The left ventricular response to aortic stenosis is not simple. It consists of a combination of wall thickening and a change in cavity size with associated effects on systolic and diastolic function. It is determined by numerous factors in addition to the transaortic resistance. These include coexistent coronary disease and hypertension, but also sex, age, and genetic factors. This review will give evidence to suggest that aortic stenosis should be regarded as a clinical syndrome involving the left ventricle as well as the aortic valve. This carries the implication that the left ventricle should be given greater priority when assessing and treating aortic stenosis.
EFFECT OF PRESSURE LOAD ON THE LEFT VENTRICLE
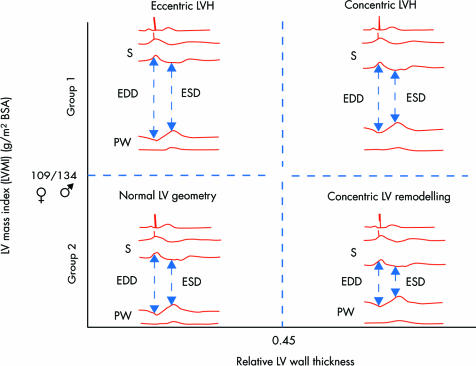
Pressure overload causes left ventricular (LV) remodelling and ultimately hypertrophy.w1 w2 This is often seen as a means of limiting wall stress in order to maintain systolic function.w2 Wall stress, by the law of Laplace, is related directly to intracavitary pressure and cavity size, but inversely to wall thickness. Concentric remodelling is defined by a normal LV mass associated with an increased relative wall thickness (fig 1). This is usually calculated as 2 × posterior wall thickness/LV diastolic diameter, and a value > 0.45 is abnormal.w3 w4 The reduction in cavity size and increase in wall thickness tend to offset the raised intracavitary pressure associated with aortic stenosis. Concentric hypertrophy is defined by a combination of left ventricular hypertrophy and increased relative wall thickness. Patients with aortic stenosis and no hypertrophy almost invariably have concentric remodelling.w4–6
Figure 1 Diagram of left ventricular hypertrophy (LVH) and geometric remodelling. Concentric remodelling is defined by normal left ventricular mass, but a relative wall thickness above 0.45. Concentric hypertrophy is defined by LVH with a relative wall thickness above 0.45. Eccentric hypertrophy as occurs in aortic regurgitation is defined by LVH, but with normal relative wall thickness. BSA, body surface area; EDD, end diastolic diameter; ESD, end systolic diameter; LV, left ventricular; PW, posterior wall. Redrawn from Seiler and Jenniw4 with permission.
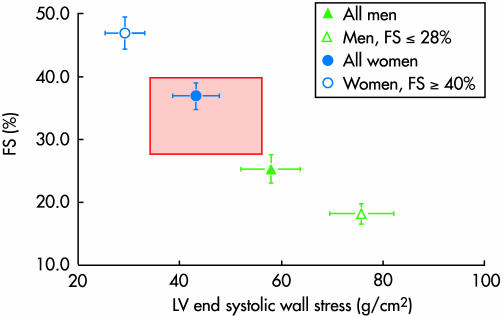
There is only a modest relation between the degree of aortic stenosis and LV mass,w7–9 although in serial studies an increase in transaortic pressure difference is associated with an increase in LV mass.w10 This is only partly explained by the fact that echocardiographic measures of valvar stenosis are imperfect surrogates for wall stress. Men have higher LV mass when indexed to body surface area,w7 w8 although LV hypertrophy is more common in women when sex specific thresholds for hypertrophy are applied (often > 134 g/m2 for men and > 110 g/m2 for women).w7 w8 w11 However, there is also a wide variation in LV geometry and function expressed in relation to wall stress (fig 2). At one extreme, seen predominantly in women, the left ventricle has a small cavity and thick walls with normal or even supra‐normal fractional shortening in association with low wall stress.1w9 w12 At the other extreme, more common in men, the left ventricle is dilated with relatively thin walls and a reduced fractional shortening in association with high wall stress (fig 2).2w13 w14 This sex effect is not adequately explained. However, there are differences in collagen deposition, occurring as parallel strands in females, but cross‐hatched in males.w15 There is also evidence that sex hormones affect cardiac adaptation to pressure load.w16–18 Oestrogen receptor β mRNA content is higher in females than males with aortic stenosis, but higher in both sexes with aortic stenosis than in controls.w19
Figure 2 Plot of fractional shortening (FS) against meridional end systolic wall stress. The red zone denotes the normal range for the stress–shortening relation. The female pattern LV response to aortic stenosis gives a thick wall and small cavity and consequently relatively low stress associated with high fractional shortening. The male pattern is a dilated left ventricle with consequently high stress associated with low fractional shortening. Redrawn from Carroll et al1 with permission.
DETERMINANTS OF LV MASS AND CAVITY SIZE
LV hypertrophy is associated with myofibrillar hypertrophy, but also fibrosis, which is the deposition of collagen and fibronectin. The renin–angiotensin system and its mediator transforming growth factor β1 are activated in aortic stenosisw20 and contribute to the fibrosis. Studies have investigated the effect on LV mass of different alleles of genes for the angiotensin converting enzyme (ACE), cardiac chymase, angiotensinogen, AT receptor 1, and aldosterone synthase.w21–23 Some show a relation between the DD ACE allele and LV mass,w21 w22 others no differencew7 w23 or a direct relation in men, but an inverse relation in women.w11 These discrepancies may partly be caused by the limited reproducibility of current techniques of genotyping.w24 There may also be interactions between gene expression and the intra‐ and extracardiac renin–angiotensin systems. Several animal studies have failed to show a correlation between LV hypertrophy induced by aortic banding, and serum ACE concentrations.3 w25 However, treatment with ACE inhibitors in these animals can cause regression of LV hypertrophy even with persisting pressure overload. This suggests an effect on local intracardiac renin–angiotensin systems, which may be more important in determining the degree of hypertrophy than systemic ACE concentrations. This is supported by the fact that ACE mRNA concentrations and activity in the non‐hypertrophied right ventricles of pressure loaded hearts are normal.4 Local renin–angiotensin systems also modulate myocardial function. The intracoronary infusion of enalaprilat causes an immediate improvement in diastolic function in patients with aortic stenosisw26 w27 despite no change in plasma renin or ACE activity.
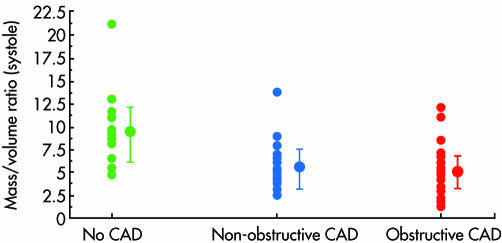
There are other determinants of LV mass and geometry. Dilatation may be caused by progressive fibrosis2w15 w28 possibly as a result of subendocardial ischaemia. This could be caused by coronary diseasew28 even if apparently minor at angiography (fig 3).2 However, there are a number of studies showing reduced flow reserve even in patients with angiographically completely normal coronary arteries.w29 w30 The coronary lumen may be relatively smallw29 w31 since, although the coronary artery lumen initially increases in size as aortic stenosis progresses, the luminal area indexed to LV mass ultimately falls.w31 The pattern of coronary flow also changes in patients with symptomatic aortic stenosis so that systolic flow is reversed or has an abnormally low peak velocity while diastolic velocities are raised.5w32 w33 This is most likely caused by a reduced transmural perfusion gradient as a result of high LV end diastolic pressurew29 and a low aortic pressure at the ostium of the artery. It is also possible that abnormal flow patterns in the aorta affect flow into the coronary arteries. Normal laminar flow is replaced by turbulence beyond the stenosed aortic valve. Furthermore, wave reflectance which normally causes augmentation of diastolic pressure is abnormal in aortic stenosis.6 The wave returns early which augments systolic rather than diastolic pressure, thus increasing afterload and reducing coronary perfusion pressure. Finally, it has also been postulated that LV dilatation could occur intrinsically as a result of cellular switchingw34—for example, in response to stimuli such as circulating catecholamines.w35
Figure 3 Plots of mass to volume ratio in systole. Ratios for non‐obstructive coronary disease (lesions < 50% of luminal diameter) (5.6 (SD 2.8)) and obstructive coronary disease (lesions > 50%) (5.2 (2.2)) are significantly smaller than for those with no coronary disease (9.2 (3.9)). This reflects a thinner, more dilated LV in patients with coronary artery disease. CAD, coronary artery disease. Redrawn from Vekshtein et al w28 with permission.
In adults systemic blood pressure and age also affect LV mass7w7 w8 w36 and cavity size.w28 LV hypertrophy occurs in even mild aortic stenosis when it is determined predominantly by systemic blood pressure,w7 while in moderate and severe stenosis, it is mainly related to the transaortic pressure difference and age.w7 LV mass regression after aortic valve replacement is also related to systemic blood pressure.8 w37
IS LV HYPERTROPHY ADAPTIVE OR MALADAPTIVE?
Contrary to the traditional view, significant LV hypertrophy is maladaptive. Both LV hypertrophy and concentric remodelling without hypertrophy are associated with increased operativew38 w39 and mid termw37 mortality and morbidity mainly in women. In one studyw28 the operative mortality was 17% in the presence of LV hypertrophy and only 1.4% with normal LV mass. In women, a relative wall thickness > 0.66 carried an in‐hospital mortality of 63% compared with only 14% for relative wall thickness < 0.66.w40 The mechanism may be worsening of diastolic dysfunction as a result of ischaemia and reperfusion.8 w40 This is supported by evidence that the functional state may be improved with volume replacement and calcium channel blockers or β blockers.w12
There is other evidence that diastolic dysfunction rather than LV mass itself affects outcome. LV filling dynamics are abnormal in one half of patients having valve replacement for aortic stenosis when systolic function appears normal, and in all who have impaired systolic function.9 w41 Pseudonormal or restrictive filling patterns at the time of surgery are associated with higher late mortality than slow filling or normal patterns.9 Measures of LV filling dynamics are affected by intrinsic LV diastolic dysfunction, but also by the filling pressure.w42 A pseudonormal filling pattern on echocardiography can develop from a slow filling pattern either from progression of fibrosis or increased filling pressure as a result of fluid loading, drugs or possibly coexistent mitral regurgitation.w43 BNP concentrations are elevated in patients with abnormal diastolic filling patterns as well as those with systolic dysfunctionw44 and may provide a surrogate for the echocardiographic assessment of filling patterns. LV mass regression after surgery is associated with an improvement in LV ejection fraction,w45 w46 but diastolic dysfunction may persist8 and affect long term mortality8,9 and function.w47 w48
Although the “female” pattern left ventricle appears to have normal or supranormal systolic function as assessed by LV ejection fraction, this has to be interpreted against the fact that wall stress may actually be lower than normal.1 Mid wall shortening may also be abnormally loww12 even if fractional shortening at the endocardium is normal or high. Finally, long axis excursion may be reduced even in the presence of normal endocardial fractional shortening.w49 w50 By contrast, in the “male” pattern dilated left ventricle, low ejection fraction and stroke volume may be normal when corrected for wall stress.w2 This means that after aortic valve replacement, measurements of systolic function should return to normalw51 and there is evidence that reduced long axis excursion can increase immediately after aortic valve replacement.w52 However, although the mean LV ejection fraction improves after surgery in those with a preoperative LV ejection fraction below 45%, about one third do not reach normal levels.w14 w53 This is either because there is another cause of LV dysfunction such as myocardial infarction or alcohol, because there has been irreversible fibrosis as a result of the aortic stenosis, or in some cases because of incomplete myocardial preservation at the time of surgery.
The left ventricle in aortic stenosis: key points
The left ventricular (LV) response to aortic stenosis includes LV remodelling, and myofibrillar hypertrophy with fibrosis
The LV response is partly determined by intrinsic factors including sex
The response is also affected by systemic hypertension, and coronary disease
LV mass is only modestly related to the degree of valvar obstruction
There is good evidence that LV hypertrophy may often be maladaptive
Animal evidence shows that blocking LV hypertrophy is associated with an improved outcome
Angiotensin converting enzyme (ACE) inhibitors are now used in patients with hypertension and mild to moderate aortic stenosis
Vasodilators including ACE inhibitors have also been used with caution in patients with symptomatic severe aortic stenosis
CLINICAL IMPLICATIONS
Since LV hypertrophy is maladaptive, it is therefore interesting that rodents in which the hypertrophic response to pressure load is blocked genetically10 or by treatment with an ACE inhibitor11,12,13 maintain systolic function with better survival than control rodents. There is also evidence that ACE inhibitors may improve diastolic function in rodents.w20 This suggests that ACE inhibitors could be used in human aortic stenosis. In mild aortic stenosis, LV mass is related to blood pressurew7 and ACE inhibitors are used for patients with hypertension and mild or moderate aortic stenosis.14 ACE inhibitors and other vasodilating agents have also been used without adverse effects in patients with severe aortic stenosis despite being traditionally contraindicated.w54 Nitroprusside produces favourable haemodynamic changes in patients with critical aortic stenosis and heart failure.15 Chockalingham and colleagues16 gave enalapril to 34 patients with aortic stenosis in New York Heart Association functional class III or IV and observed a significant increase in six minute walk time in 33% patients on enalapril compared with only 17% taking placebo.
Transverse systolic dysfunction, shown by a reduced fractional shortening or ejection fraction, is already an accepted criterion for surgery.w55 It would be intuitively sensible to use a decrease in long axis function as a more sensitive marker of a change in systolic function,9 although there is no evidence that this is useful prospectively. There is evidence, but no proof, that a change in diastolic filling pattern from slow filling to pseudo‐normal should also become a criterion for surgery.w45 w46
In patients with relatively preserved contractility, the transaortic pressure difference falls in line with the LV ejection at high wall stresses.w2 There is good evidence that surgery is at relatively low risk provided that the mean pressure difference is above 30 mm Hg on echocardiography or cardiac catheter,w56–58 which is approximately equivalent to a peak velocity on Doppler above 3.5 m/s. For patients with low flow, low gradient aortic stenosis, the operative mortality is highw58 and management decisions less easy. The management of this important clinical situation is described in the next article in this series.w59
CONCLUSION
The aortic valve is intimately related anatomically to the left ventricle and to the aorta. However, the left ventricular response to aortic stenosis is only partly related to the transaortic resistance. It is probably related to physiological events in the aorta as well as sex, genetic factors, and the presence of coronary atheroma. Independently of the grade of valve stenosis, the structure and function of the left ventricle affect the presence of symptoms and the risk and outcome of aortic valve surgery. Surgery in asymptomatic patients should be considered with minor systolic dysfunction shown by a reduced fractional shortening and in the future it is likely that evidence will support surgery at the first sign of significant diastolic dysfunction. LV hypertrophy develops early in the natural history of aortic stenosis and it is possible, but unproved, that ACE inhibitors could preserve LV function and survival if started once mild aortic stenosis is diagnosed. However, there is already evidence that ACE inhibitors can be started in patients for hypertension associated with moderate aortic stenosis and that these drugs do not need to be stopped in a patient who remains well despite more severe aortic stenosis. There is also evidence that ACE inhibitors can improve measures of haemodynamic function and exercise capacity in patients with critical stenosis who are not suitable for valve surgery.
Additional references appear on the Heart website—http://www.heartjnl.com/supplemental
Additional references appear on the Heart website—http://www.heartjnl.com/supplemental
Supplementary Material
Footnotes
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article
Additional references appear on the Heart website—http://www.heartjnl.com/supplemental
References
- 1.Carroll J D, Carroll E P, Feldman T.et al Sex‐associated differences in left ventricular function in aortic stenosis of the elderly. Circulation 1992861099–1107.One of the first studies to show striking differences in LV geometry and function between men and women with aortic stenosis. [DOI] [PubMed] [Google Scholar]
- 2.DePace N L, Ren J‐F, Iskanndrian A S.et al Correlation of echocardiographic wall stress and left ventricular pressure and function in aortic stenosis. Circulation 198367854–859.This study showed that even angiographically minor coronary disease could be associated with left ventricular dilatation. [DOI] [PubMed] [Google Scholar]
- 3.Bruckschlegal G, Holmer S R, Jandeleit K.et al Blockade of the renin‐angiotensin system in cardiac pressure‐overload hypertrophy in rats. Hypertension 199525250–259. [DOI] [PubMed] [Google Scholar]
- 4.Schunkert H, Dzau V J, Tang S S.et al Increased rat cardiac angiotensin converting enzyme activity and mRNA expression in pressure overload left ventricular hypertrophy. J Clin Invest 1990861913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hongo M, Goto T, Watanabe N.et al Relation of phasic coronary flow velocity profile to clinical and hemodynamic characteristics of patients with aortic valve disease. Circulation 199388953–960.This study showed major changes in the pattern of coronary flow in patients with aortic stenosis without coronary disease. [DOI] [PubMed] [Google Scholar]
- 6.Rajani R, Rimingtob H, Chowienczyk P.et al Vascular alterations in aortic stenosis. Heart 200389(suppl III)iii39 [abstract].This study provides preliminary data linking the presence of symptoms in aortic stenosis and measures of aortic physiology. [Google Scholar]
- 7.Villari B, Vassalli G, Schneider J.et al Age dependency of left ventricular diastolic function in pressure overload hypertrophy. J Am Coll Cardiol 199729181–186. [DOI] [PubMed] [Google Scholar]
- 8.Lund O, Erlendsen M, Dorup I.et al Predictable changes in left ventricular mass and function during ten years after valve replacement for aortic stenosis. J Heart Valve Dis 200413357–368.The study showed that persistence of LV hypertrophy after aortic valve replacement is determined by a number of factors including the systemic blood pressure after surgery and the duration of LV hypertrophy before surgery. [PubMed] [Google Scholar]
- 9.Gjertsson P, Caidahl K, Farasati M.et al Preoperative moderate to severe diastolic dysfunction: a novel Doppler echocardiographic long‐term prognostic factor in patients with severe aortic stenosis. J Thorac Cardiovasc Surg 2005129890–896.This is one of the few studies to investigate LV diastolic function in aortic stenosis and it strongly suggests that the presence of diastolic dysfunction is an important prognostic indicator and might therefore be useful as a criterion for surgery. [DOI] [PubMed] [Google Scholar]
- 10.Esposito G, Rapaccinolo A, Prasad S V N.et al Genetic alterations that inhibit in vivo pressure‐overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation 200210585–92. [DOI] [PubMed] [Google Scholar]
- 11.Weinberg E O, Schoen F J, George D.et al Angiotensin‐converting enzyme inhibition prolongs survival and modifies the transition to heart failure in rats with pressure overload hypertrophy due to ascending aortic stenosis. Circulation 1994901410–1422. [DOI] [PubMed] [Google Scholar]
- 12.Litwin S E, Katz S E, Weinberg E O.et al Serial echocardiographic‐Doppler assessment of left ventricular geometry and function in rats with pressure‐overload hypertrophy. Circulation 1995912642–2654. [DOI] [PubMed] [Google Scholar]
- 13.Turcani M, Rupp H. Heart failure development in rats with ascending aortic constriction and angiotensin‐converting enzyme inhibition. Br J Pharmacol 20001301671–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Brien K D, Zhao X Q, Shavelle D M.et al Hemodynamic effects of the angiotensin‐converting enzyme inhibitor, ramipril, in patients with mild to moderate aortic stenosis and preserved left ventricular function. J Invest Med 200452185–191.This study shows that it is safe to use an ACE inhibitor for hypertension in patients with mild or moderate aortic stenosis. [DOI] [PubMed] [Google Scholar]
- 15.Khot U N, Novaro G M, Popovi Z P.et al Nitroprusside in critically ill patients with left ventricular dysfunction and aortic stenosis. N Engl J Med 20033481756–1763.This study revisited older work showing that arterial dilators could be used safely in critical aortic stenosis. Stroke volume increased even in patients with low effective orifice area. [DOI] [PubMed] [Google Scholar]
- 16.Chockalingam A, Venkatesan S, Subramaniam T.et al Safety and efficacy of angiotensin‐converting enzyme inhibitors in symptomatic severe aortic stenosis: symptomatic cardiac obstruction‐pilot study of enalapril in aortic stenosis (SCOPE‐AS). Am Heart J 2004147L1–L8.This study gave an ACE inhibitor safely to patients with symptomatic severe aortic stenosis and showed a clinical benefit in a proportion. This suggests that such drugs could be used with caution in patients unsuitable for aortic valve surgery. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.