Abstract
Objective
To examine the association of childhood socioeconomic position (SEP) with adult cardiovascular risk factors, vascular structure, and vascular function in a contemporary population of young adults.
Design
Population based prospective cohort study with baseline assessment in 1980.
Setting
Finland.
Participants
856 men and 1066 women whose childhood SEP was determined by parental occupational status (manual, lower non‐manual, upper non‐manual) at age 3–18 years.
Main outcome measures
Cardiovascular risk factors, carotid artery intima–media thickness, and brachial artery flow mediated vasodilatation, assessed at age 24–39 years.
Results
After adjustment for age and adult SEP, systolic pressure was 2.3 mm Hg higher (p = 0.0002), high density lipoprotein (HDL) cholesterol 0.03 mmol/l lower (p = 0.02), and insulin resistance score (homeostasis model assessment index) 0.12 units greater (p = 0.05) among men; and systolic pressure was 1.3 mm Hg higher (p = 0.02), diastolic pressure 1.1 mm Hg higher (p = 0.01), and height 1.1 cm lower (p < 0.0001) among women for each step down the childhood SEP hierarchy. Lower childhood SEP was associated with a 20% increase in the odds of having a waist circumference > 102 cm in men and > 88 cm in women (overall p = 0.05). Childhood SEP was not associated with intima–media thickness, flow mediated vasodilatation, the metabolic syndrome, low density lipoprotein cholesterol, triglycerides, body mass index, alcohol consumption, or smoking.
Conclusions
Among adults under 40, low childhood SEP predicted higher blood pressure and central obesity and, among men, unfavourable HDL cholesterol and insulin resistance, independent of current SEP. No independent effects were found on adult vascular structure, vascular function, or health related behaviours at this life stage.
Keywords: socioeconomic status, cardiovascular diseases, atherosclerosis, endothelial function, risk factors
Low socioeconomic position (SEP) in childhood is predictive of increased cardiovascular disease (CVD) morbidity and mortality, but its association with adult CVD risk factors remains poorly understood.1,2 The strongest evidence relates to body mass and blood pressure, which have been found to be higher among adults with low childhood SEP.3,4,5,6,7,8,9,10,11,12,13 A study of postmenopausal women suggested that a low childhood SEP is also predictive of increased risk for the metabolic syndrome, a cluster of central obesity, high blood pressure, and impaired lipid and glucose metabolism.14,15 However, evidence with regard to lipid metabolism is inconclusive: in some studies, low childhood SEP was associated with less favourable concentrations of high (HDL) but not low density lipoprotein (LDL) cholesterol among women, whereas among men no association between childhood SEP and cholesterol concentrations was detected.5,8,16,17 Other evidence for men suggested that lower childhood SEP was associated with more favourable cholesterol concentrations.3
Evidence is also ambiguous for markers of atherosclerosis, such as carotid artery intima–media thickness.18 In one study, an association was found between lower SEP at birth and higher adult arterial intima–media thickness, but this was not replicated for SEP at 10 years of age.16 Another study reported an association between SEP in childhood and adult intima–media thickness among women but not among men.19 Reduced brachial arterial flow mediated vasodilatation is an established marker of impaired endothelial function, an important step in the atherosclerotic disease process.20 However, no previous studies have examined whether there is a link between childhood SEP and brachial arterial flow mediated vasodilatation. Some studies,3,4,5,8,12 but not all,9,12,16,21 have found an association between low childhood SEP and adult smoking, suggesting that early SEP may affect CVD risk through establishment of health related behaviours rather than through early structural or functional changes in arteries.
Most studies on childhood SEP have examined only a limited number of CVD risk factors. Hence, the relative importance of early SEP for risk factors not covered by these studies remains unknown. A further source of potential error is generated by reliance on evidence from older cohorts, since large scale studies of contemporary cohorts are scarce. Existing evidence may therefore indicate stronger SEP effects than those seen in later generations born in times of greater prosperity. For these reasons, we investigated the association between childhood SEP and a large variety of established adult risk factors, including the metabolic syndrome and markers of atherosclerosis and endothelial functioning, in a contemporary population of young adults.
METHODS
Participants
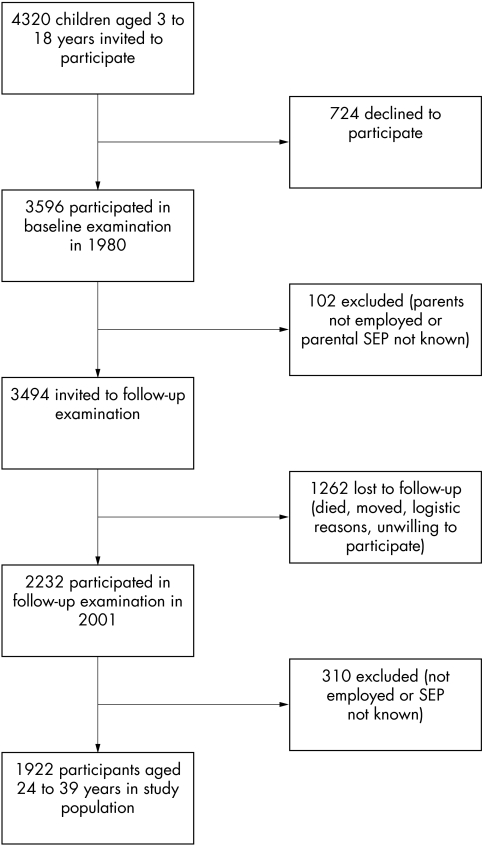
The participants were from the cardiovascular risk in young Finns study, an ongoing multicentre follow up study of CVD risk factors in Finnish children and adolescents.22,23 The original sample was 4320 children and adolescents aged 3, 6, 9, 12, 15, and 18 years. The participants were randomly chosen in five areas of Finland from the national register. Of those invited, 3596 (83%) participated in the baseline examination in 1980.22 In 2001, the participants, who by then had reached 24–39 years of age, were re‐examined. Participants in the present study were the 1922 (856 men and 1066 women) who had data on SEP in 1980 and 2001 and a measurement of CVD risk factors in 2001 (fig 1). The participants did not differ from the population at baseline in terms of age group and SEP (discrepancy in any category 3% or less), but women were slightly overrepresented (55% in the study cohort versus 51% in the baseline population).
Figure 1 Study population at baseline and follow up examination.
Socioeconomic position
SEP in childhood or adolescence was assessed in 1980 from parental occupational status as classified by Statistics Finland.24 SEP was categorised as manual (for example, factory worker, mechanic, security guard, waiter, cleaner); lower grade non‐manual (for example, clerical employee, sales representative, nurse, secretary, supervisor); and higher grade non‐manual (for example, general manager, lawyer, physician, engineer, secondary school teacher). Where SEP differed between parents, the SEP of the parent with the higher occupational status was used. The participant's own adult SEP was measured by occupational status in 2001 and categorised as for parental SEP. Childhood SEP and adult SEP were strongly associated (p < 0.0001, χ2 test). Participants whose childhood SEP was manual were more likely to work in manual or lower non‐manual occupations than in upper non‐manual occupations (corresponding frequencies 41.1%, 42.7%, and 16.2%, respectively), whereas those whose childhood SEP was upper non‐manual were more likely to be employed in upper or lower non‐manual occupations than in manual occupations (corresponding frequencies 47.4%, 39.1%, and 13.5%). Of the participants whose childhood SEP was classified as lower non‐manual, most worked in lower non‐manual occupations (46.3%) with smaller proportions working in upper non‐manual (22.2%) and manual occupations (31.5%).
Anthropometric measurements and blood pressure
Physical measurements of weight (kg), height (mm), waist circumference (mm, measured in duplicate at the level of the 12th rib or level with the navel in thin subjects), and hip circumference (mm) were obtained to calculate body mass index (weight in kg/height in m2) and waist to hip ratio. Systolic and diastolic blood pressures (mm Hg) were measured with a random zero sphygmomanometer (Hawksley & Sons Ltd, West Sussex, UK). Blood pressure was measured with the subject in a sitting position after at least five minutes' rest. Measurements were read at least three times on each participant and the average was used in the statistical analysis.
Biochemical analyses
All blood samples were taken after an overnight fast and analysed in duplicate in the same laboratory. Standard enzymatic methods were used for serum total cholesterol, HDL cholesterol, triglyceride, and plasma glucose concentrations. LDL cholesterol concentration was calculated with the Friedewald formula for subjects with < 4 mmol/l triglycerides. Serum insulin was measured by microparticle enzyme immunoassay kit (Abbott Laboratories, Diagnostic Division, Dainabot, Tokyo, Japan). Insulin resistance was estimated according to homeostasis model assessment (HOMA) as the product of fasting glucose and insulin divided by the constant 22.5.25
Assessment of risk behaviours and the metabolic syndrome
Information on smoking and alcohol consumption (units/week) was obtained by questionnaire. One unit of alcohol (12 g) was equal to a glass of wine, a single 40 ml shot of spirits, or a 330 ml bottle of beer. The metabolic syndrome was defined according to the Adult Treatment Panel III criteria with three or more of the following conditions15: waist circumference > 102 cm in men and > 88 cm in women; HDL cholesterol < 1.0 mmol/l in men and < 1.3 mmol/l in women; fasting triglycerides ⩾ 1.7 mmol/l; blood pressure > 130/85 mm Hg; and fasting plasma glucose ⩾ 6.1 mmol/l.
Ultrasonic measurements
Ultrasound studies were performed with Sequoia 512 ultrasound mainframes (Acuson, Mountain View, California, USA) to measure carotid intima–media thickness and brachial artery flow mediated dilatation.23,26 In brief, the image was focused on the posterior (far) wall of the left carotid artery. A minimum of four measurements of the common carotid far wall were taken about 10 mm proximal to the carotid bifurcation to derive maximum carotid intima–media thickness. The between‐visit coefficient of variation for the intima–media thickness measurements was 6.4%.23 To assess brachial artery flow mediated dilatation, the left brachial artery diameter was measured both at rest and during reactive hyperaemia. The vessel diameter measured from scans after reactive hyperaemia was expressed as the percentage relative to the diameter from the resting scan. The three month between‐visit coefficient of variation was 3.2% for the brachial artery diameter measurements and 26.0% for the flow mediated dilatation measurements.26
Statistical analysis
Age adjusted sex differences in risk factors were tested with logistic regression analysis for dichotomous variables (smoking, metabolic syndrome) and analysis of variance for continuous variables (all other risk factors). Distributions of insulin, glucose, and the HOMA score were slightly skewed so we replicated analyses with these variables after log transformation. As this did not materially alter the associations the untransformed data are presented. Age and current SEP adjusted associations between childhood SEP and risk factors were estimated separately for men and women with regression models (logistic models for dichotomous risk factors) with childhood SEP fitted as an ordinal variable (1, upper non‐manual; 2, lower non‐manual; 3, manual) to assess trend. Logistic regression analyses were performed to examine the associations of childhood SEP with some common, clinically defined conditions: central obesity (waist circumference > 102 cm in men and > 88 cm in women), hypertension (systolic blood pressure > 135 mm Hg or diastolic blood pressure > 80 mm Hg or antihypertensive medication), low HDL cholesterol concentration (⩽ 1.0 mmol/l in men and ⩽ 1.3 mmol/l in women), hyperinsulinaemia (insulin > 9.0 mU/l, top quartile of the study population), insulin resistance (HOMA index > 2.05, top quartile of the study population), and the metabolic syndrome.15,27 These models were adjusted for age and current SEP for men and women and, additionally, for sex in the total sample. All p values are two sided. All analyses were performed with the use of SAS software, version 8.2 (SAS Institute, Cary, North Carolina, USA).
RESULTS
Table 1 lists the characteristics of the participants. Sex differences in age, SEP in childhood, adult insulin concentrations, and insulin resistance were small. The proportions of men in the highest and lowest adult SEP categories were greater than those of women, and men had a less favourable pattern of socioeconomic mobility (p < 0.001). Men were taller and they had a greater carotid artery intima–media thickness and lower flow mediated dilatation. They also had a poorer cardiovascular risk profile indicated by higher blood pressure; higher concentrations of total cholesterol, LDL cholesterol, triglycerides, and glucose; lower HDL cholesterol; higher body mass index, waist circumference, and waist to hip ratio; higher prevalence of smoking; and higher alcohol consumption (all p < 0.001).
Table 1 Sample characteristics by sex.
| Variable | Men | Women | ||
|---|---|---|---|---|
| No | Value | No | Value | |
| Age (years) | 856 | 31.8 (0.2) | 1066 | 31.8 (0.1) |
| Childhood (parental) SEP | ||||
| Manual | 359 | 42% | 420 | 39% |
| Lower non‐manual | 345 | 40% | 471 | 44% |
| Upper non‐manual | 152 | 18% | 175 | 16% |
| Own adult SEP | ||||
| Manual | 369 | 43% | 252 | 24% |
| Lower non‐manual | 234 | 27% | 605 | 57% |
| Upper non‐manual | 253 | 30% | 209 | 20% |
| BMI (kg/m2) | 850 | 25.7 (0.1) | 1032 | 24.6 (0.1) |
| Waist circumference (cm) | 850 | 89.8 (0.3) | 1034 | 79.7 (0.3) |
| Waist to hip ratio | 850 | 0.90 (0.002) | 1032 | 0.79 (0.002) |
| Height (cm) | 850 | 179.6 (0.2) | 1032 | 166.0 (0.2) |
| Systolic pressure (mm Hg) | 848 | 121.6 (0.4) | 1052 | 112.6 (0.40 |
| Diastolic pressure (mm Hg) | 848 | 73.1 (0.4) | 1052 | 68.8 (0.3) |
| Total cholesterol (mmol/l) | 852 | 5.28 (0.03) | 1064 | 5.10 (0.03) |
| HDL cholesterol (mmol/l) | 850 | 1.16 (0.01) | 1064 | 1.40 (0.01) |
| LDL cholesterol (mmol/l) | 828 | 3.44 (0.03) | 1060 | 3.17 (0.03) |
| Triglycerides (mmol/l) | 852 | 1.52 (0.03) | 1064 | 1.19 (0.03) |
| Glucose (mmol/l) | 851 | 5.20 (0.02) | 1064 | 4.92 (0.02) |
| Insulin (mU/l) | 852 | 7.45 (0.20) | 1064 | 7.89 (0.18) |
| Insulin resistance (HOMA index)* | 844 | 1.70 (0.05) | 1060 | 1.74 (0.04) |
| Smoking | ||||
| No | 579 | 69.2% | 852 | 81.5% |
| Yes | 258 | 30.8% | 193 | 18.5% |
| Alcohol consumption (units/week)† | 846 | 8.8 (0.3) | 1060 | 3.5 (0.2) |
| Metabolic syndrome‡ | ||||
| No | 751 | 90.4% | 925 | 93.3% |
| Yes | 80 | 9.6% | 67 | 6.7% |
| Intima–media thickness (mm) | 844 | 0.594 (0.003) | 1053 | 0.574 (0.003) |
| Brachial artery flow mediated dilatation (%) | 767 | 6.80 (0.16) | 993 | 8.82 (0.14) |
Data are mean (SE) or percentage.
*Product of fasting glucose and insulin divided by the constant 22.5; †one unit (12 g) was equal to a glass of wine, a single 40 ml shot of spirits, or a 33 ml bottle of beer; ‡three or more of the following conditions: waist >102 cm in men and >88 cm in women, serum triglycerides >1.7 mmol/l, HDL cholesterol <1.04 mmol/l in men and <1.29 mmol/l in women, blood pressure >130/85 mm Hg or treated, and plasma glucose >6.1 mmol/l.
BMI, body mass index; HDL, high density lipoprotein; HOMA, homeostasis model assessment; LDL, low density lipoprotein; SEP, socioeconomic position.
Table 2 presents the age and adult SEP adjusted associations between childhood SEP and adult risk factors among men. Low SEP in childhood was associated with higher waist to hip ratio, higher systolic blood pressure, lower HDL cholesterol, higher insulin concentration, and higher insulin resistance. Exclusion of participants treated for hypertension (n = 24), hypercholesterolaemia (n = 5), or diabetes (n = 4) had little effect on these findings.
Table 2 Age adjusted levels of risk factors by childhood SEP and age and adult SEP adjusted regression coefficients for each parental SEP category in men (n = 767 to 852).
| Risk factor | Category of childhood SEP | Change/descending childhood SEP category | |||
|---|---|---|---|---|---|
| Upper non‐manual | Lower non‐manual | Manual | Adjusted* B | p Value | |
| BMI (kg/m2) | 25.2 | 25.6 | 25.8 | 0.3 (0.2) | 0.13 |
| Waist circumference (cm) | 88.1 | 89.5 | 90.2 | 1.0 (0.5) | 0.06 |
| Waist to hip ratio | 0.88 | 0.89 | 0.90 | 0.01 (0.00) | 0.006 |
| Height (cm) | 180.0 | 179.9 | 179.1 | −0.5 (0.3) | 0.10 |
| Systolic blood pressure (mm Hg) | 118.1 | 121.2 | 123.1 | 2.3 (0.6) | 0.0002 |
| Diastolic blood pressure (mm Hg) | 72.2 | 72.7 | 73.1 | 0.4 (0.5) | 0.45 |
| Total cholesterol (mmol/l) | 5.20 | 5.27 | 5.27 | 0.02 (0.05) | 0.69 |
| HDL cholesterol (mmol/l) | 1.21 | 1.15 | 1.15 | −0.03 (0.01) | 0.02 |
| LDL cholesterol (mmol/l) | 3.38 | 3.42 | 3.45 | 0.02 (0.04) | 0.60 |
| Triglycerides (mmol/l) | 1.35 | 1.60 | 1.49 | 0.06 (0.05) | 0.20 |
| Glucose (mmol/l) | 5.10 | 5.21 | 5.21 | 0.03 (0.03) | 0.29 |
| Insulin (mU/l) | 6.68 | 7.29 | 7.93 | 0.68 (0.28) | 0.02 |
| Insulin resistance (HOMA index) | 1.54 | 1.69 | 1.77 | 0.12 (0.06) | 0.05 |
| Percentage of smokers | 23.5% | 30.4% | 35.0% | −1.0 (2.2) | 0.67 |
| Alcohol consumption (units/week) | 11.2 | 8.1 | 8.7 | −0.81 (0.52) | 0.12 |
| Intima–media thickness (mm) | 0.597 | 0.587 | 0.595 | −0.000 (0.005) | 0.96 |
| Brachial artery flow mediated dilatation (%) | 6.80 | 6.89 | 6.76 | −0.12 (0.21) | 0.55 |
Data are mean (SE) or percentage.
*Adjusted for age and adult SEP except height, which is adjusted for age.
Table 3 shows the age and adult SEP adjusted associations between childhood SEP and adult risk factors among women. Those with lower SEP in childhood were shorter and had higher waist circumference, higher waist to hip ratio, and higher systolic and diastolic blood pressures. There was no material change in these findings after the exclusion of treated participants (29 for hypertension, one for hypercholesterolaemia, and three for diabetes).
Table 3 Age adjusted levels of risk factors by childhood SEP and age and adult SEP adjusted regression coefficients for each parental SEP category in women (n = 992 to 1064).
| Risk factor | Category of childhood SEP | Change/descending childhood SEP category | |||
|---|---|---|---|---|---|
| Upper non‐manual | Lower non‐manual | Manual | Adjusted* B | p Value | |
| BMI (kg/m2) | 23.9 | 24.6 | 25.0 | 0.4 (0.2) | 0.07 |
| Waist circumference (cm) | 77.2 | 79.2 | 80.7 | 1.2 (0.5) | 0.02 |
| Waist to hip ratio | 0.78 | 0.79 | 0.80 | 0.01 (0.00) | 0.0002 |
| Height (cm) | 167.7 | 166.0 | 165.3 | −1.1 (0.3) | <0.0001 |
| Systolic blood pressure (mm Hg) | 110.3 | 112.9 | 113.4 | 1.3 (0.6) | 0.02 |
| Diastolic blood pressure (mm Hg) | 67.2 | 68.7 | 69.4 | 1.1 (0.5) | 0.01 |
| Total cholesterol (mmol/l) | 5.06 | 5.07 | 5.14 | 0.04 (0.04) | 0.34 |
| HDL cholesterol (mmol/l) | 1.42 | 1.40 | 1.39 | −0.01 (0.01) | 0.59 |
| LDL cholesterol (mmol/l) | 3.13 | 3.13 | 3.22 | 0.05 (0.03) | 0.16 |
| Triglycerides (mmol/l) | 1.15 | 1.23 | 1.18 | −0.00 (0.03) | 0.88 |
| Glucose (mmol/l) | 4.95 | 4.87 | 4.95 | 0.01 (0.03) | 0.81 |
| Insulin (mU/l) | 7.72 | 8.00 | 8.05 | 0.09 (0.27) | 0.74 |
| Insulin resistance (HOMA index) | 1.62 | 1.78 | 1.76 | 0.05 (0.07) | 0.42 |
| Percentage of smokers | 11.2% | 19.6% | 20.9% | 1.7 (1.7) | 0.33 |
| Alcohol consumption (units/week) | 4.2 | 3.3 | 3.7 | −0.1 (0.3) | 0.72 |
| Intima–media thickness (mm) | 0.573 | 0.573 | 0.571 | −0.000 (0.004) | 0.99 |
| Brachial artery flow mediated dilatation (%) | 8.57 | 8.88 | 8.72 | −0.04 (0.21) | 0.86 |
*Adjusted for age and adult SEP except height, which is adjusted for age.
Table 4 presents the age and adult SEP adjusted associations between childhood SEP and common clinical conditions. Low childhood SEP was associated with increased prevalence of central obesity, hypertension, hyperinsulinaemia, and insulin resistance in the total sample with slightly stronger effects among men than among women. Among men, low childhood SEP was additionally associated with adverse HDL cholesterol concentrations. No robust association was found with the metabolic syndrome in this low risk population.
Table 4 Age and sex adjusted prevalence of some clinically defined risk factors by childhood SEP and age, sex, and adult SEP adjusted odds ratio for each childhood SEP category.
| Risk factor* | Category of childhood SEP | Change per descending childhood SEP category | |||
|---|---|---|---|---|---|
| Upper non‐manual | Lower non‐manual | Manual | Adjusted† OR (95% CI) | p Value | |
| All participants (n = 1823 to 1884) | |||||
| Central obesity | 12.3 | 17.4 | 19.1 | 1.20 (1.00 to 1.43) | 0.05 |
| Hypertension | 15.3 | 19.0 | 22.0 | 1.23 (1.03 to 1.46) | 0.02 |
| Low HDL cholesterol | 32.9 | 33.6 | 36.3 | 1.09 (0.95 to 1.26) | 0.20 |
| Hyperinsulinaemia | 16.4 | 22.9 | 24.7 | 1.23 (1.05 to 1.44) | 0.01 |
| Insulin resistance | 17.9 | 25.7 | 27.2 | 1.24 (1.07 to 1.45) | 0.005 |
| Metabolic syndrome | 5.3 | 9.1 | 8.1 | 1.14 (0.89 to 1.47) | 0.31 |
| Men (n = 831 to 850) | |||||
| Central obesity | 8.2 | 13.7 | 15.6 | 1.29 (0.95 to 1.74) | 0.10 |
| Hypertension | 22.7 | 26.3 | 32.6 | 1.27 (1.02 to 1.59) | 0.04 |
| Low HDL cholesterol | 26.4 | 29.6 | 33.0 | 1.25 (1.00 to 1.54) | 0.05 |
| Hyperinsulinaemia | 16.0 | 21.7 | 25.3 | 1.34 (1.06 to 1.71) | 0.02 |
| Insulin resistance | 19.3 | 25.6 | 28.2 | 1.29 (1.03 to 1.62) | 0.03 |
| Metabolic syndrome | 5.2 | 10.3 | 10.0 | 1.28 (0.90 to 1.82) | 0.17 |
| Women (n = 987 to 1034) | |||||
| Central obesity | 15.7 | 20.5 | 22.0 | 1.14 (0.91 to 1.43) | 0.25 |
| Hypertension | 9.4 | 13.2 | 13.4 | 1.16 (0.88 to 1.53) | 0.28 |
| Low HDL cholesterol | 41.0 | 38.5 | 42.3 | 1.03 (0.86 to 1.23) | 0.76 |
| Hyperinsulinaemia | 16.8 | 23.8 | 24.0 | 1.15 (0.93 to 1.42) | 0.21 |
| Insulin resistance | 16.7 | 25.7 | 26.4 | 1.21 (0.98 to 1.49) | 0.08 |
| Metabolic syndrome | 5.2 | 7.8 | 6.1 | 0.99 (0.69 to 1.43) | 0.97 |
*Central obesity refers to waist circumference >102 cm in men and >88 cm in women; hypertension to systolic blood pressure >130 mm Hg or diastolic pressure >85 mm Hg or antihypertensive medication; low HDL cholesterol to a concentration ⩽1.0 mmol/l in men and ⩽1.3 in women; hyperinsulinaemia to insulin concentration >9.0 mU/l (top quartile of the study population); and insulin resistance to HOMA index >2.05 (top quartile of the study population).
†Adjusted for age and adult SEP in men and women and additionally for sex in the total sample.
CI, confidence interval; OR, odds ratio.
DISCUSSION
Findings based on a contemporary, population based sample of Finns at age 24–39 years show that low SEP in childhood is associated with increased adult blood pressure and central obesity for both sexes, independent of adult SEP. Low childhood SEP is also associated with less favourable lipid and glucose metabolism in men and with shorter height in women. All these effects were relatively small and no robust evidence was found for adverse effects of low childhood SEP on adult LDL cholesterol, triglycerides, behavioural risk factors (smoking and alcohol consumption), the metabolic syndrome, carotid intima–media thickness, or brachial artery flow mediated dilatation.
One hypothesised underlying mechanism for the association between childhood SEP and adult CVD is the sustained influence of early socioeconomic circumstances on behavioural risk factors, such as smoking and diet.14 Another is that low childhood SEP is a marker of suboptimal nutrition during the intrauterine period or early childhood, which programmes later increased blood pressure, insulin resistance, and shorter height, all risk factors for CVD.14 Our findings suggest that neither smoking nor alcohol consumption is a key mediating factor in a contemporary population, although an association between childhood SEP and adult smoking has been reported in some older cohorts.3,4,5,8,12,14 In contrast, our results on the effect of childhood SEP on adult blood pressure and obesity accord with those observed for various other populations and age ranges in the USA, New Zealand, Finland, and the UK.4,7,9,10,21 Socioeconomic differences in blood pressure and obesity may start early, as they tend to track from young ages into adulthood.28 Our findings are also in line with previous studies that have found a stronger association between childhood SEP and height among women than among men,5 although reasons for this sex difference remain unknown.
The observed SEP differences in blood pressure and central obesity in young adulthood may result in a differential morbidity risk for our study population in the future. The difference in systolic blood pressure was 3.4–5.0 mm Hg between participants whose childhood SEP was manual and those whose childhood SEP was upper non‐manual. In addition, lower childhood SEP was associated with a 30% greater risk of hypertension among men. An 18 mm Hg increase in systolic blood pressure at age 60–69 years has been estimated to be associated with a 100% increase in the risk of stroke and with more than a 50% increase in coronary risk, but in younger adults the relative risks associated with a given degree of blood pressure rise may be much higher.29,30 Obesity is a significant contributor to many health problems including non‐insulin dependent diabetes mellitus, coronary heart disease, certain forms of cancer, and osteoarthritis of large and small joints.31 In our population, the relative risk of a level of central obesity that should trigger therapeutic action increased by 20% at each step down SEP hierarchy.
Insulin resistance is a central feature of the metabolic syndrome and has been linked to development of CVD. The association found between low childhood SEP and higher insulin resistance in men is consistent with the early programming hypothesis, as insulin resistance is associated with correlates of low childhood SEP such as suboptimal maternal nutrition and catch‐up growth in early childhood.32 An association between low childhood SEP and insulin resistance has previously been reported for postmenopausal women participating in the British women's heart and health study.8,14 The absence of an association between childhood SEP and insulin resistance among young women in this study may indicate a protective role for premenopausal status or younger age.
In a historical study of British men, lower childhood SEP was associated with lower total cholesterol, an association in the opposite direction to that of cardiovascular risk.3 This finding reflected less heart healthy dietary patterns among higher SEP families, but the situation has subsequently changed. Some later studies found no association between childhood SEP and adult cholesterol concentrations among men, but in the present study of young adults low childhood SEP was associated with increased risk of dyslipidaemia, as indicated by a less favourable concentration of HDL cholesterol.5,16,17
Despite the adverse levels of many metabolic syndrome factors among men with low childhood SEP, no robust association was seen between early SEP and the adult metabolic syndrome.15 However, the present data may be underpowered to observe associations with this relatively rare syndrome in young adults. Our findings in men indicate that a longer follow up may show such an association. To our knowledge, no previous prospective study has examined childhood SEP and the adult metabolic syndrome.
Major changes in vascular function and structure come relatively late in the continuum from distal risk factors to manifest CVD and death.20 According to one possible pathway, iterative exposure of endothelium to risk factors leads sequentially to endothelial dysfunction, followed by intima–media thickening, overt manifestations of atherosclerosis, development of arterial stenosis, and ultimately to plaque rupture and endovascular thrombosis.20 In our cohort of young adults, childhood SEP was not associated with the adult subclinical risk markers carotid arterial intima–media thickness and brachial artery flow mediated vasodilatation. This absence of association may be due to insufficient lag time or relatively high prosperity in childhood. Indeed, in older cohorts born in less favourable circumstances, a weak association between childhood SEP and carotid atherosclerosis has been seen. The Newcastle thousand families birth cohort study found an independent effect of SEP at birth but not at age 10 on intima–media thickness in women aged 49–51 years.19 Irrespective of the indicator of early SEP, no effect was observed for men. A retrospective study of Swedes aged 46–68 reported an association between childhood SEP and carotid atherosclerosis for women but not for men.16 In our study, imprecise measurement is an unlikely explanation for the absence of socioeconomic differences in intima–media thickness because biological and geographical factors in childhood and adult risk factors have consistently predicted intima–media thickness in this cohort.23,26 Greater body size among participants with higher SEP cannot explain the negative finding, as additional adjustment for height did not strengthen the association between childhood SEP and adult intima–media thickness.
Study limitations
At least four potential limitations are noteworthy. Firstly, 45% of the baseline cohort was lost during the 21 year follow up, the loss being slightly greater among men than women. This may not be a major source of bias because all analyses were carried out separately for men and women and the loss was not differential between age groups or socioeconomic groups. Secondly, we tested 17 associations between childhood SEP and CVD risk factors and found five with p < 0.05 for men and five with p < 0.05 for women; only one in 20 would have been expected by chance at this level of significance. Although multiple comparisons can increase the overall type I error rate in statistical testing, associations observed in this study were all in the expected direction and findings for blood pressure and waist to hip ratio were similar for both sexes. Thirdly, early SEP can be measured by using information about parental occupational group, income, or education, but the present results were reported only for parental occupational group assessed at age 3–18 years. In our cohort, parental occupational status is strongly associated with parental education (weighted κ = 0.42, p < 0.0001). Analyses of parental education as the marker of childhood SEP generally replicated the main findings, although effects were slightly weaker. For example, after adjustment for age and sex, waist to hip ratio was 0.006 higher (p = 0.003), systolic blood pressure was 1.4 mm Hg higher (p = 0.002), and, among men, insulin resistance score was 0.11 greater (p = 0.09) for each step down the educational hierarchy. These figures correspond to 0.009 (p = 0.0001), 1.8 mm Hg (p < 0.0001), and 0.12 (p = 0.05), respectively, for parental occupational group. Fourthly, corresponding to the vast majority of the Finnish population in 1980, our sample was ethnically homogeneous; thus, results cannot be generalised to other ethnic groups.
Conclusions
Data from a contemporary cohort of young adults, born in times of greater prosperity than were preceding generations, suggest that childhood SEP remains a predictor of adult CVD risk factors, such as waist circumference, waist to hip ratio, blood pressure, and, for men, HDL cholesterol and insulin resistance. At this life stage—24 to 39 years of age—the contribution of childhood SEP to adult vascular function and structure, LDL cholesterol and triglyceride concentrations, prevalence of the metabolic syndrome, and behavioural risk factors seems to be substantially smaller.
ACKNOWLEDGEMENTS
The cardiovascular risks in young Finns study has been supported by the Academy of Finland (grant 53392), the Social Insurance Institution of Finland, the Finnish Work Environment Foundation, Turku University Foundation, Juho Vainio Foundation, the Finnish Foundation of Cardiovascular Research, and the Finnish Cultural Foundation, Finland. MK, LK‐J, and JV are supported by the Academy of Finland (grants 104891, 105195, 209514, and 209518). JEF is supported by the MRC (grant number 47413). JSAV and OTR were supported by research grants from Turku University Central Hospital.
Abbreviations
CVD - cardiovascular disease
HDL - high density lipoprotein
HOMA - homeostasis model assessment
LDL - low density lipoprotein
SEP - socioeconomic position
Footnotes
The funding sources were not involved in either the submission of the manuscript nor the decision to publish the data. None of the authors has any financial disclosures.
Competing interests: None declared
This study was conducted according to the guidelines of the Declaration of Helsinki and the study protocol was approved by local ethics committees. All participants gave their informed consent.
References
- 1.Davey Smith G, Hart C, Blane D.et al Adverse socioeconomic conditions in childhood and cause‐specific adult mortality: prospective observational study. BMJ 19983161631–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch J, Davey Smith G. A life course approach to chronic disease epidemiology. Ann Rev Public Health 2005261–35. [DOI] [PubMed] [Google Scholar]
- 3.Blane D, Hart C L, Davey Smith G.et al Association of cardiovascular disease risk factors with socioeconomic position during childhood and during adulthood. BMJ 19963131434–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Power C, Matthews S. Origins of health inequalities in a national population sample. Lancet 19973501584–1589. [DOI] [PubMed] [Google Scholar]
- 5.Brunner E, Shipley M J, Blane D.et al When does cardiovascular risk start: past and present socioeconomic circumstances and risk factors in adulthood. J Epidemiol Community Health 199953757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leino M, Raitakari O T, Porkka K V.et al Cardiovascular risk factors of young adults in relation to parental socioeconomic status: the cardiovascular risk in young Finns study. Ann Med 200032142–151. [DOI] [PubMed] [Google Scholar]
- 7.Laitinen J, Power C, Järvelin M ‐ J. Family social class, maternal body mass index, childhood body mass index, and age at menarche as predictors of adult obesity. Am J Clin Nutr 200174287–294. [DOI] [PubMed] [Google Scholar]
- 8.Lawlor D A, Ebrahim S, Davey Smith G. Socioeconomic position in childhood and adulthood and insulin resistance: cross‐sectional survey using data from British women's heart and health study. BMJ 2002325805–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poulton R, Caspi A, Milne B J.et al Association between children's experience of social disadvantage and adult health: a life‐course study. Lancet 20023601640–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardy R, Kuh D, Langenberg C.et al Birthweight, childhood social class, and change in adult blood pressure in the 1946 British birth cohort. Lancet 20033621178–1183. [DOI] [PubMed] [Google Scholar]
- 11.Kivimäki M, Kinnunen M ‐ L, Pitkänen T.et al Contribution of early and adult factors to socioeconomic variation in blood pressure: 34‐year follow‐up study of school children. Psychosom Med 200466184–189. [DOI] [PubMed] [Google Scholar]
- 12.Regidor E, Banegas J R, Gutierrez‐Fisac J L.et al Socioeconomic position in childhood and cardiovascular risk factors in older Spanish people. Int J Epidemiol 200433723–730. [DOI] [PubMed] [Google Scholar]
- 13.Kivimäki M, Davey Smith G, Elovainio M.et al Socioeconomic circumstances in childhood and blood pressure in adulthood: the cardiovascular risk in young Finns study. Ann Epidemiol. (in press) [DOI] [PubMed]
- 14.Lawlor D A, Davey Smith G, Ebrahim S. Association between childhood socioeconomic status and coronary heart disease risk among postmenopausal women: findings from the British women's heart and health study. Am J Public Health 2004941386–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Cholesterol Education Program Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 20012852486–2497. [DOI] [PubMed] [Google Scholar]
- 16.Rosvall M, Östergren P O, Hedblad B.et al Life‐course perspective on socioeconomic differences in carotid atherosclerosis. Arterioscler Thromb Vasc Biol 2002221704–1711. [DOI] [PubMed] [Google Scholar]
- 17.Wannamethee S G, Whincup P H, Shaper G.et al Influence of fathers' social class on cardiovascular disease in middle‐aged men. Lancet 19963481259–1263. [DOI] [PubMed] [Google Scholar]
- 18.Burke G L, Ecans G W, Riley W A.et al Arterial wall thickness is associated with prevalent cardiovascular disease in middle‐aged adults: the atherosclerosis risk in communities (ARIC) study. Stroke 199526386–391. [DOI] [PubMed] [Google Scholar]
- 19.Lamont D, Parker L, White M.et al Risk of cardiovascular disease measured by carotid intima‐media thickness at age 49–51: lifecourse study. BMJ 2000320273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirsch A T, Folsom A R. The continuum of risk: vascular pathophysiology, function, and structure. Circulation 20041102774–2777. [DOI] [PubMed] [Google Scholar]
- 21.Power C, Graham H, Due P.et al The contribution of childhood and adult socioeconomic position to adult obesity and smoking behaviour: an international comparison. Int J Epidemiol 200534335–344. [DOI] [PubMed] [Google Scholar]
- 22.Åkerblom H K, Uhari M, Pesonen E.et al Cardiovascular risk in young Finns. Ann Med 19912335–40. [DOI] [PubMed] [Google Scholar]
- 23.Raitakari O T, Juonala M, Kähönen M.et al Cardiovascular risk factors in childhood and carotid artery intima‐media thickness in adulthood: the cardiovascular risk in young Finns study. JAMA 20032902277–2283. [DOI] [PubMed] [Google Scholar]
- 24.Statistics Finland Classification of socio‐economic groups 1989. Handbook 17. Helsinki: Statistics Finland, 1989
- 25.Matthews D R, Hosker J P, Rudenski A S.et al Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 198528412–419. [DOI] [PubMed] [Google Scholar]
- 26.Juonala M, Viikari J S A, Laitinen T.et al Interrelations between brachial endothelial function and carotid intima‐media thickness in young adults: the cardiovascular risk in young Finns study. Circulation 20041102918–2923. [DOI] [PubMed] [Google Scholar]
- 27.Han T S, van Leer E M, Seidell J C.et al Waist circumference action levels in the identification of cardiovascular risk factors: prevalence study in a random sample. BMJ 19953111401–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCarron P, Davey Smith G. Physiological measurements in children and young people, and risk of coronary heart disease in adults. In: Giles A, ed. A lifecourse approach to coronary heart disease prevention. London: National Heart Forum, the Stationery Office, 200379–118.
- 29.Collins R, Peto R, MacMahon S.et al Blood pressure, stroke, and coronary heart disease. Part 2. Short‐term reductions in blood pressure: overview of randomised drug trials in their epidemiological context, Lancet 1990335827–838. [DOI] [PubMed] [Google Scholar]
- 30.Prospective Studies Collaboration Age‐specific relevance of usual blood pressure to vascular mortality: one million adults in 61 prospective studies. Lancet 20023601903–1913. [DOI] [PubMed] [Google Scholar]
- 31.Kopelman P G. Obesity as a medical problem. Nature 2000404635–643. [DOI] [PubMed] [Google Scholar]
- 32.Parker L, Lamont D W, Unwin P.et al A life course study of risk for hyperinsulinaemia, dyslipidaemia and obesity (the central metabolic syndrome) at age 49–51 years. Diabet Med 200320406–413. [DOI] [PubMed] [Google Scholar]