac fusion was correctly diagnosed in all but one set. ICA was correctly diagnosed in all cases, although the antenatal diagnosis was revised postnatally in three cases. Abnormal ICA was found in one twin only in two group A pairs, one group B pair, and both group C pairs. All group D twins had abnormal anatomy. Ventricular function was good in all twins scanned prenatally, and postnatally function correlated well with clinical condition. Thirteen sets of twins in groups A–C were surgically separated; 16 of 26 survived. None from groups C or D survived.
Conclusions
Prenatal and postnatal echocardiography accurately delineates cardiac fusion, ICA, and ventricular function in the majority of twins with thoracic level fusion. It is integral in assessing feasibility of separation. The outcome in twins with fused hearts remains dismal.
Keywords: congenital heart disease, echocardiography, cardiac surgery, prenatal diagnosis
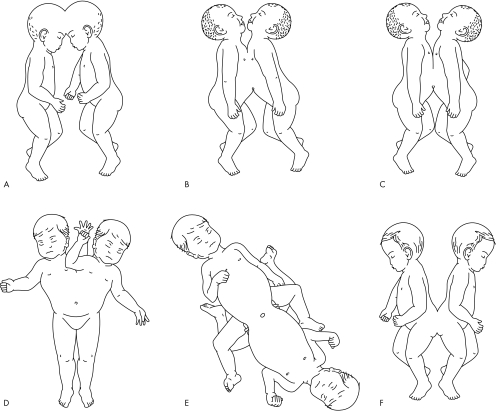
The incidence of conjoined twins is reported to be in the range of one in 50 000 to one in 100 0001 but, as 60% are stillborn or die shortly after birth, the true incidence is around one in 200 000 live births.2 Girls predominate in the ratio of 3:1. Twins are classified according to the major site of union, to which the ending “‐pagus” is added, meaning “fixed” (fig 1). Thoracopagus (joined at the thoracic level) twins are the most common, accounting for 40% of cases, followed by omphalopagus (joined at the abdomen, but often including the lower thorax), accounting for 32%.2 Other forms are pyopagus (sacral fusion), ischiopagus, and craniopagus. The term parapagus is used to describe twins fused extensively side to side (formerly known as thoraco‐omphalo‐ischiopagus, or dicephalus). Although there may be cardiac abnormalities in any type of fusion, thoracopagus twins have the highest incidence of abnormal cardiovascular findings with a 90% incidence of shared pericardium3 and major myocardial connections in some 75% of cases.4 The extent of cardiac fusion and intracardiac anatomy (ICA) in conjoined twins not only determines the potential for surgical separation but also long term survival. Surgical separation is rarely feasible in complex fused hearts although there may be occasional exceptions.5,6
Figure 1 Common forms of conjoined twins: (A) craniopagus, (B) thoracopagus, (C) omphalopagus, (D) parapagus, (E) ischiopagus, and (F) pyopagus.
We report the effectiveness of both prenatal and postnatal echocardiography in the evaluation of 23 sets of conjoined twins with thoracic level fusion over a 20 year period at a single centre. To our knowledge, this is the largest single centre series of conjoined twins with cardiac involvement.
PATIENTS AND METHODS
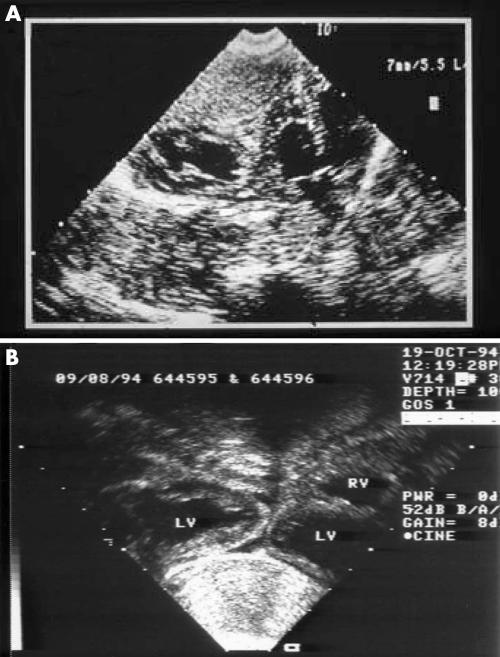
Between 1985 and 2004, 23 sets of conjoined twins with thoracic level fusion were referred to assess the feasibility of surgical separation. Two further sets without thoracic level fusion (one omphalopagus and one pyopagus) were excluded from this analysis. There were 18 sets of female and four sets of male twins. In the remaining set, sex was not determined at prenatal assessment. Six sets of twins were referred from overseas and the remaining 17 sets were from the UK and the Republic of Ireland. There were 13 sets of thoracopagus twins, five sets of thoraco‐omphalopagus twins, and five sets of parapagus twins. All except two sets were diagnosed prenatally, between 14 and 38 weeks' gestation, and delivery was by caesarean section. Fetal echocardiography was performed on 12 sets of twins later in the series, 10 at Great Ormond Street Hospital and two at other fetal medicine units. Diagnostic echocardiographic images were obtained as early as 14 weeks' gestation. In general the left sided twin was termed twin 1 and the right sided twin termed twin 2. The remaining 11 sets underwent postnatal echocardiography between 1 day and 3 years of age. Standard echocardiographic views were modified as dictated by the accessibility of subcostal and precordial views. Cardiac catheterisation was undertaken in three sets of twins early in our experience. The twins were classified according to the degree of cardiac fusion: group A had separate hearts and pericardium, group B had separate hearts and a common pericardium (fig 2), group C had fused atria and separate ventricles, and group D had fused atria and ventricles (figs 3 and 4).
Figure 2 Contiguous but non‐fused hearts with no dividing pericardial wall between them. (A) Twins B1, 1985, with subaortic ventricular septal defect (VSD) in the twin on the right. (B) Twins B5, 1994, with improved resolution compared with (A). LV, left ventricle; RV, right ventricle.
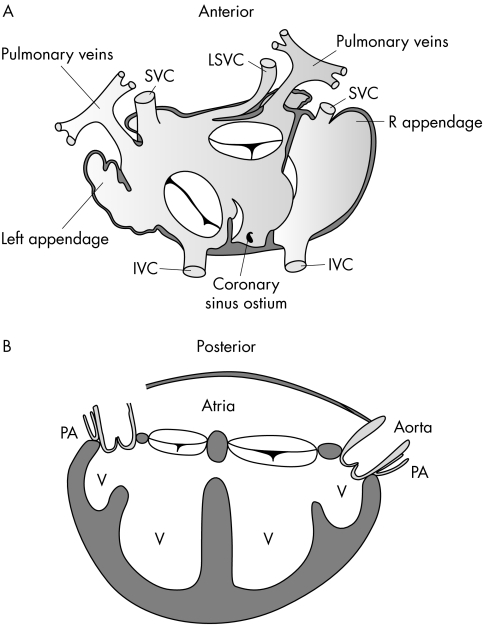
Figure 3 Drawings from the postmortem examination of twins D1. (A) Anatomical drawing of the systemic and pulmonary venous connections. IVC, inferior vena cava; LSVC, left superior vena cava; SVC, superior vena cava. (B) Schematic drawing of the arrangement of the ventricles (V) and great arteries. PA, pulmonary artery.
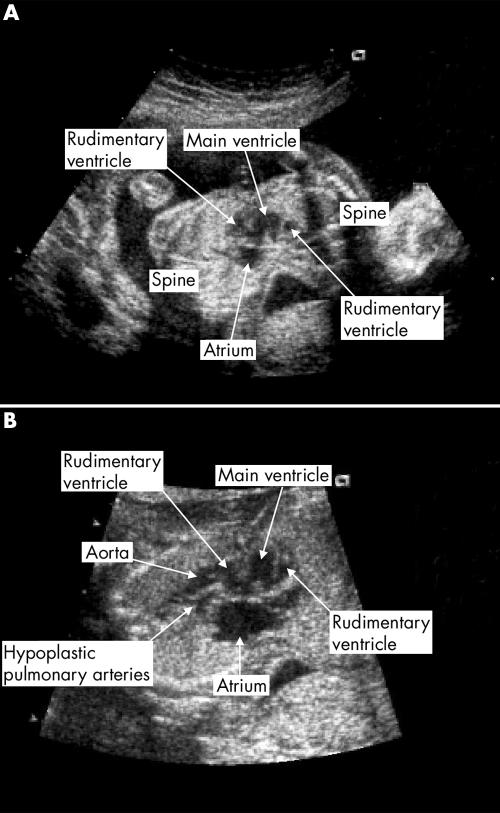
Figure 4 Fetal echocardiographic images at 20 weeks' gestation (twins D8, 2004). (A) Fused atria and ventricles. (B) Great arteries arising in parallel from a rudimentary ventricle. The pulmonary artery is hypoplastic.
RESULTS
Cardiac fusion
The type of conjoin was the major determinant of the degree of cardiac fusion, with the majority of thoraco‐omphalopagus twins in group A (table 1), the majority of parapagus twins in group B (table 2), and the majority of thoracopagus twins in groups C and D (tables 3 and 4).7,8,9 In the 15 sets of twins with anatomy verifiable by surgery or postmortem examination, the degree of cardiac fusion was correctly diagnosed in all but one set (twins C2). In these twins fetal echocardiography at 22 weeks was suggestive of cardiac fusion, with a common atrial mass and two atrioventricular valves overriding a complex ventricular structure. Two great arteries arose towards the right sided twin, but no outflow tracts could be seen arising towards the left sided twin. However, postnatal echocardiography suggested contiguous but non‐fused hearts, although the pericardium was shared. Twin 2 (right) had normal ICA but twin 1 had a severely hypoplastic heart, which was only about 20% of the expected size. The atrioventricular valves were severely regurgitant and there was a large VSD. Two small great arteries arose in parallel from the ventricular mass. The twins underwent surgery when twin 1 had an acute haemodynamic decompensation on day 1 of life. At separation, a single pericardial sac was identified and the atria were found to be connected. When this connection was divided, twin 1 died. Soon after pericardial closure twin 2 developed severe pulmonary hypertension and she died 33 hours later.
Table 1 Features of group A: separate hearts, separate pericardium.
| Twins and sex | Type | Timing of diagnosis | Twin 1 ICA/twin 2 ICA | Surgery | Outcome |
|---|---|---|---|---|---|
| A1 female | Thoraco‐omphalopagus | 17 weeks' gestation, postnatal echocardiogram | Normal/normal | Elective separation at 10 weeks | Both survived, now 7 years |
| A2 female | Thoraco‐omphalopagus | 17 weeks' gestation, postnatal echocardiogram | Normal/normal | Emergency separation at 1 day (volvulus) | Both survived, now 6 years |
| A3 male | Thoraco‐omphalopagus | 17 weeks' gestation, fetal echocardiogram | Normal/normal | Emergency separation at 1 day | One died during transfer, other now 5 years |
| A4 female | Parapagus | 14 weeks' gestation, fetal echocardiogram | Normal/AVSD, DORV, ant Ao, PAT | Emergency separation at 1 day for PHT | Both died |
| A5 female | Thoraco‐omphalopagus | 20 weeks' gestation, fetal echocardiogram | Normal/VSD (repaired at 8 weeks) | Elective separation at 7 weeks | Both survived, now 1 year |
ant Ao, anterior aorta; AVSD, atrioventricular septal defect; DORV, double outlet right ventricle; ICA, intracardiac anatomy; PAT, pulmonary atresia; PHT, pulmonary hypertension; VSD, ventricular septal defect.
Table 2 Features of group B: separate hearts, common pericardium.
| Twins and sex | Type | Timing of diagnosis | Twin 1 ICA/twin 2 ICA | Surgery | Outcome |
|---|---|---|---|---|---|
| B1 female | Thoracopagus | 38 weeks, postnatal echocardiogram and catheter | Normal/PAT and VSD, given prostaglandin | Emergency separation at 3 days | Twin 2 died at 5 weeks, other now 19 years |
| B2 male | Parapagus | At delivery, postnatal echocardiogram | Normal/normal | Elective separation at 8 months7 | Both survived, now 17 years |
| B3 female | Parapagus | 18 weeks' gestation, postnatal echocardiogram | Normal/normal | Elective separation at 3 years8 | Twin 2 died after surgery, other 12 years |
| B4 male | Parapagus | 22 weeks' gestation, postnatal echocardiogram | Normal/normal | Elective separation at 10 months | One died later at home, other 11 years |
| B5 female | Thoracopagus | 15 weeks' gestation, postnatal echocardiogram | Normal/normal | Elective separation at 3 months9 | Both survived, now 10 years |
| B6 female | Parapagus + normal triplet | 20 weeks, fetal echocardiogram | Normal/normal | Separation declined | Died at 5 days |
| B7 female | Thoraco‐omphalopagus | At delivery, postnatal echocardiogram | Normal/normal | Elective separation at 6 months | Both survived, now 1 year |
Table 3 Features of group C: fused atria, separate ventricles.
| Twins and sex | Type | Timing of diagnosis | Twin 1 ICA/twin 2 ICA | Surgery | Outcome |
|---|---|---|---|---|---|
| C1 male | Thoracopagus | 18 weeks' gestation, postnatal echocardiogram | Normal/AVSD, hypoplastic LV | Emergency separation at 5 days | Twin 2 died in surgery, other died at home |
| C2 female | Thoracopagus | 22 weeks' gestation, fetal echocardiogram | Hypoplastic heart, large VSD, ant Ao/normal | Emergency surgery at 1 day | Twin 1 died in surgery, other shortly after |
LV, left ventricle.
Table 4 Features of group D: atrial and ventricular fusion (all thoracopagus).
| Twins and sex | Timing of diagnosis | No of AV valves/ventricles | Twin 1/twin 2 great arteries | Timing of delivery/death |
|---|---|---|---|---|
| D1 female | Unknown time of gestation, postnatal echocardiogram and catheter | 2/4 | DOV, PAT/DOV, PAT | Term/3 days |
| D2 female | 28 weeks' gestation, postnatal echocardiogram and catheter | 2/2 | DOV with ant Ao, PS/DOV, NRGA, hypoplastic Ao | 37 weeks/4 weeks |
| D3 unknown | 18 weeks' gestation, fetal echocardiogram | 1/3 | DOV with ant Ao/single outlet | TOP at 20 weeks |
| D4 female | 17 weeks' gestation, fetal echocardiogram | 2/4 | NRGA/VA discordance, IAA | Term/first week of life |
| D5 female | 28 weeks' gestation, fetal echocardiogram | 1/4 | VA discordance/single outlet | 34 weeks/1 hour |
| D6 female | 14 weeks gestation, fetal echocardiogram | 1/2 | DOV, ant small Ao/PAT, MAPCAS | 36 weeks/4 weeks |
| D7 female | 15 weeks' gestation, fetal echocardiogram | 1/4 | Aortic atresia/single outlet | 28 weeks/30 minutes |
| D8 female | 20 weeks gestation, fetal echocardiogram | 2/3 | NRGA/DOV with ant Ao, PS | TOP at 20 weeks |
| D9 female | 15 weeks gestation, fetal echocardiogram | 2/3 | DOV, ant Ao/VA discordance | 35 weeks/30 hours |
AV, atrioventricular; DOV, double outlet ventricle; IAA, interrupted aortic arch; MAPCAS, multiple aortopulmonary collateral arteries; NRGA, normally related great arteries; PS, pulmonary stenosis; TOP, termination of pregnancy; VA, ventriculoarterial.
In one further set of twins (C1), a left atrial communication was correctly diagnosed on the postnatal echocardiogram but at surgery an additional aberrant artery was found arising from the descending aorta of twin 1, which inserted into the aortic arch of twin 2 between the origin of the left carotid artery and the left subclavian. It became clear that twin 1 was partially perfusing twin 2 and when this vessel was clamped twin 2 died. Twin 1 survived surgery but died unexpectedly at 6 weeks of age. Despite extensive investigation, no cause was found.
Intracardiac anatomy
As far as could be assessed by repeated echocardiography, catheterisation, surgery, or postmortem examination, the ICA was correctly diagnosed in all 18 sets seen postnatally. Of the seven sets who underwent both prenatal and postnatal echocardiography, the prenatal diagnosis had to be revised postnatally in three: one VSD turned out to be an atrioventricular septal defect (twins A4); one perimembranous VSD was seen only on postnatal echocardiography (twins A5); and one suspected single outlet heart had normal ventriculoarterial connections (twins B6). Abnormal ICA was far more common in thoracopagus twins (23 of 26 (88%)) than in either thoraco‐omphalopagus twins (one of 10) or parapagus twins (one of 10). It affected one twin only in two sets from group A, one set from group B, and both sets from group C. All twins in group D had very abnormal anatomy, which was discordant in eight of nine pairs as described below.
Twins D1 shared a single atrial chamber into which two inferior venae cava, three superior venae cava, and the pulmonary veins from each twin drained ipsilaterally. Two separate atrioventricular valves were connected to ventricles that communicated through a large muscular VSD. A rudimentary anterior ventricle on each side, connected to each main ventricle through a VSD, gave rise to a patent aorta and an atretic pulmonary artery in each twin. Figure 3 shows drawings of the postmortem findings.
Twins D2 shared a large common atrium, into which hepatic veins drained from twin 2 and a single inferior vena cava drained from twin 1. The pulmonary veins of each twin drained ipsilaterally. These twins had two atrioventricular valves and two ventricles, each of which were double outlet. From the ventricle on the side of twin 1, the great arteries arose in parallel, with the aorta anterior and a stenotic pulmonary artery posterior. From the ventricle on the side of twin 2, the great arteries were normally related but the ascending aorta and arch were hypoplastic.
Twins D3 shared a common atrium, which received systemic and pulmonary veins from each twin, and a common atrioventricular valve committed to two large ventricles. Two great arteries arose in parallel from the ipsilateral ventricle to one twin. One great artery to the other twin arose from a rudimentary ventricle that communicated with the other large ventricle.
Twins D4 shared a large common atrium. Twin 1 had a common atrioventricular valve orifice overriding two ventricles, of which the left was smaller. The great arteries were normally related. Twin 2 had a single atrioventricular valve connected to a good sized left ventricular cavity, a small restrictive VSD, and a hypoplastic right ventricle. They had ventriculoarterial discordance with a small aorta arising from the hypoplastic right ventricle and type B interruption of the aortic arch. The left ventricular cavities of each twin were fused, with at least two large defects allowing communication between them.
Twins D5 shared a complex fused heart with two atrial cavities. A common atrioventricular orifice overrode four ventricles, which were all in communication. Two great arteries arose in parallel in twin 1 but only a single arterial trunk arose in twin 2. Ventricular function was good but there was severe atrioventricular valve regurgitation accompanied by severe ascites most notable in twin 2.
Twins D6 shared a complex fused heart, which lay mainly in the thorax of twin 1. There was total anomalous pulmonary venous drainage from both twins to an enlarged right atrium and a common atrioventricular orifice overriding two ventricles. The left ventricle was hypoplastic. Two great arteries to twin 1 arose in parallel from the right ventricle but the aorta was smaller than the pulmonary artery. The aorta to twin 2 arose from the hypoplastic left ventricle but the pulmonary artery in this twin was atretic with multiple aortopulmonary collateral arteries.
Twins D7 shared a complex conjoined heart with two atria and four ventricles, of which two were rudimentary. The common atrioventricular valve had moderate regurgitation. Two great arteries arose towards twin 1, with the pulmonary artery much larger than the aorta at 15 weeks' gestation, but only one great vessel arising towards twin 2. By 20 weeks' gestation the aortic valve in twin 1 had become atretic, with retrograde flow around the aortic arch from the duct.
Twins D8 shared a common atrium, into which the systemic and pulmonary veins drained ipsilaterally. Two atrioventricular valves were committed to one large central ventricle, which was connected to a rudimentary ventricle on each side through a VSD. Twin 1 had normally related great arteries but in twin 2 the arteries arose in parallel from the ipsilateral rudimentary ventricle, with the aorta anterior. The pulmonary artery was hypoplastic. Figure 4 shows the echocardiographic images.
Twins D9 shared a heart that lay mainly in the thorax of twin 1 and consisted of a common atrium and two atrioventricular valves opening into two main ventricles, which were connected by an inlet VSD. An additional rudimentary ventricle on the left was also connected by a VSD. This gave rise to two great arteries to twin 1 that arose in parallel. The two main ventricles each gave rise to a great artery to twin 2 but there was ventriculoarterial discordance.
Ventricular function
Ventricular function was good in all sets scanned prenatally and postnatally correlated well with clinical condition. Poor preoperative ventricular function with high postoperative inotrope requirements was of concern in some twins early in the series, most notably twins B3.8 These parapagus twin girls were referred at 3 years of age for separation, and twin 2 had moderately reduced left ventricular function preoperatively. After separation her ventricular function deteriorated further despite aggressive fluid and blood replacement and inotropic support, and she died on the third postoperative day. At postmortem examination, the myocardium appeared pale and poorly developed, although myocardial histological analysis was normal. In view of this, subsequent sets of twins were given prophylactic preoperative inotropes if there was any concern over cardiac function, with good effect.
Separation
Thirteen sets of twins were surgically separated: five from group A, six from group B, and two from group C. Surgery was not offered to twins in group D. Six separations were undertaken as emergency procedures within the first week of life and seven were performed electively between 3 months and 3 years of age. In one emergency separation, one twin had died on the journey to our unit and the conjoined area had to be compressed digitally until the surviving twin could be separated (twins A3). There were 16 of a possible 26 survivors: seven from group A (70%) and nine from group B (75%)—four after emergency separation (33%) and 12 after elective separation (86%). None from group C or D survived. Three babies died relatively late after separation, from sets B1, B4, and C1 (see above). After separation of the B1 twins, the duct dependent circulation of twin 2 was maintained with prostaglandin. She subsequently developed obstructive jaundice, which necessitated a choledochoenterostomy and then a further laparotomy five weeks later. Two days after this she had a cardiopulmonary arrest and died. Postmortem examination confirmed the cardiac anatomy and showed the arterial duct to have closed. The B4 twins had a very prolonged postoperative course because of difficulties with wound closure and were discharged after 11 months. One twin subsequently died after an episode of aspiration.
DISCUSSION
Although the literature relating to conjoined twins is extensive, few large series have been reported and comparison is difficult because of continued discrepancies in nomenclature, despite standardised systems being proposed.10 Classification of thoracopagus, thoraco‐omphalopagus, and omphalopagus twins is particularly difficult. In this series we have described twins with ventral thoracic level fusion as thoracopagus and with ventral abdominal fusion extending to the lower part of the thorax as thoraco‐omphalopagus. We have excluded omphalopagus twins with ventral lower abdominal fusion only. Among the 23 sets of twins, thoracopagus connections were the most common and were associated with a greater degree of cardiac fusion and a higher risk of abnormal ICA than either thoraco‐omphalopagus or parapagus connections. This finding agrees with that of both postmortem and surgical series.6,11,12,13 In Group D, the number of ventricles varied between two and four, although one or two were often rudimentary. The anatomy of the atrioventricular junction was very variable but even hearts with more than two ventricles never had more than two atrioventricular valves. Four of nine sets had a common valve. Each pair had either three or four great arteries but one or more were hypoplastic or atretic in eight of nine. The concordance rate for abnormal ICA was low, consistent with non‐conjoined monochorionic twins.14
Ventricular fusion has been reported in parapagus twins but this was not seen in our series, although four of five had a common pericardium.11,12,13,15 Defects of lateralisation, including right and left atrial isomerism and mirror imagery, are particularly common in parapagus twins, although they are also seen in other forms.15,16 Usually the right sided twin is affected. In one pair of parapagus twins from this series, the right sided twin was isomeric (twins A4). Unfortunately no postmortem examination was done to confirm this. Although postmortem examinations were always requested, the majority of parents declined (11 of 16 pairs in which one or both babies died).
Historically, conjoined twins have been classified on the basis of cardiac fusion into groups with separate hearts with common pericardium, fused atria with normal ventricles, and fused atria and ventricles.4 However, this does not take into account twins with separate hearts and pericardium, which occurred in both thoraco‐omphalopagus and parapagus twins in this series. Our classification includes this group of patients. Although surgical survival rates were not significantly different in pairs with or without pericardial fusion, detailed knowledge of the anatomy allows meticulous planning of the operative strategy, which is a key factor in successful surgery.2
An accurate understanding of the degree of cardiac fusion and the ICA is essential to determine the potential for surgical separation of twins with thoracic level fusion. Our policy has been to offer surgery to twins in groups A–C. All parents of twins with type D anatomy were counselled that separation would not be feasible. In addition, accurate assessment of ventricular function is essential for successful surgery. The unexpected death of twin B3‐2 secondary to ventricular dysfunction caused us to institute prophylactic inotropy in twins B5, with good effect.
The experience reported in this series was accumulated over 20 years, and the quality and availability of imaging modalities has changed considerably over this time. Echocardiography has provided accurate assessment of cardiac fusion, ICA, and ventricular function throughout, so that cardiac catheterisation and angiography were performed in only three cases early in the experience. Angiography may occasionally still be required if the anatomy severely restricts the echocardiographic windows or if uncertainty about the degree of cardiac fusion persists.17,18,19
As the resolution of ultrasound has improved, assessing the heart prenatally has become possible at earlier gestations. Diagnostic images have been reported as early as nine weeks' gestation.12 In our series, although all twins from the UK and Ireland were assessed antenatally, not all were referred for prenatal echocardiography, even as late as 1998. Referral rates were higher for thoracopagus twins, but as abnormal ICA may be seen in all three types of thoracic level fusion, we would recommend that all types should be referred. Prenatal diagnosis is technically easier for the operator, as it avoids the anatomical constraints of scanning fused chests postnatally, and fetal fluid filled lungs may allow better images. Recently it has become possible to assess conjoined fetuses by three dimensional echocardiography, and this may be helpful for the parents, as the images are easier to understand.20
Magnetic resonance imaging has an increasing role in multisystem evaluation of conjoined twins before separation and has the advantage of being able to produce three dimensional reconstructed images in any orientation as well as providing information about intracardiac and great vessel blood flow.21,22,23 Multislice computed tomography with its rapid acquisition times may also be useful, particularly in assessing details of arterial and venous anatomy. However, these sophisticated imaging techniques will continue to be used as adjuncts to echocardiography, which is likely to remain the bedrock of cardiac assessment for the foreseeable future.
Results from surgical separation have improved over time, helped by advances in imaging allowing for increasingly meticulous preoperative planning, and in intensive care.1,24 Echocardiography performed by an experienced operator is a key part of both preoperative and postoperative management and provides an accurate, safe, non‐invasive, and easily portable method of assessing the cardiovascular system of these complex infants.
ACKNOWLEDGEMENTS
The authors thank the Pathology Department of Bristol Royal Infirmary for supplying the postmortem drawings of the shared heart of twins D1.
Footnotes
Conflicts of interest: none
References
- 1.Spitz L, Kiely E M. Conjoined twins. JAMA 20032891307–1310. [DOI] [PubMed] [Google Scholar]
- 2.Spitz L, Kiely E M. Experience in the management of conjoined twins. Br J Surg 2002891188–1192. [DOI] [PubMed] [Google Scholar]
- 3.Nichols B L, Blattner R J, Rudolph A J. Obstetric management of conjoined twins. Birth Defects 1967338–51. [Google Scholar]
- 4.Leachman R D, Latson J R, Kohler C M.et al Cardiovascular evaluation of conjoined twins. Birth Defects 1967352–62. [Google Scholar]
- 5.Synhorst D, Matlak M, Roan Y.et al Separation of conjoined twins joined at the right atria. Am J Cardiol 197943662–665. [DOI] [PubMed] [Google Scholar]
- 6.Cywes S, Millar A J, Rode H.et al Conjoined twins: the Cape Town experience. Pediatr Surg Int 199712234–248. [DOI] [PubMed] [Google Scholar]
- 7.Spitz L, Capps S N J, Kiely E M. Xiphoomphaloischiopagus tripus conjoined twins: successful separation following abdominal wall expansion. J Pediatr Surg 19912626–29. [DOI] [PubMed] [Google Scholar]
- 8.Spitz L, Stringer M D, Kiely E M.et al Separation of brachio‐thoraco‐omphalo‐ischiopagus bipus conjoined twins. J Pediatr Surg 199429477–481. [DOI] [PubMed] [Google Scholar]
- 9.Spitz L, Crabbe D C, Kiely E M. Separation of thoraco‐omphalopagus conjoined twins with complex hepatobiliary anatomy. J Pediatr Surg 199732787–789. [DOI] [PubMed] [Google Scholar]
- 10.Spencer R. Anatomic description of conjoined twins: a plea for standardized terminology. J Pediatr Surg 199631941–944. [DOI] [PubMed] [Google Scholar]
- 11.Gerlis L M, Seo J W, Ho S Y.et al Morphology of the cardiovascular system in conjoined twins: spatial and sequential segmental arrangements in 36 cases. Teratology 19934791–108. [DOI] [PubMed] [Google Scholar]
- 12.Mackenzie T C, Crombleholme T M, Johnson M P.et al The natural history of prenatally diagnosed conjoined twins. J Pediatr Surg 200237303–309. [DOI] [PubMed] [Google Scholar]
- 13.Barth R A, Filly R A, Goldberg M D.et al Conjoined twins: prenatal diagnosis and assessment of associated malformations. Radiology 1990177201–207. [DOI] [PubMed] [Google Scholar]
- 14.Dumontier J, Fermont L, Le Bidois J. Congenital heart defects in monochorionic twin gestations [abstract]. Cardiol Young 200212(suppl 1)42 [Google Scholar]
- 15.Cunniff C, Jones K L, Jones M C.et al Laterality defects in conjoined twins: implications for normal asymmetry in human embryogenesis. Am J Med Genet 198831669–677. [DOI] [PubMed] [Google Scholar]
- 16.Levin M, Roberts D J, Holmes L B.et al Laterality defects in conjoined twins. Nature 1996384321. [DOI] [PubMed] [Google Scholar]
- 17.McMahon C J, Mullins C E, Vick G W., 3rdet al Cardiac catheterization in the diagnosis and management of congenital heart disease in thoracopagus conjoined twins. Catheter Cardiovasc Interv 200051159–167. [DOI] [PubMed] [Google Scholar]
- 18.Beghetti M, Abdel‐Massih T, Bonhoeffer P. Images in cardiology. Thoraco‐omphalagus twins: heart to heart, Heart 200287278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMahon C J, Vick G W, 3rd, Nihill M R. Contiguous but non‐fused ventricles in conjoined thoracopagus twins. Cardiol Young 200212284–285. [DOI] [PubMed] [Google Scholar]
- 20.Bonilla‐Musoles F, Machado L E, Osborne N G.et al Two dimensional and three dimensional sonography of conjoined twins. J Clin Ultrasound 20023068–75. [DOI] [PubMed] [Google Scholar]
- 21.Kingston C A, McHugh K, Kumaradevan J.et al Imaging in the preoperative assessment of conjoined twins. Radiographics 2001211187–1208. [DOI] [PubMed] [Google Scholar]
- 22.Martinez L, Fernandez J, Pastor I.et al The contribution of modern imaging to planning separation strategies in conjoined twins. Eur J Pediatr Surg 200313120–124. [DOI] [PubMed] [Google Scholar]
- 23.Norwitz E R, Hoyte L P, Jenkins K J.et al Separation of conjoined twins with the twin reversed‐arterial‐perfusion sequence after prenatal planning with three‐dimensional modeling. N Engl J Med 2000343399–402. [DOI] [PubMed] [Google Scholar]
- 24.Spitz L, Kiely E M. Success rate for surgery of conjoined twins [letter]. Lancet 20003561765. [DOI] [PubMed] [Google Scholar]