Abstract
Objective
To assess aortic valve probes for valvar C reactive protein (CRP) presence, the relation between valvar and serum CRP, and a possible modification of CRP by statin medication.
Setting
Tertiary referral centre.
Patients and design
End stage, degenerative valve tissue was taken from 81 patients, 57 with non‐rheumatic aortic valve stenosis (AS) and 24 with degenerative aortic valve bioprosthesis (BP). Five non‐stenosed valves served as controls. Tissue from four non‐implanted bioprostheses was also examined. The presence and location of CRP was analysed by use of immunostaining and morphometry. Serum CRP concentrations were measured preoperatively.
Results
The majority of AS and BP valves exhibited CRP labelled cells, predominantly localised to the valvar fibrosa. The expression of CRP was much higher in BP than in AS (by a factor of 3.7, p = 0.03). Notably, non‐stenosed aortic valves and non‐implanted bioprostheses did not have CRP signalling. Serum CRP was also increased with BP (by a factor of 2.5, p = 0.02) and was significantly correlated with valvar CRP expression (r = 0.54, p < 0.001). The main finding in patients with (n = 26) and without statin treatment (n = 55) was that both valvar CRP expression (p = 0.02) and serum CRP concentrations (p = 0.04) were lower in the statin treated group.
Conclusions
CRP was found in a large series of degenerative aortic valves, more often in bioprostheses than in native cusps. Serum CRP concentrations may reflect inflammatory processes within the aortic valve. The association of statin treatment with decreases in both valvar and serum CRP concentrations may explain known pleiotropic effects of statins in patients with aortic stenosis.
Keywords: aortic stenosis, valve bioprosthesis, inflammation, C reactive protein
Although calcific aortic stenosis is recognised as the most common form of valve disease among the elderly, its underlying aetiology is still poorly understood.1 Valve degeneration has been shown to be preceded by focal endothelial damage at the fibrosa, with subsequent inflammatory cell infiltration and cellular transformation into an osteogenic phenotype, making these cells capable of initiating mineralisation.2,3,4,5,6 Specifically, T lymphocytes and macrophages indicating chronic inflammation are known to aggregate in aortic valve stenosis, in many ways similar to atherosclerotic plaques.4,5,7,8,9,10,11,12,13 Also, increased serum concentrations of C reactive protein (CRP) have recently been observed in patients with non‐rheumatic aortic stenosis (AS) and CRP concentrations decreased six months after aortic valve replacement, suggesting that the diseased aortic valve is the site of active inflammation.4,14,15 As yet, no data on the valvar expression of CRP have been reported, in particular in degenerative bioprostheses.
The hypothesis of an active disease process suggests that aortic stenosis may be amenable to medical treatment to prevent or slow disease progression. Indeed, four recent retrospective studies consistently showed an association between statin treatment and much lower haemodynamic progression of aortic stenosis.16,17,18,19 Furthermore, two of these studies showed that this effect is independent of cholesterol concentrations, suggesting pleiotropic or anti‐inflammatory statin effects.18,19
Therefore, we assessed the presence and frequency of CRP in degenerative native and bioprosthetic valves, elucidated the relation between valvar and serum CRP concentrations, and evaluated a possible modification of CRP concentrations by long term statin medication.
METHODS
Patients and valve specimens
In the present study, we assessed a series of surgically removed diseased aortic valves. Valve tissues were obtained from 57 consecutive patients with AS who underwent aortic valve replacement. All valves examined were tricuspid. In addition, 24 severely degenerative aortic valve (porcine) bioprostheses (BP) were obtained during surgical replacement (11 Carpentier‐Edwards and 13 Hancock devices, mean (SD) implant time 13 (4) years). Specific exclusion criteria were evidence of rheumatic disease, other coexisting valve diseases, endocarditis, or other systemic inflammatory processes. The diagnosis of aortic valve disease was based on detailed history and physical examination of the patients, as well as echocardiographic and invasive findings. Information on the type and dosage of long term statin treatment was recorded. Non‐stenotic aortic valve specimens (n = 5) were obtained as controls from surgical patients affected by aortic valve insufficiency caused by aneurysms on the ascending aorta (three men and two women; mean (SD) age 59 (4) years; two with arterial hypertension, two treated with angiotensin converting enzyme inhibitors, and two treated with aspirin). In addition, four non‐implanted bioprostheses (Hancock) were examined. Written informed consent for the subsequent analysis of valve tissue was given by each patient; the local ethics committee had approved the present study.
Immunohistochemistry
Valve probes were fixed in 4.5% buffered formaldehyde and embedded in paraffin. Serial sections (4 μm) were cut from diseased tissue adjacent to calcified areas for immunohistochemical analysis. First the paraffin embedded tissue was dewaxed and rehydrated, followed by saponin (2%; Sigma, Deisenhofen, Germany) retrieval for 20 minutes at room temperature. Then the tissue was incubated for 30 minutes at room temperature in fetal bovine serum (1:25) to block non‐specific binding sites. In the next step, the polyclonal antibody specific for CRP (1:250; ICN, Eschwege, Germany) was added and the tissue sections were incubated overnight at 4°C. After addition of mouse anti‐rabbit IgG (1:75, 30 minutes at room temperature) the colour reaction was done with alkaline phosphatase antialkaline phosphatase marking (Dianova, Hamburg, Germany) according to a standard protocol.10,13,20 The colour substrate of alkaline phosphatase was fast red (Sigma). Lastly, nuclear counterstaining was done by haematoxylin. Negative controls were omission of the antibodies (mouse monoclonal IgG1, clone NCG01, Dianova) and staining with unspecific antibody (ChromPure rabbit IgG, code number 011‐000‐003, Dianova) in known concentrations for primary antibodies.
Histological analysis
Haematoxylin stained histological sections allowed for the detection of cellular nuclei. Cell density was assessed with a computer assisted morphometric system (VFG 1 graphic card, VIBAM 0.0. software) to count nuclei per area (0.04 mm2) and to calculate cell density.10,13,20 Pictures were obtained by an Optiphot‐2 microscope (Nikon, Düsseldorf, Germany) and a downstream KP‐C 553 charge coupled device video camera (Hitachi, Rodgau, Germany). Five randomly selected areas were assessed for each distinct leaflet layer—that is, fibrosa, spongiosa, and ventricularis. The percentage of immunostained cells was determined as the proportion of positive cells in the total number of cells in each layer. Two independent examiners evaluated all morphometric data.
Serum CRP
In 72 patients high sensitive CRP was measured with a commercially available kit (N high sensitivity CRP; Dade Behring, Marburg, Germany). From all patients, serum was collected at admission before operative valve replacement.
Statistical analysis
Data are presented as the percentage (mean (SEM)) of positively stained cells among five randomly chosen high power fields. We assessed group differences by use of the χ2 test for categorical variables. Probability was calculated with the Mann‐Whitney U test for differences in cell density and expression of valvar determinants. Values of p < 0.05 were considered to be significant.
RESULTS
Diseased valve probes from 81 patients undergoing aortic valve replacement were examined for the presence and location of CRP. Fifty seven valves were from patients with AS and 24 from those with BP. Table 1 presents patient characteristics such as age, sex, cardiovascular risk factors, and medication, which did not differ between groups.
Table 1 Characteristics of patients (n = 81) undergoing aortic valve replacement.
| Variable | Patients with AS | Patients with BP | p Value |
|---|---|---|---|
| Number | 57 | 24 | |
| Age (years) | 68 (10) | 68 (6) | 0.9 |
| Men | 34 (60%) | 13 (54%) | 0.6 |
| CAD | 30 (53%) | 11 (46%) | 0.5 |
| Hypertension | 33 (58%) | 11 (46%) | 0.2 |
| Diabetes | 9 (16%) | 3 (13%) | 0.7 |
| Smoker | 25 (44%) | 11 (46%) | 0.9 |
| Hyperlipidaemia | 30 (53%) | 10 (42%) | 0.3 |
| Obesity | 37 (65%) | 13 (54%) | 0.4 |
| Family history | 12 (21%) | 5 (21%) | 0.9 |
| Aspirin | 34 (60%) | 13 (54%) | 0.6 |
| Statin | 20 (35%) | 6 (25%) | 0.4 |
| ACE inhibitor | 32 (56%) | 12 (50%) | 0.6 |
Age is mean (SD).
ACE, angiotensin converting enzyme; AS, non‐rheumatic aortic valve stenosis; BP, degenerative aortic valve bioprosthesis; CAD, coronary artery disease.
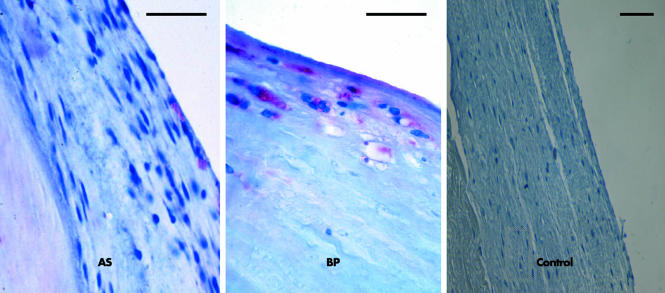
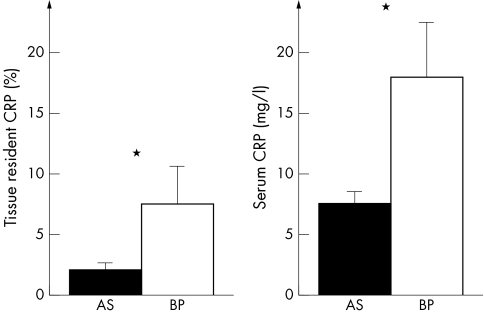
CRP was found in the majority of degenerative valves. Specifically, CRP was detected in 34 of 57 (60%) AS and 16 of 24 (67%) BP. Stained cells were predominantly localised to the fibrosa of AS and the superficial regions of BP (fig 1). Figure 2 summarises quantitative immunohistochemical data for these compartments. Mean expression of CRP was 3.8% and expression was maximal in 65% of valve cells. Remarkably, the percentage of CRP labelled cells was increased by a factor 3.7 in BP compared with AS (p = 0.03). Non‐degenerative native valves and non‐implanted bioprostheses did not show any signalling and served as negative controls.
Figure 1 Photomicrographs of representative valve lesions showing detection of C reactive protein (CRP) in degenerative aortic valves by immunohistochemistry. Signals occur in valvar fibrosa but not in the valvar spongiosa, with a higher percentage of cell bound signals in degenerative aortic valve bioprostheses (BP) with a typically decreased cellularity than in degenerative native valves (non‐rheumatic aortic valve stenosis (AS)). Non‐degenerative native valves did not show any signalling and served as negative controls. Bar = 25 μm.
Figure 2 Quantitative analysis of valvar CRP expression and serum CRP concentrations comparing both groups of aortic valve degeneration. Expression of CRP at the tissue level and systemic CRP were significantly increased in BP versus AS. *p < 0.05.
Of note, a subgroup of 72 patients were analysed for serum CRP concentrations. Mean serum CRP was 10.7 mg/l. Serum CRP was increased in patients with BP compared with those with AS (factor 2.5, p = 0.02) (fig 2). Furthermore, a significant positive correlation between tissue resident CRP and circulating CRP was found (r = 0.54, p < 0.001). Comparison of patients with (n = 50) versus without (n = 31) tissue resident CRP found serum CRP concentrations to be higher in the first group (17.7 (2.6) v 4.4. (1.1) mg/l, p < 0.001). In this first group, 12 of 50 (24%) patients were treated with statins versus 14 of 31 (45%) in the second group (p = 0.047).
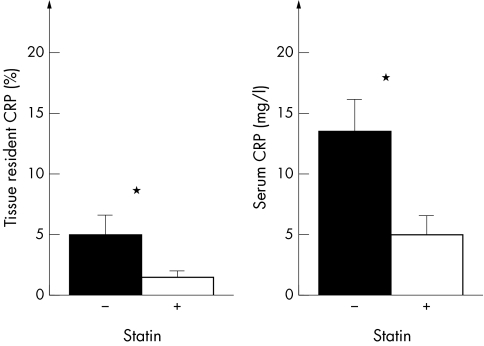
Of the 81 patients, 26 were treated with a statin (eight with simvastatin, eight atorvastatin, four cerivastatin, four pravastatin, and two fluvastatin). A central finding when patients were categorised with (n = 26) versus without statin treatment (n = 55) was that both valvar CRP expression (p = 0.02) and serum CRP concentrations (p = 0.04) were lower in the statin group (fig 3). Beyond this, we observed no significant relation between CRP concentrations, different statins, or different doses of statins.
Figure 3 Expression of valvar CRP and serum CRP concentrations dependent on statin treatment. In the statin treated group (+), valvar expression of CRP and serum CRP concentrations were significantly decreased compared with the non‐statin treated group (−). *p < 0.05.
DISCUSSION
The present study documented the ex vivo presence of CRP in degenerative aortic valves, showing, firstly, that increasing valvar CRP is associated with increasing serum CRP concentrations; secondly, that valvar and serum CRP significantly increased in degenerative prostheses compared with their native counterparts; and thirdly, that statin treatment is associated with notable decreases in both valvar CRP expression and serum CRP concentrations.
Our study showed, for the first time, the presence of tissue resident CRP in degenerative aortic valves (figs 1 and 2). Recently, others reported increased serum CRP concentrations in patients with degenerative aortic stenosis.4,14 Although those authors favoured local actions at the valve tissue level to be responsible for their observation, they did not report the tissue level data.14 In addition, the increased CRP concentrations in patients with AS were found to have decreased after valve replacement, suggesting also that the aortic valve is the key site of active inflammation.15 Beyond confirming this, the present study extends these previous observations with evidence of valvar CRP expression and of a significant association of the staining intensity of valvar and serum CRP concentrations. This finding is in accordance with recent postmortem data from sudden death coronary lesions that found correlations between intimal immunostaining intensity and serum CRP concentrations.21 Our present data show maximal local CRP expression in 65% of all valve cells. These high values do not support the suggestion that serum CRP concentrations are increased by continuous hepatic CRP synthesis but rather they suggest local intravalvar CRP generation. This hypothesis has recently been strengthened by studies that detected CRP mRNA within atherosclerotic plaques and aneurysmal tissue.22,23,24 CRP mRNA content was sevenfold higher in atheroma than in the liver and 10‐fold higher than in undiseased arteries.22 Possibly, the majority of high serum CRP found in patients with AS may be attributed to the direct release of CRP from the diseased valve, thereby reflecting the degree of individual valve inflammation.
Of course, the present study cannot definitively prove whether CRP is an active player in the inflammatory, degenerative process in the valvar fibrosa or is induced by the disease itself. The concept of direct deleterious effects of CRP on valve tissue is supported by several experimental and in vitro studies on atherosclerosis.22,23,24,25,26,27,28,29 CRP leads to induction of the adhesion molecules intercellular adhesion molecule 1, vascular cell adhesion molecule 1, and monocyte chemoattractant protein 1 in endothelial cells and macrophages, exerts chemotactic effects on monocytes/macrophages, propagates inflammation by release of the cytokines interleukin 1β, interleukin 6, and tumour necrosis factor α from monocytes, and, recently, was reported to cause accelerated aortic atherosclerosis in apolipoprotein E−/− mice.26,27,28,29 Whereas undiseased control valves in the present study were not found to have signals of CRP, tissue expression and serum concentrations of CRP are more extensive in patients with degenerative bioprostheses than in those with degenerative native valves. Thus, CRP may be an active participant in both types of valve degeneration and, thereby, may have an even more important role in prosthetic degeneration.
A central finding of the present study was that both valvar CRP expression and serum CRP concentrations were significantly lower in patients with valve disease who were treated with a statin. Analogous to our present data, several clinical studies on patients with atherosclerosis have shown that statins reduce CRP concentrations rapidly and for extended periods, associated with a better clinical outcome.30 Likewise, statins had already been shown to slow the progression of aortic stenosis in retrospective studies but failed to do so in a prospective trial.16,17,18,19,31 It was suggested that this effect is independent of cholesterol lowering.18,19 Indeed, our findings further support the concept that these effects of statins work at the valve tissue level and may be caused by pleiotropic or anti‐inflammatory properties, or both.
In conclusion, the presence of local and systemic CRP provides further evidence that inflammation has an important role in valve degeneration that is more striking in bioprosthetic valves than in native valves. Our findings may suggest the generous use of statins in particular to prevent degeneration of bioprostheses. Future clinical trials are mandatory to prove this attractive concept.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the expert technical assistance of Nicole Kuhn. This work was supported in part by grants to GB from the Deutsche Forschungsgemeinschaft (DFG Ba 1076/2‐2).
Abbreviations
AS - non‐rheumatic aortic valve stenosis
BP - degenerative aortic valve bioprosthesis
CRP - C reactive protein
Footnotes
Competing interests: none declared
References
- 1.Carabello B A, Crawford F A., Jr Valvular heart disease. N Engl J Med 199733732–41. [DOI] [PubMed] [Google Scholar]
- 2.Chan K L. Is aortic stenosis a preventable disease? J Am Coll Cardiol 200342593–599. [DOI] [PubMed] [Google Scholar]
- 3.Farzaneh‐Far A, Proudfoot D, Shanahan C.et al Vascular and valvar calcification: recent advances. Heart 20018513–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazzone A, Epistolato M C, De Caterina R.et al Neoangiogenesis, T‐lymphocyte infiltration, and heat shock protein‐60 are biological hallmarks of an immunomediated inflammatory process in end‐stage calcified aortic valve stenosis. J Am Coll Cardiol 2004431670–1676. [DOI] [PubMed] [Google Scholar]
- 5.Mohler ER I I I, Gannon F, Reynolds C.et al Bone formation and inflammation in cardiac valves. Circulation 20011031522–1528. [DOI] [PubMed] [Google Scholar]
- 6.Rajamannan N M, Subramaniam M, Rickad D.et al Human aortic valve calcification is associated with an osteoblast phenotype. Circulation 20031072181–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ollson M, Dalsgard C J, Haegerstrand A.et al Accumulation of T lymphocytes and expression of interleukin‐2 receptors in nonrheumatic stenotic aortic valves. J Am Coll Cardiol 1994231162–1170. [DOI] [PubMed] [Google Scholar]
- 8.Otto C M, Kuusisto J, Reichenbach D D.et al Characterization of the early lesions of ‘degenerative' valvular aortic stenosis: Histologic and immunohistochemical studies. Circulation 199490844–854. [DOI] [PubMed] [Google Scholar]
- 9.Satta J, Melkko J, Pöllännen R.et al Progression of human aortic valve stenosis is associated with tenascin‐C expression. J Am Coll Cardiol 20023996–101. [DOI] [PubMed] [Google Scholar]
- 10.Skowasch D, Yeghiazaryan K, Schrempf S.et al Persistence of Chlamydia pneumoniae in degenerative aortic valve stenosis indicated by heat shock protein 60 homologues. J Heart Valve Dis 20031268–75. [PubMed] [Google Scholar]
- 11.Kaden J J, Dempfle C E, Grobholz R.et al Interleukin‐1 beta promotes matrix metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis 2003170205–211. [DOI] [PubMed] [Google Scholar]
- 12.Kuusisto J, Rösänen K, Särkioja T.et al Atherosclerosis‐like lesions of the aortic valve are common in adults of all stages: a necropsy study. Heart 200591576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skowasch D, Jabs A, Andrié R.et al Pathogen burden, inflammation, proliferation and apoptosis in human in‐stent restenosis: tissue characteristics compared to primary atherosclerosis. J Vasc Res 200441525–534. [DOI] [PubMed] [Google Scholar]
- 14.Galante A, Pietroiusti A, Vellini M.et al C‐reactive protein is increased in patients with degenerative aortic valvular stenosis. J Am Coll Cardiol 2001381078–1082. [DOI] [PubMed] [Google Scholar]
- 15.Gerber I L, Stewart R A, Hammett C J.et al Effect of aortic valve replacement on C‐reactive protein in nonrheumatic aortic stenosis. Am J Cardiol 2003921129–1132. [DOI] [PubMed] [Google Scholar]
- 16.Aronow W S, Ahn C, Kronzon I.et al Association of coronary risk factors and use of statins with progression of mild valvular aortic stenosis in older persons. Am J Cardiol 200188693–695. [DOI] [PubMed] [Google Scholar]
- 17.Novaro G M, Tiong I Y, Pearce G L.et al Effect of hydroxymethylglutaryl coenzyme A reductase inhibitors on the progression of calcific aortic stenosis. Circulation 20011042205–2209. [DOI] [PubMed] [Google Scholar]
- 18.Bellamy M F, Pellikka P A, Klarich K W.et al Association of cholesterol levels, hydroxymethylglutaryl coenzyme‐A reductase inhibitor treatment, and progression of aortic stenosis in the community. J Am Coll Cardiol 2002401723–1730. [DOI] [PubMed] [Google Scholar]
- 19.Rosenhek R, Rader F, Loho N.et al Statins but not angiotensin‐converting enzyme inhibitors delay progression of aortic stenosis. Circulation 20041101291–1295. [DOI] [PubMed] [Google Scholar]
- 20.Bauriedel G, Jabs A, Skowasch D.et al Dendritic cells in neointima formation after rat carotid balloon injury: coordinated expression with anti‐apoptotic Bcl‐2 and HSP47 in arterial repair. J Am Coll Cardiol 200342930–938. [DOI] [PubMed] [Google Scholar]
- 21.Burke A P, Tracy R P, Kolodgie F.et al Elevated C‐reactive protein values and atherosclerosis in sudden coronary death: association with different pathologies. Circulation 20021052019–2023. [DOI] [PubMed] [Google Scholar]
- 22.Yasojima K, Schwab C, McGeer E G.et al Generation of C‐reactive protein and complement components in atherosclerotic plaques. Am J Pathol 20011581039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vainas T, Lubbers T, Stassen F R.et al Serum C‐reactive protein level is associated with abdominal aortic aneurysm size and may be produced by aneurysmal tissue. Circulation 20031071103–1105. [DOI] [PubMed] [Google Scholar]
- 24.Jabs W J, Theissing E, Nitschke M.et al Local generation of C‐reactive protein in diseased artery venous bypass grafts and normal vascular tissue. Circulation 20031081428–1431. [DOI] [PubMed] [Google Scholar]
- 25.Skowasch D, Jabs A, Andrié R.et al Progression of native coronary plaques and in‐stent restenosis are associated and predicted by increased preprocedural C reactive protein. Heart 200591535–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasceri V, Cheng J, Willerson J T.et al Modulation of C‐reactive protein‐mediated monocyte chemoattractant protein‐1 induction in human endothelial cells by anti‐atherosclerosis drugs. Circulation 20011032531–2534. [DOI] [PubMed] [Google Scholar]
- 27.Torzewski M, Rist C, Mortensen R F.et al C‐reactive protein in the arterial intima: role of C‐reactive protein receptor‐dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol 2000202094–2099. [DOI] [PubMed] [Google Scholar]
- 28.Calabro P, Willerson J T, Yeh E T H. Inflammatory cytokines stimulated C‐reactive protein production by human coronary artery smooth muscle cells. Circulation 20031081930–1932. [DOI] [PubMed] [Google Scholar]
- 29.Paul A, Ko K W S, Li L.et al C‐reactive protein accelerates the progression of atherosclerosis in apolipoprotein E‐deficient mice. Circulation 2004109647–655. [DOI] [PubMed] [Google Scholar]
- 30.Ridker P M, Cannon C P, Morrow D.et al C‐reactive protein levels and outcomes after statin therapy. N Engl J Med 200535220–28. [DOI] [PubMed] [Google Scholar]
- 31.Cowell S J, Newby D E, Prescott R J.et al A randomized trial of intensive lipid‐lowering therapy in calcific aortic stenosis. N Engl J Med 20053522441–2443. [DOI] [PubMed] [Google Scholar]