Abstract
Objective
To evaluate the role of factor V Leiden, prothrombin G20210A polymorphism, plasminogen activator inhibitor type 1 (PAI‐1) 4G/5G polymorphism, PAI‐1, homocysteine, and lipoprotein (a) (Lp(a)) in the occurrence of major adverse cardiac events (MACE) in patients with acute coronary syndromes who underwent coronary stenting.
Design
520 patients (375 men and 145 women) with acute coronary syndromes and 520 age and sex matched controls were enrolled. MACE were recorded for 109 patients. Heterozygosity for factor V Leiden, prothrombin G20210A polymorphism, and 4G/5G polymorphism did not significantly differ between patients with and without MACE. A significantly higher percentage of patients with increased homocysteine (28% v 19%, p < 0.001) and PAI‐1 concentrations (25% v 16%, p < 0.001) had MACE with respect to those who did not. In Kaplan‐Meier survival analysis, the overall risk of MACE was significantly higher among patients with increased PAI‐1 (p = 0.006) and homocysteine concentrations (p = 0.04). Cox regression analysis adjusted for age, sex, traditional cardiovascular risk factors, renal function, systolic left ventricular function, the number of stenosed vessels, and history of percutaneous coronary intervention or coronary artery bypass grafting showed that homocysteine (odds ratio 7.5, 95% confidence interval (CI) 1.1 to 57.7, p < 0.05) and PAI‐1 concentrations (odds ratio 5.3, 95% CI 1.2 to 23.8, p < 0.05) within the fifth quintile (with respect to the first) were significant and independent risk factors for the future occurrence of MACE.
Conclusions
Increased PAI‐1 and homocysteine concentrations are independent risk factors for MACE after successful coronary stenting, whereas Lp(a) and thrombophilic polymorphisms are not predictive.
Keywords: coronary stenting, MACE, genetic polymorphisms, homocysteine, PAI‐1
The introduction of intracoronary stents into clinical practice dramatically changed the strategy of treatment of coronary artery disease (CAD). In particular, in patients with acute coronary syndromes (ACS), primary coronary angioplasty with stent implantation has been shown to reduce in‐hospital mortality.1,2 As regards the incidence of major adverse cardiac events (MACE)—defined as death, myocardial infarction (MI), or target lesion revascularisation—several clinical factors have been documented to be associated with an adverse outcome after ACS. In particular, the rapid increase in the number of stent implantations shows in‐stent restenosis to be a new entity with significant clinical and socioeconomic implications. Repeated angioplasty is the treatment of choice even if it is characterised by a 30% rate of MACE at a mean (SD) of 9 (4) months after the procedure. In 90% of cases, the rate of MACE is driven by the need for target lesion revascularisation, particularly as a result of recurrent restenosis.2
Ongoing secondary prevention efforts include the identification and validation of novel biochemical and genetic factors that increase the rate of MACE.
Thrombophilic polymorphisms such as prothrombin gene mutation G20210A and factor V gene mutation G1619A (factor V Leiden) are established risk factors for venous thromboembolism,3 but their role in arterial thrombosis4 is controversial and no data are available on their possible role in the occurrence of MACE after coronary stenting. Data obtained on a limited number of patients are available on the association between plasminogen activator inhibitor type 1 (PAI‐1) circulating concentration and MACE after coronary stenting.5,6,7,8 Contrasting data have been obtained on the association between homocysteine concentration and both restenosis and mortality9,10,11,12,13,14,15 in patients who underwent coronary revascularisation with stent implantation, whereas two studies on lipoprotein (a) (Lp(a)) concentration and coronary stenting provided inconsistent results.16,17
The objective of our study was to thoroughly evaluate the association between the occurrence of MACE in patients with ACS who underwent successful percutaneous coronary intervention (PCI) with stent implantation and the presence of genetic and metabolic thrombophilic risk factors: factor V Leiden, prothrombin G20210A and PAI‐1 4G/5G polymorphisms, and PAI‐1, homocysteine, and Lp(a) concentrations.
METHODS
Patients investigated
The study population consisted of 530 consecutive adult patients admitted to the coronary care unit of the University of Florence with a diagnosis of acute MI or unstable angina. All patients underwent coronary angiography performed by the Judkins method and PCI with bar metal stent implantation for de novo stenosis in one or more native coronary arteries. Ten patients were excluded, as PCI was not successful (> 30% residual stenosis), so that the final patient group comprised 520 patients. The diagnosis of unstable angina at presentation was based on a history of crescendo angina or angina of new onset (within one month) in the absence of ECG and cardiac enzyme changes indicative of an acute MI. Acute MI was diagnosed as an increase in creatine kinase (or its MB isoenzyme) concentration at least twice the upper normal limit with at least one of the following: acute onset of prolonged typical ischaemic chest pain; and ST segment elevation or depression in two or more leads, ⩾ 0.2 mV in leads V1–V3, or > 0.1 mV in other leads.
The patients were considered to have hypertension if they had hypertension diagnosed according to the guidelines of the European Society of Hypertension/European Society of Cardiology or were taking antihypertensive drugs.18 A positive family history was defined as the presence of at least one first degree relative who had developed CAD before the age of 55 years for men and 65 years for women. Dyslipidaemia was defined according to the third report of the National Cholesterol Education Program19 and diabetes was defined in agreement with the American Diabetes Association.20
The control group consisted of 520 volunteers, sex and age (by 10 years) matched, recruited from the staff of the University of Florence and Careggi Hospital and from partners or friends of patients.
A structured questionnaire identified symptom‐free controls and excluded patients who were suspected of having any form of vascular disease.
Informed written consent was obtained from all participants and the study was approved by the local ethics review board.
Follow up
Data were obtained by a structured telephone interview and a clinical evaluation in case of clinical recurrences or new PCI.
The clinical end point was a composite of MACE: cardiac death (defined as death from MI, pump failure, sudden cardiac death, or death because of arrhythmias), MI, and target lesion revascularisation for symptomatic restenosis. Restenosis was defined as a diameter stenosis ⩾ 50% in patients needing target vessel revascularisation because of symptoms or signs of ischaemia.
Experimental procedures
Blood samples were collected from the basilic vein at the time of admission to hospital.
Homocysteine concentration was measured by an immunoassay method (fluorescence polarisation immunoassay, IMX system; Abbott) in plasma samples obtained after centrifuging blood collected into tubes containing EDTA. Lp(a) concentration was measured in serum by an enzyme linked immunoassay (ELISA) method with a commercial kit (Apo(a) ELISA; Mercodia), which contains monoclonal antibodies that minimise the possible interference of heterogeneity in apolipoprotein(a) isoforms with the results. PAI‐1 activity was determined by a chromogenic method (Spectrolyse Biopool, Umea, Sweden) in plasma obtained after centrifuging blood collected into tubes containing sodium citrate. For detection of factor V Leiden, G20210A polymorphism in the factor II gene, and PAI‐1 4G/5G polymorphism, genomic DNA was extracted from peripheral blood leucocytes with a MagNA Pure (Roche, Mannheim, Germany) and QUIAmp Blood Kit (QUIAGEN, Hilden, Germany), respectively. Factor V Leiden and G20210A polymorphism in the factor II gene were identified by the LightCycler Capillaries method (Roche). PAI‐1 4G/5G polymorphism was identified by polymerase chain reaction amplification and digestion with BslI restriction enzyme.8
Statistical analysis
Data were analysed with SPSS (version 11.5; Chicago, Illinois, USA) software for Windows. Values are presented either as mean (SD) or as median (range). The non‐parametric Mann‐Whitney test for unpaired data and χ2 test for categorical variables were used for comparisons between groups. Fasting hyperhomocysteinaemia was defined based on the 95th centile cut off of the control population (19 μmol/l for men; 15 μmol/l for women). Increased PAI‐1 concentration was defined based on the 90th centile of the control population (14.9 IU/mol). An Lp(a) concentration of 300 mg/l, which is widely accepted as the cut off of increased vascular risk, was considered to separate normal from pathological samples. The continuous variables—homocysteine, PAI‐1, and Lp(a)—were divided into quintiles (q1–5) based on the distribution of these parameters in patients and controls (homocysteine: q1, ⩽ 9; q2, 9.1–10.2; q3, 10.3–12; q4, 12.1–15.6; q5, > 15.6 μmol/l; PAI‐1: q1, ⩽ 7.2; q2, 7.3–9; q3, 9.1–13.4; q4, 13.5–20.3; q5, > 20.3 IU/ml; Lp(a): q1, ⩽ 68; q2, 69–109; q3, 110–226; q4, 227–410; q5, > 410 mg/l).
For multivariate analysis, unconditional logistic regression analyses were used with age (continuous variable), sex, traditional cardiovascular risk factors (hypertension, smoking habit, diabetes, dyslipidaemia, and family history of CAD), creatinine concentration, and thrombophilic risk factors as the independent variables and presence versus absence of the disease as the dependent variable.
For the analysis of MACE in relation to the time of the event, Cox regression analysis was used with age (continuous variable), sex, traditional cardiovascular risk factors (hypertension, smoking habit, diabetes, dyslipidaemia, and family history of CAD), creatinine concentration, thrombophilic risk factors, ejection fraction, the number of stenosed coronary vessels, and history of PCI or coronary artery bypass grafting (CABG) as the independent variables.
In addition, we analysed the interval from hospital admission to either the occurrence of MACE (uncensored observations) or to the telephone interview (censored observation) to estimate the probability of MACE as a function of time, according to the method of Kaplan‐Meier. The probability of MACE was compared between groups with use of the log rank test. All odds ratios (ORs) are given with their 95% confidence intervals (CIs). All probability values are two tailed with p < 0.05 considered to be significant.
RESULTS
Table 1 lists the clinical characteristics of patients and controls. Two hundred and eighty two (54.2%) patients were admitted to our coronary care unit with a diagnosis of MI and 238 (45.8%) with unstable angina. At coronary angiography, one vessel disease was present in 364 (70%) patients, whereas two vessel and multivessel disease was observed in 115 (22.1%) and 41 (7.9%) patients, respectively.
Table 1 Baseline characteristics of patients and controls.
| Characteristic | Patients (n = 520) | Controls (n = 520) |
|---|---|---|
| Age (years) | 67 (32–95) | 67 (32–89) |
| Men | 375 (72.1%) | 375 (72.1%) |
| Smoking habit | 279 (53.7%) | 117 (22.5%)* |
| Hypertension | 311 (59.8%) | 167 (32.1%)* |
| Diabetes | 134 (25.8%) | 20 (3.8%)* |
| Dyslipidaemia | 235 (45.2%) | 119 (22.8%)* |
| Family history | 177 (34%) | 49 (9.4%)* |
| Creatinine (μmol/l) | 84.0 (16.5) | 82.2 (17.7) |
| BMI (kg/m2) | 25.8 (2.8) | 24.5 (3.1) |
| History of MI | 18 (3.5%) | 0 |
| PCI | 38 (7.3%) | 0 |
| CABG | 17 (3.3%) | 0 |
Data are median (range), mean (SD), or number (%).
*p<0.0001.
BMI, body mass index; CABG, coronary artery bypass grafting; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Prevalence of thrombophilic risk factors
No significant differences between patients and controls were documented either in the prevalence of heterozygosity for factor V Leiden or prothrombin polymorphism or in the prevalence of homozygosity for PAI‐1 4G/5G polymorphism (table 2).
Table 2 Thrombophilic risk factors.
| Risk factor | Patients (n = 520) | Controls (n = 520) | p Value |
|---|---|---|---|
| Homocysteine (µmol/l) | 12.7 (3–95) | 10 (6–24) | 0.001 |
| PAI‐1 (IU/ml) | 16 (1–64) | 8 (4–38) | 0.001 |
| Lp(a) (mg/l) | 170 (1–2141) | 135 (7–1390) | 0.02 |
| Heterozygosity for factor V Leiden | 20 (3.8%) | 20 (3.8%) | 0.99 |
| Heterozygosity for factor II G20210A | 21 (4.0%) | 19 (3.6%) | 0.87 |
| Homozygosity for PAI‐1 4G/5G | 166 (31.9%) | 156 (30%) | 0.55 |
Data are median (range) or number (%).
PAI‐1, plasminogen activator inhibitor type 1.
Homocysteine, Lp(a), and PAI‐1 plasma concentrations were significantly higher in patients than in controls (table 2). Hyperhomocysteinaemia was diagnosed in 115 of 520 patients (22.1%) and in 26 of 520 controls (5%). Lp(a) concentrations above 300 mg/l were found in 185 of 520 patients (35.6%) and in 108 of 520 (20.8%) controls. PAI‐1 concentration was increased in 292 of 520 (56.2%) patients and in 52 of 520 (10%) controls. No significant difference was observed in the circulating PAI‐1 concentrations according to PAI‐1 4G/5G genotype either in patients or in controls (table 3).
Table 3 PAI‐1 activity (IU/ml) according to PAI‐4G/5G genotype.
| 5G/5G | 4G/5G | 4G/4G | p Value | |
|---|---|---|---|---|
| Patients (n = 520) | 15.2 (1–64) | 16.0 (1–58) | 17.3 (1–64) | 0.33 |
| Controls (n = 520) | 7.9 (4–35) | 8.0 (4–38) | 8.3 (4–36) | 0.39 |
Data are median (range).
At multivariate regression analysis adjusted for age, sex, creatinine concentration, and the traditional cardiovascular risk factors—homocysteine, PAI‐1, and Lp(a) within the fifth quintile with respect to the first—were significant and independent risk factors for ACS (PAI‐1: OR 24.2, 95% CI 11.1 to 32.5.0, p < 0.0001; homocysteine: OR 12.1, 95% CI 5.3 to 19.2, p < 0.0001; Lp(a): OR 3.5, 95% CI 1.7 to 5.6, p < 0.005).
No significant differences in the prevalence of thrombophilic risk factors were documented between patients with unstable angina and those with MI (data not shown).
Follow up
All patients were followed up for a mean (SD) duration of 22.2 (3.9) months (median 24, range 12–26 months). At the end of follow up, MACE were recorded in 109 patients. Fifty four (10.4%) patients died. Fifty two of 54 deaths were classified as due to cardiovascular causes: 21 acute MI, 10 sudden deaths, 10 congestive heart failure, 10 cerebrovascular events, and one ruptured abdominal aneurysm. Two non‐cardiovascular deaths were due to cancer. Of 52 patients who died of cardiovascular causes, 47 had a diagnosis at admission of MI and five had unstable angina.
Table 4 shows clinical characteristics of patients with and without MACE. The prevalence of MI among patients with MACE was significantly higher than the prevalence of unstable angina. On the other hand, systolic left ventricular function measured before discharge from hospital and estimated by the ejection fraction was significantly different between the two groups. Concerning previous revascularisation procedures, a higher percentage of patients in the MACE group had a previous PCI or CABG (table 4). A similar percentage of patients with and without traditional cardiovascular risk factors had MACE (MACE in smoking versus no smoking: 21.5% v 25.5%; in hypertension versus no hypertension: 21.5% v 20%; in dyslipidaemia versus no dyslipidaemia: 22.1% v 22.2%; in diabetes versus no diabetes: 23.1% v 20%; and in overweight versus no overweight: 19% v 22%). A significantly higher percentage of patients with ejection fraction ⩽ 40% than with ejection fraction > 40% had MACE (25.4% v 19.8%, p < 0.01).
Table 4 Clinical characteristics of patients according to the occurrence of major adverse cardiac events (MACE).
| MACE (n = 109) | No MACE (n = 411) | p Value | |
|---|---|---|---|
| Age (years) | 70 (32–95) | 67 (35–95) | 0.2 |
| Men | 86 (71%) | 289 (70.3%) | 0.2 |
| Smoking habit | 64 (58.7%) | 215 (52.3%) | 0.7 |
| Hypertension | 67 (61.5%) | 244 (59.4%) | 0.7 |
| Diabetes | 31 (28.4%) | 103 (25%) | 0.4 |
| Dyslipidaemia | 52 (47.7%) | 183 (44.5%) | 0.5 |
| BMI >25 kg/m2 | 50 (45.8%) | 188 (45.7%) | 0.4 |
| Ejection fraction (%) | 45 (12.6) | 50 (12.8) | 0.007 |
| Diagnosis at admission | |||
| Unstable angina | 41 (37.6%) | 197 (47.9%) | 0.05 |
| MI | 68 (62.4%) | 214 (52.1%) | 0.05 |
| History of MI | 18 (16.5) | 0 | NA |
| PCI | 31 (28.4%) | 7 (1.7%) | 0.001 |
| CABG | 12 (11%) | 5 (1.2%) | 0.001 |
Data are median (range), mean (SD), or number (%).
NA, not applicable.
Thrombophilic risk factors and MACE
Heterozygosity for factor V Leiden and prothrombin polymorphism and homozygosity for PAI‐1 4G/5G polymorphism did not differ significantly between patients with and without MACE (table 5).
Table 5 Thrombophilic risk factors according to the occurrence of MACE.
| MACE (n = 109) | No MACE (n = 411) | p Value | |
|---|---|---|---|
| Homocysteine (μmol/l) | 16 (3–95) | 12.3 (3.3–90.7) | 0.001 |
| PAI‐1 (IU/ml) | 19 (2–48) | 16 (1–64) | 0.001 |
| Lp(a) (mg/l) | 191.5 (1–1962) | 168 (2–2141) | 0.8 |
| Heterozygosity for factor V Leiden | 4 (3.7%) | 16 (3.9%) | 0.8 |
| Heterozygosity for factor II G20210A | 4 (3.7%) | 17 (4.1%) | 0.8 |
| Homozygosity for PAI‐14G/5G | 37 (33.9%) | 129 (31.4%) | 0.5 |
Data are median (range) or number (%).
Homocysteine and PAI‐1 plasma concentrations, measured at hospital admission, were significantly higher in patients with MACE (table 5). A significantly higher percentage of patients with increased homocysteine (28% v 19%, p < 0.001) and PAI‐1 concentrations (25% v 15.7%, p < 0.001) had MACE.
Lp(a) plasma concentrations were not significantly different between patients with and without MACE (table 5). A similar percentage of patients with and without increased Lp(a) concentrations had MACE (22.7% v 20%).
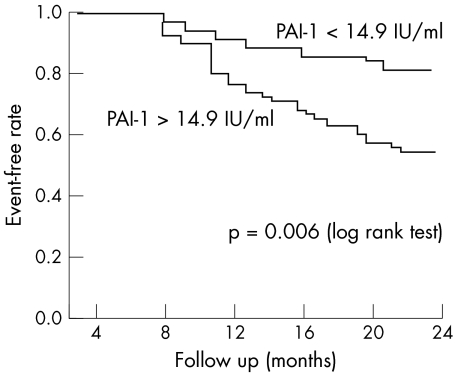
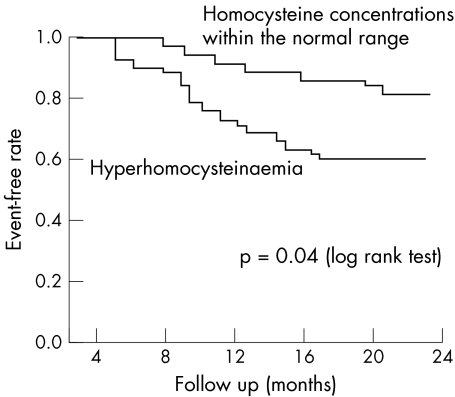
Kaplan‐Meier survival curves showed that the overall risk of MACE was significantly higher among patients with increased PAI‐1 (p = 0.006) (fig 1) or homocysteine concentration (p = 0.04) (fig 2), whereas no significant differences were found for Lp(a) concentration or for the presence of the investigated polymorphisms.
Figure 1 Kaplan‐Meier event‐free rates according to plasminogen activator inhibitor type 1 (PAI‐1) concentrations.
Figure 2 Kaplan‐Meier event‐free rates according to homocysteine concentrations.
Cox regression analysis adjusted for age, sex, traditional cardiovascular risk factors, creatinine concentration, systolic left ventricular function, number of coronary stenosed vessels, and history of PCI or CABG showed that homocysteine and PAI‐1 within the fifth quintile with respect to the first were significant and independent risk factors for the occurrence of MACE (homocysteine: q1, reference group; q2, OR 3.5, 95% CI 0.3 to 41, not significant; q3, OR 3.4, 95% CI 0.3 to 38.2, not significant; q4, OR 3.8, 95% CI 0.5 to 45, not significant; q5, OR 7.5, 95% CI 1.1 to 57.7, p < 0.05; PAI‐1: q1, reference group; q2, OR 1.7, 95% CI 0.2 to 13.1, not significant; q3, OR 2.8, 95% CI 0.5 to 14.1, not significant; q4, OR 4.4, 95% CI 1.0 to 19.1, not significant; q5, OR 5.3, 95% CI 1.2 to 23.8, p < 0.05).
DISCUSSION
We have thoroughly investigated, for the first time, the possible role of some genetic and metabolic thrombophilic risk factors in the occurrence of MACE after successful coronary stenting. The main finding of our study is that increased PAI‐1 and homocysteine concentrations at hospital admission are independent risk factors for the occurrence of MACE in patients with ACS who undergo PCI with stent implantation. Furthermore, we failed to find any significant role of the thrombophilic polymorphisms investigated (factor V Leiden, prothrombin polymorphism, and PAI 4G/5G) and of increased Lp(a) concentration as prognostic risk factors for MACE. In addition, PAI‐1 4G/5G polymorphism did not significantly affect PAI‐1 concentration measured at the time of the acute event, suggesting that the influence of the inflammatory state on PAI‐1 concentration is scarcely genetically modulated.
Some studies have suggested, in addition to the traditional risk factors, the existence of metabolic, haemostatic, and genetic risk markers for CAD. Relations have been found between increased baseline concentrations of Lp(a), homocysteine, and PAI‐1 and the risk of CAD.21,22,23 Other studies have associated baseline PAI‐1 concentration with subsequent cardiac events in patients with angina, MI, or angiographic CAD.5,6,7,8 However, increased PAI‐1 concentration was shown to predict new acute coronary events, including death and restenosis after coronary stenting, in only two studies of a very low number of patients.7,8
Contrasting data are available on the association between homocysteine concentration and MACE after coronary stenting,9,10,11,12,13,14,15 whereas only two studies—with contrasting results—have investigated the role of Lp(a) after stent implantation.16,17 Lastly, no data are available on the prevalence of thrombophilic polymorphisms—factor V Leiden and prothrombin polymorphism—in patients with MACE after coronary stenting.
Data from the literature have documented that PAI‐1, homocysteine, and Lp(a) act as acute phase reactants.24,25,26,27,28 Therefore, baseline concentrations measured at hospital admission have been influenced, at least in part, by the acute coronary event. Furthermore, our result could have varied if we had assayed concentrations at different days after admission. Nevertheless, these concentrations seem to predict the future occurrence of MACE, suggesting that a more pronounced increase during the acute phase identifies a subgroup of patients at higher risk for new events. Determining the thrombophilic risk profile, and in particular PAI‐1 and homocysteine concentrations of patients with ACS at the time of the acute event, makes possible the selection of patients who will need more intensive follow up after hospital discharge. In addition, these markers may be new potential pharmacological targets. Previous interventional trials with angiotensin converting enzyme inhibitors and statins have suggested that this class of drugs benefits the fibrinolytic system.29,30 Furthermore, it has recently been reported that thiazolidinediones, the new class of insulin sensitising drugs, can reduce PAI‐1 protein expression in human pre‐adipocytes under basal conditions and after stimulation of the cells with transforming growth factor β.31 Accordingly, patients with insulin resistance may benefit from this class of drugs, even in terms of reduction of PAI‐1 concentrations.
We and others have previously shown that homocysteine is an independent risk factor for restenosis after PCI with and without stenting, although other authors did not confirm these data.9,10,11,12,13,14 In the present study, we found a significant association between hyperhomocysteinaemia and MACE. Increased homocysteine concentrations may be easily reduced by vitamin supplementation with folic acid, vitamin B6, and vitamin B12.32 However, contrasting data are available on the possible clinical benefits resulting from the pharmacological correction of homocysteine. Indeed, some authors reported a positive effect in terms of reduced restenosis rate, reduced progression of carotid intima–media thickness, or reduced number of positive ergometric tests.33,34,35 On the other hand, other authors recently reported a negative effect of vitamin supplementation on the occurrence of restenosis.10 Contradictory data may be attributed to the different doses of vitamins used in the studies, to the different homocysteine concentrations in the patients enrolled, or to the possibility that homocysteine is only a marker of a metabolic derangement in which the leading actor is methionine instead of homocysteine.36 Interventional randomised trials designed to test the effect of homocysteine lowering treatment on multiple clinical end points are ongoing.
An original finding of our study is the lack of a role of thrombophilic polymorphisms in the occurrence of MACE after ACS. No association was reported between factor V Leiden and restenosis after PCI (with a follow up of six months) without stent implantation. No data at all are available on the prevalence of prothrombin polymorphism and MACE after PCI with or without stenting. As regards MACE, factor V Leiden and prothrombin polymorphism were shown not to be associated with increased susceptibility to sudden cardiac death among apparently healthy adults.37 Our present study did not find independent and consistent associations between the investigated genes and the risk of MACE after PCI with stent implantation, but the number of MACE is probably not enough to obtain a definitive result, given the prevalences of genes investigated in the general population.
In conclusion, our study showed that increased PAI‐1 and homocysteine concentrations, but not Lp(a) and thrombophilic polymorphisms, are independent risk factors for MACE after successful coronary stenting. Determining the concentrations of PAI‐1 and homocysteine at the time of the acute event may allow selection of patients at higher risk for future new events.
Abbreviations
ACS - acute coronary syndromes
CABG - coronary artery bypass grafting
CAD - coronary artery disease
CI - confidence interval
ELISA - enzyme linked immunoassay
Lp(a) - lipoprotein (a)
MACE - major adverse cardiac events
MI - myocardial infarction
OR - odds ratio
PAI‐1 - plasminogen activator inhibitor type 1
PCI - percutaneous coronary intervention
q1–5 - first to fifth quintile
References
- 1.Keeley E C, Grines C L. Primary percutaneous coronary intervention for every patient with ST‐segment elevation myocardial infarction: what stands in the way? Ann Intern Med 2004141298–304. [DOI] [PubMed] [Google Scholar]
- 2.Radke P W, Kaiser A, Frost C.et al Outcome after treatment of coronary in‐stent restenosis; results from a systematic review using meta‐analysis techniques. Eur Heart J 200324266–273. [DOI] [PubMed] [Google Scholar]
- 3.Margaglione M, Brancaccio V, De Lucia D.et al Inherited thrombophilic risk factors and venous thromboembolism: distinct role in peripheral deep venous thrombosis and pulmonary embolism. Chest 20001181405–1411. [DOI] [PubMed] [Google Scholar]
- 4.Kim R J, Becker R C. Association between factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations and events of the arterial circulatory system: a meta‐analysis of published studies. Am Heart J 2003146948–957. [DOI] [PubMed] [Google Scholar]
- 5.Soeki T, Tamura Y, Shinohara H.et al Plasma concentrations of fibrinolytic factors in the subacute phase of myocardial infarction predict recurrent myocardial infarction or sudden cardiac death. Int J Cardiol 200285277–283. [DOI] [PubMed] [Google Scholar]
- 6.Anvari A, Schuster E, Gottsauner‐Wolf M.et al PAI‐1 4G/5G polymorphism and sudden cardiac death in patients with coronary artery disease. Thromb Res 2001103103–107. [DOI] [PubMed] [Google Scholar]
- 7.Strauss B H, Lau H K, Bowman K A.et al Plasma urokinase antigen and plasminogen activator inhibitor‐1 antigen levels predict angiographic coronary restenosis. Circulation 19991001616–1622. [DOI] [PubMed] [Google Scholar]
- 8.Prisco D, Fedi S, Antonucci E.et al Postprocedural PAI‐1 activity is a risk marker of subsequent clinical restenosis in patients both with and without stent implantation after elective balloon PTCA. Thromb Res 2001104181–186. [DOI] [PubMed] [Google Scholar]
- 9.Genser D, Prachar H, Hauer R.et al Relation of homocysteine, vitamin B12 and folate to coronary in‐stent restenosis. Am J Cardiol 200289495–499. [DOI] [PubMed] [Google Scholar]
- 10.Lange H, Suryapranata H, De Luca G.et al Folate therapy and in‐stent restenosis after coronary stenting. N Engl J Med 20043502673–2681. [DOI] [PubMed] [Google Scholar]
- 11.Marcucci R, Prisco D, Brunelli T.et al Tissue factor and homocysteine levels in ischemic heart disease are associated with angiographically documented clinical recurrences after coronary angioplasty. Thromb Haemost 200083826–832. [PubMed] [Google Scholar]
- 12.Schnyder G, Roffi M, Flammer Y.et al Association of plasma homocysteine with restenosis after percutaneous coronary angioplasty. Eur Heart J 200223726–733. [DOI] [PubMed] [Google Scholar]
- 13.Koch W, Ndrepepa G, Mehilli J.et al Homocysteine status and polymorphisms of methylenetetrahydrofolate reductase are not associated with restenosis after stenting in coronary arteries. Arterioscler Thromb Vasc Biol 2003232229–2234. [DOI] [PubMed] [Google Scholar]
- 14.Kojoglanian S A, Jorgensen M B, Wolde‐Tsadik G.et al Restenosis in intervened coronaries with hyperhomocysteinemia. Am Heart J 20031461077–1081. [DOI] [PubMed] [Google Scholar]
- 15.Nygard O, Nordrehaug J E, Refsum H.et al Plasma homocysteine and mortality in patients with coronary artery disease. N Engl J Med 1997337230–236. [DOI] [PubMed] [Google Scholar]
- 16.Zairis M N, Ambrose J A, Manousakis S J.et al The impact of plasma levels of C‐reactive protein, lipoprotein(a) and homocysteine on the long‐term prognosis after successful coronary stenting. J Am Coll Cardiol 2002401375–1382. [DOI] [PubMed] [Google Scholar]
- 17.Gazzaruso C, Garzaniti A, Falcone C.et al Lipoprotein (a), apolipoprotein (a) polymorphism and restenosis after intracoronary stent placement in type 2 diabetic patients. J Diabetes Complications 200317135–140. [DOI] [PubMed] [Google Scholar]
- 18.Chobanian A V, Bakris G L, Black H R, and the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure et al National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003421206–1252. [DOI] [PubMed] [Google Scholar]
- 19.NCEP National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 20021063143–3421. [PubMed] [Google Scholar]
- 20.Anon Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 200326(suppl 1)S5–20. [DOI] [PubMed] [Google Scholar]
- 21.Scanu A M. Atherothrombogenicity of lipoprotein(a): the debate. Am J Cardiol 19988226Q–33Q. [DOI] [PubMed] [Google Scholar]
- 22.Homocysteine Studies Collaboration Homocysteine and risk of ischemic heart disease and stroke: a meta‐analysis. JAMA 20022882015–2022. [DOI] [PubMed] [Google Scholar]
- 23.Huber K. Plasminogen activator inhibitor type‐1: basic mechanisms, regulation, and role for thromboembolic disease. J Thromb Thrombolysis 200111183–193. [DOI] [PubMed] [Google Scholar]
- 24.Hoekstra T, Geleijnse J M, Schouten E G.et al Plasminogen activator inhibitor‐type 1: its plasma determinants and relation with cardiovascular risk. Thromb Haemost 200491861–872. [DOI] [PubMed] [Google Scholar]
- 25.Dudman N P. An alternative view of homocysteine. Lancet 19993542072–2074. [DOI] [PubMed] [Google Scholar]
- 26.Korte W, Greiner J, Feldges A.et al Increased Lp(a) levels are not a steady prothrombotic defect [letter]. Blood 2001981993–1994. [DOI] [PubMed] [Google Scholar]
- 27.Lee Y H, Choi S J, Ji J D.et al Lp(a) and lipids in relation to inflammation in rheumatoid arthritis. Clin Rheumatol 200019324–325. [DOI] [PubMed] [Google Scholar]
- 28.Ramharack R, Barkalow D, Spahr M A. Dominant negative effect of TGF‐beta1 and TNF‐alpha on basal and IL6 induced Lp(a) and apoLp(a) mRNA expression in primary monkey hepatocyte cultures. Arterioscler Thromb Vasc Biol 199818984–990. [DOI] [PubMed] [Google Scholar]
- 29.Tsikouris J P, Suarez J A, Meyerrose G E. Plasminogen activator inhibitor‐1: physiologic role, regulation, and the influence of common pharmacologic agents. J Clin Pharmacol 2002421187–1199. [DOI] [PubMed] [Google Scholar]
- 30.Krysiak R, Okopien B, Herman Z. Effects of HMG‐CoA reductase inhibitors on coagulation and fibrinolysis processes. Drugs 2003631821–1854. [DOI] [PubMed] [Google Scholar]
- 31.Zirlik A, Leugers A, Lohrmann J.et al Direct attenuation of plasminogen activator inhibitor type‐1 expression in human adipose tissue by thiazolidinediones. Thromb Haemost 200491674–682. [DOI] [PubMed] [Google Scholar]
- 32.Selhub J. Homocysteine metabolism. Annu Rev Nutr 199919217–246. [DOI] [PubMed] [Google Scholar]
- 33.Schnyder G, Roffi M, Flammer Y.et al Effect of homocysteine‐lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention: the Swiss heart study: a randomized controlled trial. JAMA 2002288973–979. [DOI] [PubMed] [Google Scholar]
- 34.Marcucci R, Zanazzi M, Bertoni E.et al Vitamin supplementation reduces the progression of atherosclerosis in hyperhomocysteinemic renal‐transplant recipients. Transplantation 2003751551–1555. [DOI] [PubMed] [Google Scholar]
- 35.Vermeulen E G J, Stehouwer C D A, Twisk J W R.et al Effect of homocysteine‐lowering treatment with folic acid plus vitamin B6 on progression of subclinical atherosclerosis: a randomised, placebo‐controlled trials. Lancet 2000355517–522. [DOI] [PubMed] [Google Scholar]
- 36.Troen A M. Lutgens E, Smith DE, et al. The atherogenic effect of excess methionine intake. Proc Natl Acad Sci USA 200310015089–15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reiner A P, Rosendaal F R, Reitsma P H.et al Factor V Leiden, prothrombin G20210A, and risk of sudden coronary death in apparently healthy persons. Am J Cardiol 20029066–68. [DOI] [PubMed] [Google Scholar]