Abstract
Objective
To establish the prevalence of chronotropic incompetence in a cohort of patients with chronic heart failure (CHF) taking modern medications for heart failure, and whether this affected exercise capacity and predicted prognosis.
Methods
Heart rate response to exercise was examined in 237 patients with CHF in sinus rhythm, who were compared with 118 control volunteers. The percentage of maximum age predicted peak heart rate (%Max‐PPHR) and percentage heart rate reserve (%HRR) were calculated, with a cut off of < 80% as the definition of chronotropic incompetence for both. Patients were followed up for an average (SD) of 2.8 (9) years. Mortality was related to peak oxygen consumption (pVo2), and the presence or absence of chronotropic incompetence.
Results
%Max‐PPHR < 80% identified 103 (43%) and %HRR < 80% identified 170 patients (72%) as having chronotropic incompetence. Chronotropic incompetence was more common in patients taking β blockers than in those not taking β blockers as assessed by both methods (80 (49%) v 23 (32%) by %Max‐PPHR and 123 (75%) v 47 (64%) by %HRR, respectively). Patients with chronotropic incompetence by either method had a lower pVo2 than those without. These differences remained significant for both patients taking and not taking a β blocker. %HRR, Max‐PPHR%, and HRR were related to New York Heart Association class and correlated with pVo2. There was no difference in the slopes relating heart rate to pVo2 between patients with and those without chronotropic incompetence (6.1 (1.7) v 5.1 (1.8), p = 0.34). During an average 2.8 year follow up 40 patients (17%) died. In Cox proportional hazard models, pVo2 was the most powerful predictor of survival and neither measure of chronotropic incompetence independently predicted outcome.
Conclusions
pVo2 is a powerful marker of prognosis for patients with CHF whether they are taking β blockers or not. A low heart rate response to exercise in patients with CHF correlates with worse exercise tolerance but is unlikely to contribute to exercise impairment.
Keywords: chronic heart failure, heart rate, β blockers
Patients with chronic heart failure (CHF) are exercise intolerant, usually due to breathlessness and fatigue.1 These can be objectively assessed as a reduction in peak oxygen consumption (pVo2) and an increase in the ventilatory response to exercise (the relation of ventilation (Ve) to carbon dioxide production (Vco2) or Ve/Vco2 slope) during incremental exercise testing with metabolic gas exchange analysis.2 The impaired exercise tolerance is probably due to an interaction of cardiac and peripheral factors, each under the influence of the chronic sympathetic overactivity seen in CHF.3,4,5 This overactivity leads to β receptor downregulation and reduced myocardial sensitivity to β agonists, which may in turn lead to a reduction in heart rate response to exercise in patients with heart failure (chronotropic incompetence) and thereby contribute to exercise intolerance.6,7,8,9,10
The frequency of a poor heart rate response to exercise in patients with CHF increases with increasing severity of the heart failure.11 Poor heart rate response is also seen in asymptomatic people with left ventricular (LV) dilatation and reduced LV ejection fraction.12 Some, but not all, studies have suggested that chronotropic incompetence may contribute to reduced exercise tolerance.13,14
Little work has been done on chronotropic incompetence and prognosis in heart failure populations but one study has suggested that the prognosis for patients with CHF with poor chronotropic reserve is particularly bad.15 β Blockers reduce resting (RHR) and peak heart rate (PHR) but improve prognosis in CHF. These mortality data may therefore not hold true in the modern era of CHF management.
We wanted to establish the prevalence of chronotropic incompetence in a cohort of patients with CHF taking modern medications for heart failure and whether this affected exercise capacity and predicted prognosis.
METHODS
We enrolled 237 patients into the study and 118 controls. CHF was defined as the presence of symptoms of fatigue or breathlessness on exertion and an LV ejection fraction on echocardiography of < 40% with no other apparent cause of breathlessness. For patients to be included in the analysis, their condition had to be of at least three months' duration before the initial visit, with no recent exacerbation or change in medication. We did not include patients with neurological conditions, inducible ischaemia, or a history of pulmonary disease or if their forced expiratory volume in one second was < 80% of predicted. We also excluded patients with atrial fibrillation. The control population consisted of people of a similar age chosen at random from the patient lists of local general practitioners with no history or symptoms of cardiovascular disease.
At the initial visit each participant had an echocardiographic examination with a Vingmed Vivid 5 scanner (Horten, Norway). M mode was used to determine LV end diastolic diameter, and the modified Simpson's rule was used to calculate LV volumes and ejection fraction. Each subject then underwent symptom limited treadmill based maximal exercise testing according to a Bruce protocol modified by the addition of a stage 0 at onset consisting of three minutes of exercise at 1.61 km/h (1 mph) with a 5% gradient. Patients were encouraged to exercise to exhaustion. During the tests subjects wore a tight fitting facemask to which was connected a capnograph and a sample tube enabling online Ve and metabolic gas exchange measurements (Jaeger Oxycon Delta, Würtzburg, Germany). A respiratory exchange ratio (Vco2/Vo2) of 1.1 was taken to indicate maximum effort. The anaerobic threshold was calculated by the Vo2/Vco2 slope method.16 The Ve/Vco2 slope was calculated by simple regression of data collected throughout exercise.17
Before the exercise test, subjects were seated comfortably and asked not to talk for three minutes. The heart rate over this time was averaged and taken as the RHR. Baseline metabolic gas exchange was read at this time and the monitoring period was extended as necessary to achieve three minutes of true resting oxygen consumption. PHR was the averaged heart rate over the final 30 seconds of exercise.
Chronotropic incompetence is defined as an inadequate heart rate response to exercise. Maximum age predicted heart rate is most commonly calculated by the formula of Astrand (220 − age).18 Chronotropic incompetence can be defined as a peak exercise heart rate less than 80–85% of the maximum age predicted PHR (Max‐PPHR).19 As most of the more recent reports have used < 80% Max‐PPHR, we used this figure in our study. We also calculated percentage heart rate reserve (%HRR). This is a measure of the change in heart rate from rest to peak exercise as a proportion of the possible maximum change from rest to expected PHR. This may be more useful in a β blocked group, as it incorporates the RHR (calculated by [(PHR − RHR)/220 − age − RHR)] × 100). We took a %HRR < 80% as abnormal.
Follow up was censored at 1 July 2004. We used a computerised local health records database to find whether each subject was alive at the censorship date. For subjects without data and those who were reported as dead, we contacted the general practitioners to confirm the information. We analysed data from patients taking a β blocker at the time of the test separately from those not taking a β blocker. Patients were not taking β blockers at the time of the initial test either because it was their first visit or because they had not tolerated the drugs. We also examined the ability of Max‐PPHR and peak HRR to predict survival during follow up.
Results are reported as mean (SD). We used unpaired Student's t test for between group comparisons and paired t test for within group analyses. A value of p < 0.05 was taken to be significant. We used linear regression analysis to explore the relation between continuous variables and the χ2 to compare between nominal variables. Cox proportional hazard analyses were used to assess prognostic associations. The hazard ratio with 95% confidence intervals and p values by the likelihood ratio test are given. Hazard ratios for continuous variables apply for each unit of the analysed variable. Kaplan‐Meier cumulative survival plots were constructed to illustrate the results (StatView 5.0, Abacus Concepts, Berkeley, USA).
RESULTS
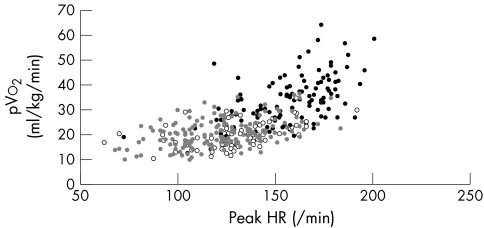
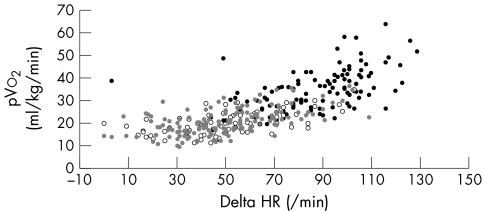
Table 1 shows control and patient characteristics split by treatment. The groups were similar in height, age, and sex. The patient groups did not differ in terms of aetiology, LV function and diameters, exercise time, respiratory exchange ratio, and predicted PHR (table 1). Patients taking β blockers had a lower RHR and achieved a lower PHR, lower %Max‐PPHR, and lower %HRR than did patients not taking β blockers, but these two groups did not differ in pVo2, Ve/Vco2 slope, or the ratio between pVo2 and PHR. Patients taking a β blocker exercised longer than those not taking a β blocker. Figures 1 and 2 show PHR and change in heart rate relative to pVo2 for patients taking and not taking a β blocker and controls.
Table 1 Characteristics of patients and controls.
| Characteristic | Patients | Controls (n = 118) | p Value between patient groups | |
|---|---|---|---|---|
| With β blocker (n = 164) | Without β blocker (n = 73) | |||
| Age (years) | 66 (10) | 68 (11) | 65 (12) | 0.11 |
| Height (cm) | 171 (8) | 168 (11) | 169 (10) | 0.09 |
| Weight (kg) | 80.5 (15.9) | 76.0 (17.3) | 74.2 (12.9)* | <0.05 |
| Men/women | 123/41 | 55/18 | 62/56 | |
| LVEDD (cm) | 6.5 (0.9) | 6.3 (1.2) | 4.9 (0.7)† | 0.12 |
| LVEF (%) | 32.0 (7.0) | 33.3 (9.8) | 59.3 (8.0)† | 0.40 |
| Aetiology | ||||
| Dilated cardiomyopathy | 29 | 21 | ||
| Hypertensive HF | 10 | 2 | ||
| Ischaemic heart disease | 121 | 50 | ||
| IDDM | 4% | 4% | 0 | |
| NIDDM | 12% | 10% | ||
| Hypertension | 38% | 21% | 19% | |
| Drugs | ||||
| Furosemide (mg)‡ | 68 (46) | 72 (38) | 0 | 0.45 |
| ACEI/AIIA | 84% | 78% | 2% | |
| Amiodarone (currently) | 5% | 7% | 0 | |
| Amiodarone (last 6 months) | 2% | 0% | ||
| Digoxin | 7% | 14% | 0 | |
| Aspirin | 70% | 68% | 7/0 | |
| Warfarin | 29% | 33% | ||
| NYHA class | ||||
| I | 6% | 5% | ||
| II | 74% | 72% | ||
| III | 19% | 23% | ||
| RHR (beats/min) | 72 (15) | 80 (17) | 71 (11) | 0.0002 |
| PHR (beats/min) | 123 (25) | 133 (26) | 157 (21)† | 0.0045 |
| Predicted PHR (beats/min) | 154 (9.6) | 153 (10.8) | 155 (12) | 0.23 |
| %Max‐PPHR | 80 (15) | 87 (17) | 102 (11)† | 0.0005 |
| HRR | 31 (23) | 19 (25) | −3 (17)† | 0.0004 |
| %HRR | 63 (26) | 73 (32) | 104 (21)† | 0.017 |
| pVo2 (ml/kg/min) | 20.4 (5.7) | 19.5 (5.3) | 34.4 (9.4)† | 0.35 |
| Ve/Vco2 slope | 36.0 (8.2) | 36.6 (6.7) | 28.3 (3.9)† | 0.55 |
| Exercise time (s) | 498 (198) | 435 (218) | 788 (290)† | 0.02 |
| RER | 1.02 (0.1) | 1.02 (0.1) | 1.02 (0.1) | 0.31 |
| pVo2/PHR ratio | 0.13 (0.03) | 0.13 (0.03) | 0.22 (0.05)† | 0.34 |
| Follow up (days alive) | 1032 (337) | 898 (410) | 909 (326) | 0.029 |
Values are mean (SD).
*p<0.05 for the difference between all patients and controls; †p<0.0001 for the difference between all patients and controls; ‡mean daily dose of furosemide (or furosemide equivalent).
ACEI, angiotensin converting enzyme inhibitor; AIIA, angiotensin II inhibitor; HF, heart failure; HRR, heart rate reserve; HR/Vo2 slope, slope relating heart rate to oxygen consumption; IDDM, insulin dependent diabetes mellitus; LVEDD, left ventricular end diastolic dimension from M mode echocardiography; LVEF, left ventricular ejection fraction; NIDDM, non‐insulin dependent diabetes mellitus; NYHA, New York Heart Association; PHR, peak heart rate; pVo2, peak oxygen consumption; %HRR, percentage heart rate reserve; %Max‐PPHR, percentage maximum age predicted peak heart rate; RER, respiratory exchange ratio (Vco2/Vo2); RHR, resting heart rate; Ve/Vco2 slope, slope relating ventilation to carbon dioxide production.
Figure 1 Relation between peak heart rate (HR) and peak oxygen consumption (pVo2) in patients taking β blockers (grey circles; r = 0.45, p < 0.001)) and not taking β blockers (unfilled circles; r = 0.49, p < 0.001) and controls (black circles; r = 0.44, p < 0.001).
Figure 2 Relation between change in HR and pVo2 in patients taking β blockers (grey circles; r = 0.56, p < 0.001) and not taking β blockers (unfilled circles; r = 0.60, p < 0.001) and controls (black circles; r = 0.53, p < 0.001).
Prevalence of chronotropic incompetence and the effect of β blockers
We used two definitions for chronotropic incompetence. With %Max‐PPHR < 80%, 103 (43%) patients had chronotropic incompetence (change in heart rate 37 (17) v 60 (19) beats/min, p < 0.001). Patients with chronotropic incompetence had a lower pVo2 than those without (18.6 (5.3) v 21.2 (5.5) ml/kg/min, p = 0.0007) and a steeper Ve/Vco2 slope (37.9 (9.0) v 35.0 (6.4), p = 0.005). Patients taking β blockers were more likely to have chronotropic incompetence than those not taking β blockers (80 (49%) v 23 (32%), χ2 p = 0.015).
When we used %HRR < 80% to define chronotropic incompetence, 170 patients (72%) were identified as having a poor rise in heart rate on exercise (change in heart rate 41 (16) v 71 (18) beats/min, p < 0.0001). Patients with chronotropic incompetence had a lower pVo2 than those without (17.8 (4.8) v 22.4 (4.8) ml/kg/min, p = 0.0002) and a steeper Ve/Vco2 slope (34.3 (7.0) v 37.0 (8.0), p = 0.02). Patients taking β blockers were more likely to have chronotropic incompetence than those not taking β blockers (123 (75%) v 47 (64%), χ2 p = 0.04).
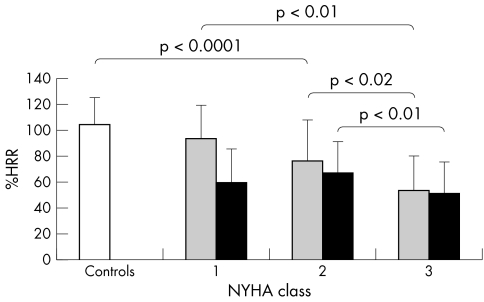
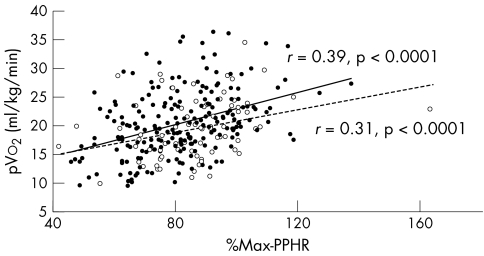
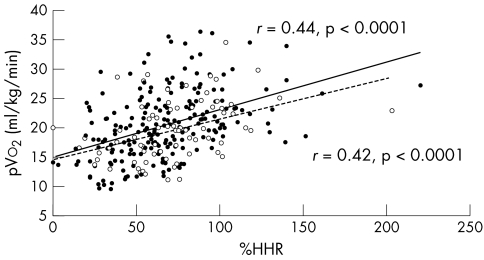
Figures 3 and 4 show the %Max‐PPHR and %HRR of heart failure patients and the relation to New York Heart Association class split by β blocker use. pVo2 correlated with %Max‐PPHR and %HRR both in patients taking and those not taking β blocker (figs 5 and 6).
Figure 3 Percentage maximum peak heart rate (%Max‐PPHR) in patients taking (black bars) and not taking β blockers (grey bars) and the relation to New York Heart Association (NYHA) class.
Figure 4 Percentage heart rate reserve (%HRR) in patients taking β blockers (black bars) and not taking β blockers (grey bars) and the relation to NYHA class.
Figure 5 Relation between pVo2 and %Max‐PPHR in patients taking β blockers (filled circles and solid trend line; r = 0.39, p < 0.0001) and not taking β blockers (unfilled circles and dashed trend line; r = 0.31, p < 0.0001).
Figure 6 Relation between pVo2 and %HRR in patients taking β blockers (filled circles and solid trend line; r = 0.44, p < 0.0001) and not taking β blockers (unfilled circles and dashed trend line; r = 0.42, p < 0.0001).
Patients taking β blocker identified as having chronotropic incompetence by either method had a lower pVo2 (%Max‐PPHR 18.9 (5.3) v 21.7 (5.6) ml/kg/min, p = 0.002 and %HRR 19.2 (5.3) v 24.0 (5.3) ml/kg/min, p < 0.0001) and a steeper Ve/Vco2 slope (37.5 (9.7) v 34.8 (6.3), p = 0.03 and 36.8 (8.7) v 34.0 (6.3), p = 0.03, respectively) than those taking β blocker but without chronotropic incompetence. Patients not taking β blockers but having chronotropic incompetence by either method also had a lower pVo2 (%Max‐PPHR 17.9 (5.6) v 20.0 (5.0) ml/kg/min, p < 0.05 and %HRR 17.8 (4.9) v 22.4 (4.8) ml/kg/min, p = 0.0002) and steeper Ve/Vco2 slope (39.1 (6.1) v 35.5 (6.8), p = 0.03 and 39.1 (6.1) v 35.6 (6.8), p = 0.03, respectively) than those not taking β blockers without chronotropic incompetence.
Survival results
During an average follow up period of 2.8 (0.9) years, 40 patients died, of whom 19 were taking a β blocker at the time of the initial exercise test. Many of the patients not taking a β blocker at the time of the test eventually started taking them (n = 48, 66% of those not taking β blocker at baseline) and absolute mortality at follow up was lower for those who did eventually start a β blocker than for those who did not (8 of 48 (17%) v 10 of 27 (37%), p = 0.05). Overall, patients who died during the follow up period had a lower baseline pVo2 (17.5 (4.0) v 20.6 (5.7) ml/kg/min, p = 0.0015) and steeper Ve/Vco2 slope (40.3 (7.0) v 35.5 (7.7), p = 0.0004) than the survivors.
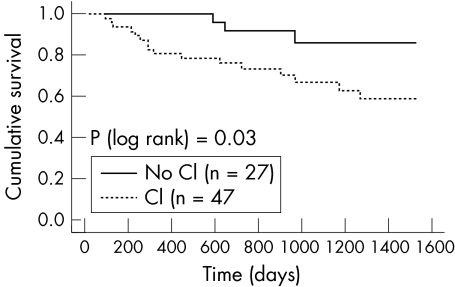
The only relation between chronotropic incompetence and outcome in Kaplan‐Meier analysis that we found was between the presence and absence of chronotropic incompetence assessed by the %HRR definition in that group of patients not taking β blockers at baseline (fig 7). Table 2 gives univariate predictors of survival relating to heart rate during exercise. In a model including RHR and PHR only, increasing RHR was an adverse predictor (hazard ratio (HR) 1.03, 95% confidence interval (CI) 1.01 to 1.05; p = 0.003) and increasing peak rate was a predictor of better outcome (HR 0.98, 95% CI 0.97 to 0.99; p = 0.008). pVo2 was the most powerful single predictor of outcome. In a multivariate model constructed with age, β blocker use, pVo2, and chronotropic incompetence, pVo2 was the most powerful predictor of outcome (table 3).
Figure 7 Survival grouped by chronotropic incompetence (CI) based on %HRR in patients not taking a β blocker.
Table 2 Cox proportional survival analyses according to measures of chronotropic incompetence (univariate analysis).
| Variable | HR | 95% CI | p Value | χ2 |
|---|---|---|---|---|
| RHR | 1.02 | 1.00 to 1.04 | 0.07 | 3.3 |
| PHR | 0.99 | 0.98 to 1.00 | 0.14 | 2.3 |
| Delta HR | 0.98 | 0.96 to 0.99 | 0.003 | 9.2 |
| %Max‐PPHR | 1.0 | 0.98 to 1.01 | 0.76 | 0.1 |
| %HRR | 0.99 | 0.98 to 1.00 | 0.20 | 1.7 |
| Determination of chronotropic incompetence | ||||
| %Max‐PPHR | 1.13 | 0.62 to 2.08 | 0.69 | 0.2 |
| %HRR | 0.87 | 0.45 to 1.68 | 0.67 | 0.2 |
| β Blocker use | 0.52 | 0.29 to 0.95 | 0.03 | 4.3 |
| Age | 1.06 | 1.02 to 1.10 | 0.0004 | 13.9 |
| Ve/Vco2 slope | 1.05 | 1.05 to 1.08 | <0.0001 | 13.1 |
| pVo2 | 0.88 | 0.82 to 0.93 | <0.0001 | 18.6 |
Hazard ratios for continuous variables apply per unit of the analysed variable.
CI, confidence interval; HR, hazard ratio.
Table 3 Cox proportional survival analyses according to measures of chronotropic incompetence (multivariate analysis excluding Ve/Vco2 slope).
| Variable | HR | 95% CI | p Value | χ2 |
|---|---|---|---|---|
| Age | 1.04 | 1.00 to 1.07 | 0.07 | 3.4 |
| pVo2 | 0.89 | 0.83 to 0.96 | 0.002 | 9.6 |
| β Blocker use | 0.69 | 0.37 to 1.30 | 0.19 | 1.7 |
| Chronotropic incompetence (%Max‐PPHR) | 1.22 | 0.63 to 2.38 | 0.55 | 0.36 |
Hazard ratios for continuous variables apply per unit of the analysed variable.
Combined χ2 = 26.4 (p<0.0001).
DISCUSSION
Patients with CHF have a reduced heart rate rise on exercise (chronotropic incompetence). Different methods of defining chronotropic incompetence lead to different estimates of its prevalence. Previous estimates based on %Max‐PPHR had suggested that < 30% of patients with CHF had chronotropic incompetence.14 Our data suggest that 32% of patients not yet taking β blockers have chronotropic incompetence when defined by < 80% Max‐PPHR and 64% have it as defined by < 80% HRR. In β blocked patients the figures are higher: 49% with %Max‐PPHR and 75% with %HRR.
Chronotropic incompetence was related to the severity of heart failure as measured by symptom assessment (New York Heart Association class) and pVo2. Patients with chronotropic incompetence had a lower pVo2 and a steeper Ve/Vco2 slope. We found a close relation between pVo2 and %HRR, %Max‐PPHR, and HRR in patients whether or not they were taking β blockers. These results support previous data that chronotropic incompetence is linked to the severity of heart failure. It was thought that chronotropic incompetence may be an important cause of the reduction in exercise capacity in patients with CHF, but previous work and the present study do not support this view.20 Patients with chronotropic incompetence did have reduced exercise capacity but the slope relating heart rate to pVo2 was the same for those with and without chronotropic incompetence. This suggests that at matched work loads, the heart rates were similar and that reduced heart rates may merely reflect work being performed or, in incremental tests, the duration of exercise. Patients taking β blockers had significantly lower PHRs and more chronotropic incompetence than those not taking β blockers, with no overall difference in pVo2 despite a longer exercise time. Longitudinal studies examining the effects of acute and long term β blockade on patients with heart failure have not shown a reduction of pVo2 despite reductions in PHR with β blockers.20,21
Poor chronotropic response to exercise predicts a poor outcome in patients having stress myocardial perfusion scans, independent of severity of coronary disease and LV function.22,23,24,25,26,27 In patients with heart failure chronotropic incompetence is associated with poor heart rate variability and disturbed cardiac autonomic status.28,29
%HRR, perhaps because it includes the RHR, may be useful in populations treated with heart rate limiting agents. It is possibly a more reliable marker of prognosis than percentage maximum predicted heart rate in patients undergoing myocardial perfusion scanning and may therefore also be more useful in assessing the modern heart failure population, many of whom will be taking a β blocker, than measures based on percentage of predicted heart rate.30 The present study is the first to use this calculation in a large heart failure population taking contemporary drugs.
Assessing chronotropic response in patients with CHF may not only be important as a simple way to further stratify patients at higher risk of cardiac death. Data also suggest that chronotropic incompetence can be improved with physical training. The identification of patients with chronotropic incompetence may allow further targeting of medical and device therapy and exercise training to those who will most benefit from it.31
Previous data have suggested that the presence of chronotropic incompetence may be a more powerful predictor of mortality than pVo2 and Ve/Vco2 slope.15 It has also been suggested in a review of 127 patients with CHF that for patients who tolerate β blockers, pVo2 is not useful for predicting mortality.32 In our study, pVo2 was a stronger predictor of mortality than %HRR and %Max‐PPHR, and patients who died during follow up had lower pVo2 and steeper Ve/Vco2 slopes than those who survived, whether they were taking β blocker at baseline or not.
The heart rate slowing effects of β blockade are important in improving prognosis: patients with greater heart rate reductions gain additional prognostic benefits.33,34 No data on the influence of heart rate reductions during exercise and outlook in patients with CHF have been published. However, higher sympathetic hormone concentrations during exercise are associated with a worse outcome.35 Recent small non‐randomised studies have suggested that target doses and heart rate lowering may be less important and that any dose of β blocker may be sufficient to improve outlook.36,37 In our study, among patients not taking β blockers at the time of the test, chronotropic incompetence or a poor heart rate response to exercise was associated with increased mortality over the subsequent three years. In contrast, patients taking a β blocker with a poor heart rate response to exercise did not have a higher mortality. Patients taking a β blocker with a lower heart rate response to exercise, implying more aggressive β blockade, did not have a worse outcome, even though the pVo2 was lower in patients with %HRR < 80%. Pure heart rate limiting agents without some of the potential side effects or contraindications of β blockers are under investigation and may be useful for patients unable to tolerate β blockade.38 Our data support the concept that chronotropy is not a major factor in determining exercise capacity in patients with CHF, and chronotropic limitation may be an important goal for patients taking β blockers. It is therefore not surprising that pVo2 remains an important predictor of mortality in patients treated with β blockers.
Conclusions
Chronotropic incompetence is common among patients with CHF and its prevalence is greater among patients taking β blockers. Patients with more severe symptoms of CHF and greater reductions in exercise tolerance have a greater reduction in heart rate response to exercise and a higher mortality. pVo2 was the most powerful and consistent predictor of mortality in all subgroups of patients including patients taking β blockers. Patients with chronotropic incompetence who were not taking β blockers had a higher mortality than those without. Chronotropic incompetence due to aggressive β blocker treatment does not predict mortality.
Abbreviations
CHF - chronic heart failure
HRR - heart rate reserve
LV - left ventricular
Max‐PPHR - maximum age predicted peak heart rate
PHR - peak heart rate
pVo2 - peak oxygen consumption
RHR - resting heart rate
Vco2 - carbon dioxide production
Ve - ventilation
Footnotes
Financial support: None
Competing interests: None declared
The study was approved by the local ethics committee.
References
- 1.Clark A L, Sparrow J L, Coats A J S. Muscle fatigue and dyspnoea in chronic heart failure: two sides of the same coin? Eur Heart J 19951649–52. [DOI] [PubMed] [Google Scholar]
- 2.Clark A L, Poole‐Wilson P A, Coats A J S. Exercise limitation in chronic heart failure: central role of the periphery. J Am Coll Cardiol 1996281092–1102. [DOI] [PubMed] [Google Scholar]
- 3.Piepoli M, Clark A, Volterrani M.et al Contribution of muscle afferents to the hemodynamic, autonomic and ventilatory responses to exercise in patients with chronic heart failure. Circulation 199693940–952. [DOI] [PubMed] [Google Scholar]
- 4.Francis G S, Cohn J N, Johnson G.et al Plasma norepinephrine, plasma rennin activity and congestive heart failure: relations to survival and the effects of therapy in V‐HeFT II follow‐up. Circulation 199387(suppl VI)40–48. [PubMed] [Google Scholar]
- 5.Witte K K, Thackray S D, Nikitin N P.et al The effects of alpha and beta blockade on ventilatory responses to exercise in chronic heart failure. Heart 2003891169–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ungerer M, Bohm M, Elce J S.et al Altered expression of beta‐adrenergic receptor kinase and beta 1‐adrenergic receptors in the failing human heart. Circulation 199387454–463. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert E M, Olsen S L, Renlund D G.et al Beta‐adrenergic receptor regulation and left ventricular function in idiopathic dilated cardiomyopathy. Am J Cardiol 19937123C–9C. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein R E, Beiser G D, Stampfer M.et al Impairment of autonomically mediated heart rate control in patients with cardiac dysfunction. Circ Res 197536571–578. [DOI] [PubMed] [Google Scholar]
- 9.White M, Yanowitz F, Gilbert E M.et al Role of beta‐adrenergic receptor downregulation in the peak exercise response in patients with heart failure due to idiopathic dilated cardiomyopathy. Am J Cardiol 1995761271–1276. [DOI] [PubMed] [Google Scholar]
- 10.Colucci W S, Ribeiro J P, Rocco M B.et al Impaired chronotropic response to exercise in patients with congestive heart failure: role of postsynaptic beta‐adrenergic desensitization. Circulation 198980314–323. [DOI] [PubMed] [Google Scholar]
- 11.Yamabe H, Kobayashi K, Takata T.et al Reduced chronotropic reserve to the metabolic requirement during exercise in advanced heart failure with old myocardial infarction. Jpn Circ J 198751259–264. [DOI] [PubMed] [Google Scholar]
- 12.Lauer M S, Larson M G, Evans J C.et al Association of left ventricular dilatation and hypertrophy with chronotropic incompetence in the Framingham heart study. Am Heart J 1999137903–909. [DOI] [PubMed] [Google Scholar]
- 13.Samejima H, Omiya K, Uno M.et al Relationship between impaired chronotropic response, cardiac output during exercise, and exercise tolerance in patients with chronic heart failure. Jpn Heart J 200344515–525. [DOI] [PubMed] [Google Scholar]
- 14.Clark A L, Coats A J. Chronotropic incompetence in chronic heart failure. Int J Cardiol 199549225–231. [DOI] [PubMed] [Google Scholar]
- 15.Robbins M, Francis G, Pashkow F J.et al Ventilatory and heart rate responses to exercise: better predictors of heart failure mortality than peak oxygen consumption. Circulation 19991002411–2417. [DOI] [PubMed] [Google Scholar]
- 16.Beaver W L, Wasserman K, Whipp B J. A new method for detecting the anaerobic threshold by gas exchange. J Appl Physiol 1986602020–2027. [DOI] [PubMed] [Google Scholar]
- 17.Witte K K A, Clark A L. Is the elevated slope relating ventilation to carbon dioxide production in chronic heart failure a consequence of slow metabolic gas kinetics? Eur J Heart Fail 20024469–472. [DOI] [PubMed] [Google Scholar]
- 18.Astrand I. Aerobic work capacity in men and women with special reference to age. Acta Physiol Scand 196049(suppl 169)1–92. [PubMed] [Google Scholar]
- 19.Katritsis D, Camm A J. Chronotropic incompetence: a proposal for definition and diagnosis. Br Heart J 199370400–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witte K K A, Thackray S D R, Nikitin N P.et al The effects of α‐ and β‐blockade on ventilatory responses to exercise in chronic heart failure. Heart 2003891169–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witte K K A, Thackray S, Nikitin N P.et al The effects of long‐term β‐blockade on the ventilatory responses to exercise in chronic heart failure. Eur J Heart Fail 20057612–617. [DOI] [PubMed] [Google Scholar]
- 22.Lauer M S, Francis G S, Okin P M.et al Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA 1999281524–529. [DOI] [PubMed] [Google Scholar]
- 23.Lauer M S, Mehta R, Pashkow F J.et al Association of chronotropic incompetence with echocardiographic ischemia and prognosis. J Am Coll Cardiol 1998321280–1286. [DOI] [PubMed] [Google Scholar]
- 24.Lauer M S, Okin P M, Larson M G.et al Impaired heart rate response to graded exercise: prognostic implications of chronotropic incompetence in the Framingham heart study. Circulation 1996931520–1526. [DOI] [PubMed] [Google Scholar]
- 25.Endo A, Kinugawa T, Ogino K.et al Cardiac and plasma catecholamine responses to exercise in patients with type 2 diabetes: prognostic implications for cardiac‐cerebrovascular events. Am J Med Sci 200032024–30. [DOI] [PubMed] [Google Scholar]
- 26.Dresing T J, Blackstone E H, Pashkow F J.et al Usefulness of impaired chronotropic response to exercise as a predictor of mortality, independent of the severity of coronary artery disease. Am J Cardiol 200086602–609. [DOI] [PubMed] [Google Scholar]
- 27.Elhendy A, Mahoney D W, Khandheria B K.et al Prognostic significance of impairment of heart rate response to exercise: impact of left ventricular function and myocardial ischemia. J Am Coll Cardiol 200342823–830. [DOI] [PubMed] [Google Scholar]
- 28.Fei L, Keeling P J, Sadoul N.et al Decreased heart rate variability in patients with congestive heart failure and chronotropic incompetence. Pacing Clin Electrophysiol 199619477–483. [DOI] [PubMed] [Google Scholar]
- 29.Roche F, Pichot V, Da Costa A.et al Chronotropic incompetence response to exercise in congestive heart failure, relationship with the cardiac autonomic status. Clin Physiol 200121335–342. [DOI] [PubMed] [Google Scholar]
- 30.Azarbal B, Hayes S W, Lewin H C.et al The incremental prognostic value of percentage of heart rate reserve achieved over myocardial perfusion single‐photon emission computed tomography in the prediction of cardiac death and all‐cause mortality: superiority over 85% of maximal age‐predicted heart rate. J Am Coll Cardiol 200444423–430. [DOI] [PubMed] [Google Scholar]
- 31.Keteyian S J, Brawner C A, Schairer J R.et al Effects of exercise training on chronotropic incompetence in patients with heart failure. Am Heart J 1999138233–240. [DOI] [PubMed] [Google Scholar]
- 32.Shakar S F, Lowes B D, Lindenfeld J.et al Peak oxygen consumption and outcome in heart failure patients chronically treated with beta‐blockers. J Card Fail 20041015–20. [DOI] [PubMed] [Google Scholar]
- 33.Lechat P, Hulot J S, Escolano S.et al Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II trial. Circulation 20011031428–1433. [DOI] [PubMed] [Google Scholar]
- 34.Poole‐Wilson P A, Swedberg K, Cleland J G.et al Carvedilol Or Metoprolol European Trial Investigators. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the carvedilol or metoprolol european trial (COMET): randomised controlled trial, Lancet 20033627–13. [DOI] [PubMed] [Google Scholar]
- 35.Kinugawa T, Ogino K, Osaki S.et al Prognostic significance of exercise plasma noradrenaline levels for cardiac death in patients with mild heart failure. Circ J 200266261–266. [DOI] [PubMed] [Google Scholar]
- 36.Mehta P A, McDonagh S, Poole‐Wilson P A.et al Heart failure in a district general hospital: are target doses of beta‐blockers realistic? QJM 200497133–139. [DOI] [PubMed] [Google Scholar]
- 37.Arnold R H, Kotlyar E, Hayward C.et al Relation between heart rate, heart rhythm, and reverse left ventricular remodelling in response to carvedilol in patients with chronic heart failure: a single centre, observational study. Heart 200389293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DiFrancesco D, Camm J A. Heart rate lowering by specific and selective I(f) current inhibition with ivabradine: a new therapeutic perspective in cardiovascular disease. Drugs 2004641757–1765. [DOI] [PubMed] [Google Scholar]