Abstract
Objective
To evaluate the effects of valsartan on cardiac sympathetic nerve activity, plasma brain natriuretic peptide (BNP) concentration, cardiac function, and symptoms in patients with congestive heart failure (CHF) by comparison with those of enalapril.
Methods
50 patients with CHF (left ventricular ejection fraction (LVEF) < 40%) were randomly assigned to valsartan (80 mg/day; n = 25) or enalapril (5 mg/day; n = 25). All patients were also treated with a loop diuretic. The delayed heart to mediastinum count (H/M) ratio, delayed total defect score (TDS), and washout rate were determined from 123I‐meta‐iodobenzylguanidine (MIBG) images. Plasma BNP concentrations were measured before and after six months of treatment. The left ventricular end diastolic volume (LVEDV) and LVEF were also determined by echocardiography.
Results
In patients receiving valsartan, TDS decreased from a mean (SD) of 43 (8) to 39 (10) (p < 0.01), H/M ratio increased from 1.70 (0.17) to 1.78 (0.22) (p < 0.05), washout rate decreased from 46 (11)% to 41 (10)% (p < 0.05), and plasma BNP concentration decreased from 237 (180) pg/ml to 143 (93) pg/ml (p < 0.05). In addition, LVEDV decreased from 172 (42) ml to 151 (45) ml (p < 0.05) and LVEF increased from 31 (7)% to 39 (10)% (p < 0.001). However, these parameters did not change significantly in patients receiving enalapril.
Conclusion
Plasma BNP concentration and 123I‐MIBG scintigraphic and echocardiographic parameters improved significantly after six months of treatment with valsartan. These findings indicate that valsartan can improve cardiac sympathetic nerve activity and left ventricular performance in patients with CHF.
Keywords: 123I‐meta‐iodobenzylguanidine, heart failure, angiotensin receptor blocker
Myocardial imaging with 123I‐meta‐iodobenzylguanidine (123I‐MIBG), an analogue of noradrenaline, is a useful tool for detecting abnormalities of the myocardial adrenergic nervous system in patients with congestive heart failure (CHF).1,2,3 Moreover, cardiac 123I‐MIBG scintigraphic findings and left ventricular function are correlated,3 and 123I‐MIBG scintigraphy has a useful prognostic value in patients with CHF.2,3,4 Furthermore, the concentration of plasma brain natriuretic peptide (BNP), secreted mainly from the ventricle,5 is a useful prognostic indicator in patients with CHF.6
Activation of the renin–angiotensin–aldosterone system (RAAS) promotes structural remodelling of the heart and the progression of heart failure.7,8 Several reports have suggested, based on cardiac 123I‐MIBG scintigraphic studies, that inhibition of RAAS can improve cardiac sympathetic nerve activity in patients with CHF.9,10,11,12,13,14,15 The angiotensin receptor blocker (ARB) valsartan has beneficial haemodynamic and hormonal effects in patients with CHF treated conventionally.16,17 Cohn et al18 reported that the addition of valsartan significantly improved New York Heart Association (NYHA) functional class, left ventricular remodelling, left ventricular ejection fraction (LVEF), and signs and symptoms of heart failure. However, its influence on cardiac sympathetic nerve activity and BNP concentration has not been sufficiently determined.
In the present study, we evaluated the effects of valsartan on cardiac sympathetic nerve activity, plasma BNP concentration, cardiac function, and symptoms in patients with CHF by comparison with those of enalapril.
METHODS
Study patients
From December 2001 through March 2003, 54 patients were admitted to our institution with a first episode of CHF. A detailed history and physical examination were obtained before their inclusion in the study. None of the patients had a history of heart failure. All patients underwent chest radiography, standard ECG, echocardiography, and thallium‐201 and 123I‐MIBG scintigraphy. Patients were in NYHA functional class II or III at the time of enrolment and had an echocardiographic LVEF < 40% (mean (SD) 32 (8)%).
Patients were excluded from the study if they had congenital heart disease, unstable angina, recent acute myocardial infarction, primary hepatic failure, or active cancer. Patients with stenotic valvar heart disease were also excluded from the study because cardiac function in these patients may respond differently to vasodilating treatment depending on intravascular volume, cardiac sympathetic nerve system, and RAAS activity.
This study was approved by the ethics review board of our institution. The nature and purpose of the study and risks involved were explained to all patients. Written informed consent to participate in the study was obtained.
Study protocol
Twenty seven patients were randomly assigned to valsartan (80 mg/day) and the remaining 27 patients were assigned to enalapril (5 mg/day). All patients were also treated with a loop diuretic. Treatment with digitalis and vasodilators was allowed. We performed a series of examinations before and after six months of treatment.
123I‐MIBG imaging
The method used for 123I‐MIBG imaging has been described.9,10,11,12,13,14,19,20123I‐MIBG was obtained from a commercial source (Daiichi Radioisotope Laboratories, Tokyo, Japan). Patients were intravenously injected with 123I‐MIBG (111 MBq) while in the supine position. At 15 minutes and at four hours after the injection, static data were acquired in the anterior view with a single head gamma camera (Millennium MPR, GE Medical Systems, Waukesha, Wisconsin, USA) equipped with a low energy, general purpose, parallel hole collimator. Static images on a 128 × 128 matrix were collected for five minutes with a 20% window centred on 159 keV, corresponding to the 123I photopeak. After the static planar images were acquired, single photon emission computed tomographic (SPECT) images were obtained. The camera was rotated over 180° from the 45° right anterior oblique position to the 45° left posterior oblique position in 32 views with an acquisition time of 40 s/view. Scans were acquired in a 64 × 64 matrix by a filtered back projection method for reconstruction.
The heart to mediastinum count (H/M) ratio was determined from the anterior planar delayed 123I‐MIBG image. The washout rate (WR) was calculated from the formula: [(H − M) early − (H − M) delayed]/(H − M) early × 100 (%), where H is mean count/pixel in the left ventricle and M is mean count/pixel in the upper mediastinum. In this study, time decay was not corrected for the calculation of WR.
The delayed myocardial SPECT images of each patient were divided into 17 segments as recommended by the American Heart Association.21 Regional tracer uptake was assessed semiquantitatively by a five point scoring system (0, normal uptake; 1, mildly reduced uptake; 2, moderately reduced uptake; 3, significantly reduced uptake; and 4, no uptake). The total defect score (TDS) was calculated as the sum of all defect scores.
Two independent observers with no knowledge of patient clinical status or medical treatment determined interobserver variability in a blinded fashion. The interobserver correlation was highly significant (r = 0.90, p < 0.001).
Plasma BNP concentration
Blood samples were collected into test tubes containing EDTA after the patient had rested in the supine position for at least 30 minutes. Plasma was separated by centrifugation and was frozen at −84°C. Then the plasma concentration of BNP was measured with a specific immunoradiometric assay for human BNP with a commercially available kit (Shionogi, Osaka, Japan) as previously reported.13,14,22
Echocardiography
Echocardiographic measurements were taken by standard methods in a blinded manner before and after six months of treatment. Two independent and experienced echocardiographers who had no knowledge of the study performed all measurements. Left ventricular end diastolic volume (LVEDV) and LVEF were calculated with the modified Simpson's method. The interobserver and intraobserver correlations for LVEDV were r = 0.90, p < 0.001 and r = 0.94, p < 0.0001, respectively, and for LVEF were r = 0.90, p < 0.001 and r = 0.93, p < 0.0001, respectively.
Statistical analysis
Data were statistically analysed with Statview (Abacus Concepts, Berkeley, California, USA) for Macintosh (Apple Computer, Inc, Cupertino, California, USA). Numerical results are expressed as the mean (SD). Baseline categorical data of the two groups were compared by the χ2 test. The differences between continuous variables were evaluated with an unpaired t test. Changes in NYHA functional class were assessed with the Wilcoxon matched pairs signed ranks test. For patients who underwent repeated assessments, changes from baseline were evaluated within each treatment group with a paired t test and between the valsartan and enalapril groups, with two way analysis of variance. A value of p < 0.05 was considered significant.
RESULTS
Clinical characteristics
In the group of patients receiving valsartan, one patient had a cerebral embolism and one patient died of an arrhythmia. In the group of patients receiving enalapril, one patient died of CHF during the follow up period and one was excluded because of the onset of unstable angina. Therefore, 50 of the 54 patients (31 men and 19 women, mean (SD) age 68 (9) years, range 42 to 80 years) enrolled in the trial completed the entire protocol. The causes of heart failure were idiopathic dilated cardiomyopathy (n = 25), old myocardial infarction (n = 17), or valve disease (n = 8; six with mitral regurgitation and two with aortic regurgitation).
The two groups did not differ significantly in haemodynamic characteristics or cardiac medications on entry into the study. Before treatment, TDS, H/M ratio, WR, plasma BNP concentration, LVEDV, LVEF, and NYHA functional class in both groups were similar (table 1). In this study, baseline cardiac medication was not changed for any of the patients during the follow up period. The mean dose of furosemide was 56 (23) mg/day in the valsartan group versus 58 (22) mg/day in the enalapril group (not significant). The mean dose of carvedilol was 14 (6) mg/day in the valsartan group versus 13 (6) mg/day in the enalapril group (not significant). The mean dose of isosorbide mononitrate was 37 (8) mg/day in the valsartan group versus 36 (9) mg/day in the enalapril group (not significant). Furthermore, the dose of spironolactone was only 25 mg/day in both groups.
Table 1 Baseline patient characteristics.
| Valsartan (n = 25) | Enalapril (n = 25) | p Value | |
|---|---|---|---|
| Age (years) | 67 (10) | 68 (9) | NS |
| Men/women | 16/9 | 15/10 | NS |
| Height (cm) | 160 (9) | 162 (10) | NS |
| Weight (kg) | 58 (8) | 59 (9) | NS |
| SBP (mm Hg) | 132 (16) | 131 (14) | NS |
| DBP (mm Hg) | 74 (8) | 72 (8) | NS |
| NYHA functional class | |||
| II/III | 6/19 | 6/19 | NS |
| Cause of CHF | |||
| DCM | 12 (48%) | 13 (52%) | NS |
| OMI | 8 (32%) | 9 (36%) | NS |
| Valve disease | 5 (20%) | 3 (12%) | NS |
| 123I‐MIBG | |||
| TDS | 43 (8) | 43 (9) | NS |
| H/M ratio | 1.70 (0.17) | 1.68 (0.36) | NS |
| WR | 46 (11) | 47 (8) | NS |
| Echocardiography | |||
| LVEDV (ml) | 172 (42) | 173 (29) | NS |
| LVEF(%) | 31 (7) | 32 (8) | NS |
| Plasma BNP (pg/ml) | 237 (180) | 235 (154) | NS |
| Medical treatment | |||
| Loop diuretic | 25 (100%) | 25 (100%) | NS |
| β Blocker | 15 (60%) | 17 (68%) | NS |
| Spironolactone | 8 (32%) | 7 (28%) | NS |
| Nitrate | 6 (24%) | 5 (20%) | NS |
| Calcium antagonist | 6 (24%) | 9 (36%) | NS |
| Digitalis | 2 (8%) | 3 (12%) | NS |
Values are mean (SD) or number (%).
BNP, brain natriuretic peptide; CHF, congestive heart failure; DBP, diastolic blood pressure; DCM, dilated cardiomyopathy; H/M, heart to mediastinum count; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; 123I‐MIBG, iodine‐123 labelled meta‐iodobenzylguanidine; NS, not significant; NYHA, New York Heart Association; OMI, old myocardial infarction; SBP, systolic blood pressure; TDS, total defect score; WR, washout rate.
Comparison of cardiac 123I‐MIBG scintigraphic findings
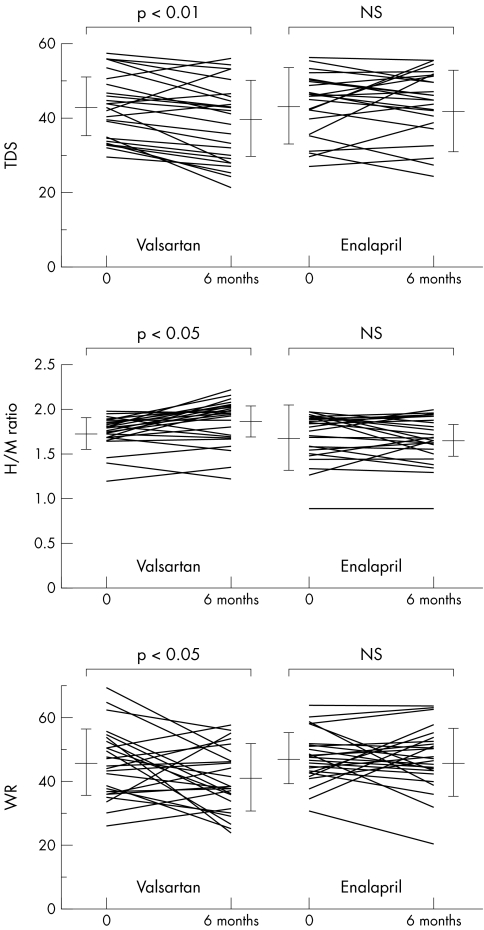
Table 2 and fig 1 summarise TDS, H/M ratio, and WR. In patients receiving valsartan, TDS was significantly decreased at six months compared with the baseline value (p < 0.01). In contrast, in patients receiving enalapril, TDS at baseline and after six months of treatment did not differ significantly. Segmental analysis of TDS showed that this tended to improve due to uptake of the inferior wall in both groups, although the improvement was not significant. In patients receiving valsartan, the H/M ratio was significantly increased at six months compared with the baseline values (p < 0.05). In contrast, in patients receiving enalapril, H/M ratio at baseline and after six months of treatment did not differ significantly. In patients receiving valsartan, the WR was significantly decreased at six months compared with the baseline values (p < 0.05). In contrast, in patients receiving enalapril, WR at baseline and after six months of treatment did not differ significantly.
Table 2 Changes in TDS, H/M ratio, and WR of patients in valsartan and enalapril groups.
| Valsartan | Enalapril | |||
|---|---|---|---|---|
| Baseline | 6 months | Baseline | 6 months | |
| 123I‐MIBG | ||||
| TDS | 43 (8) | 39 (10)* | 43 (9) | 42 (10) |
| H/M ratio | 1.70 (0.17) | 1.78 (0.22)** | 1.68 (0.36) | 1.67 (0.22) |
| WR | 46 (11) | 41 (10)** | 47 (8) | 46 (10) |
Values are mean (SD).
*p<0.01 v baseline; **p<0.05 v baseline.
Figure 1 Comparison of cardiac iodine‐123 labelled meta‐iodobenzylguanidine scintigraphic findings during treatment in the two groups. H/M, heart to mediastinum count; NS, not significant; TDS, total defect score; WR, washout rate.
Comparison of echocardiographic findings
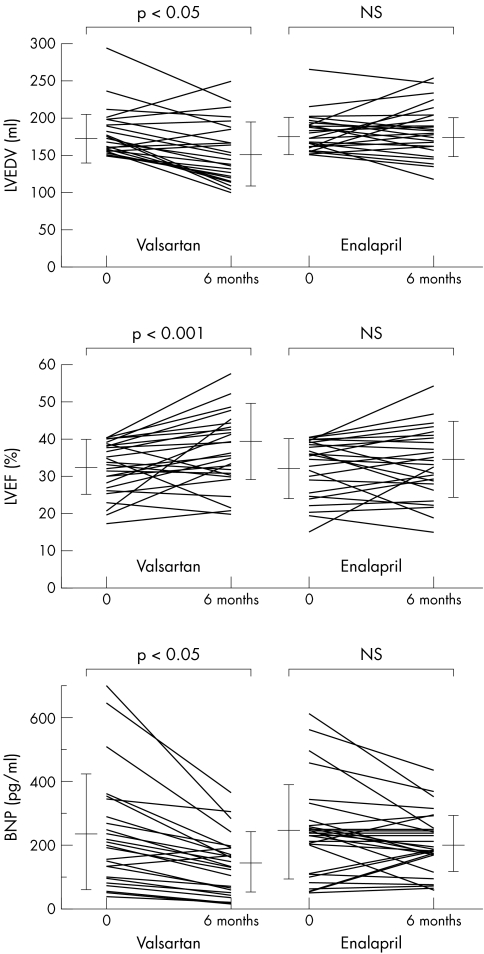
Table 3 and fig 2 summarise the changes in LVEDV and LVEF. In patients receiving valsartan, LVEDV was significantly decreased at six months compared with baseline values (p < 0.05). In contrast, in patients receiving enalapril, LVEDV at baseline and after six months of treatment did not differ significantly. In patients receiving valsartan, LVEF was significantly increased at six months compared with baseline values (p < 0.001). In contrast, in patients receiving enalapril, LVEF at baseline and after six months of treatment did not differ significantly.
Table 3 Changes of plasma BNP concentration, LVEDV, LVEF, and NYHA functional class of patients in the valsartan and enalapril groups.
| Valsartan | Enalapril | |||
|---|---|---|---|---|
| Baseline | 6 months | Baseline | 6 months | |
| Plasma BNP (pg/ml) | 237 (180) | 143 (93)* | 235 (154) | 200 (95) |
| LVEDV (ml) | 172 (42) | 151 (45)* | 173 (29) | 172 (33) |
| LVEF (%) | 31 (7) | 39 (10)** | 32 (8) | 35 (10) |
| NYHA class | ||||
| I/II/III | 0/6/19 | 8/15/2*** † | 0/6/19 | 2/14/9* |
Values are mean (SD).
*p<0.05 v baseline; **p<0.001 v baseline; ***p<0.001 v baseline; †p<0.01 v enalapril at 6 months.
Figure 2 Comparison of echocardiographic findings and plasma brain natriuretic peptide (BNP) concentrations during treatment in the two groups. LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction.
Comparison of plasma BNP concentration
Table 3 and fig 2 show plasma BNP concentrations. In patients receiving valsartan, plasma BNP concentrations were significantly decreased at six months compared with the baseline values (p < 0.05). In contrast, BNP concentration at baseline and after six months of treatment did not differ significantly in the patients receiving enalapril.
Comparison of NYHA functional class
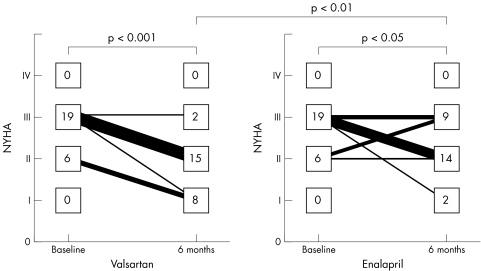
Table 3 and fig 3 summarise the NYHA functional class of the patients. Patients in both groups improved after six months of treatment compared with the baseline values (in patients receiving valsartan, p < 0.001; in patients receiving enalapril, p < 0.05). After treatment, the NYHA functional class of the patients receiving valsartan was significantly better than in those receiving enalapril (p < 0.01).
Figure 3 Changes in New York Heart Association (NYHA) functional class during treatment in the two groups.
DISCUSSION
Angiotensin converting enzyme (ACE) inhibitors reduce angiotensin II concentrations, mortality, and morbidity in patients with CHF.23,24 ACE inhibitors are believed to act principally by blocking the formation of angiotensin II, a potent vasoconstrictor and cardiovascular growth stimulator that may contribute to increased impedance, left ventricular ejection, and cardiac remodelling.25 However, growing evidence supports an important role for non‐ACE mediated enzymatic pathways (for example, chymase pathway) in the conversion of angiotensin I to angiotensin II.26,27 Therefore, a strategy of providing greater blockade of angiotensin with valsartan than with enalapril in the treatment of heart failure appears rational. Furthermore, subtypes of the angiotensin II receptor have been reported.28 One of the subtypes, the type 2 receptor, is known to have antagonistic actions against the type 1 receptor and to have favourable effects on the myocardium.28 Therefore, because the ARB valsartan selectively inhibits the angiotensin II type 1 receptor, this drug may have more cardioprotective effects than ACE inhibitors.
The growth promoting and apoptotic effects of angiotensin II have been well documented and may contribute to the structural remodelling that promotes the progression of heart failure.7,8,29 A long term increase in LVEF has been identified as a marker of beneficial left ventricular remodelling that is manifested as a reduced chamber volume.30 This structural effect is associated with an improvement in survival.31 Cohn et al18 reported that the addition of valsartan significantly improved cardiac function and reduced mortality and morbidity in patients with heart failure. In our study, left ventricular volume and cardiac function were significantly improved by valsartan compared with enalapril. Moreover, in our study, valsartan improved the symptoms of heart failure as measured by changes in the NYHA functional class.
Plasma BNP concentration is a useful prognostic indicator in patients with CHF, since it is a ventricular hormone.5,6 Patient plasma BNP concentration is reported to correlate with abnormalities of LVEF and left ventricular end diastolic pressure, as well as with left ventricular mass.5,32 Therefore, the decrease in plasma BNP concentrations after treatment with valsartan was probably due to decreased left ventricular filling pressure, an improvement in left ventricular remodelling, or both factors.32 Moreover, treatment of CHF guided by plasma BNP concentration has been reported to reduce cardiovascular events33; thus, a decrease in BNP concentrations may be associated with a better outcome, as was observed in the Val‐HeFT (valsartan heart failure trial) study.18 Furthermore, Latini et al34 found that plasma BNP concentration was the most powerful indicator after valsartan treatment in patients with CHF. In our study, plasma BNP concentrations were significantly decreased by valsartan compared with enalapril.
123I‐MIBG, an analogue of noradrenaline, can be used to detect abnormalities in the myocardial adrenergic nervous system in patients with CHF.1,2,3 Activation of the renin–angiotensin system in patients with CHF facilitates cardiac noradrenaline release; therefore, treatment with ACE inhibitors may affect cardiac sympathetic activity.35,36 Somsen et al15 reported that ACE inhibitors can improve cardiac sympathetic nerve activity on the basis of cardiac 123I‐MIBG scintigraphic findings in patients with CHF. However, no reports have compared the effect of the two drugs (valsartan and enalapril) on cardiac sympathetic nerve activity in patients with CHF. In the present study, we examined whether valsartan improved cardiac sympathetic nerve activity in patients with CHF by using 123I‐MIBG scintigraphy. Whereas TDS, H/M ratio, and WR were significantly improved in the valsartan group after six months of treatment, we observed no significant changes in the enalapril group.
On the basis of cardiac 123I‐MIBG scintigraphy, our study showed that treatment with the ACE inhibitor enalapril did not improve cardiac sympathetic nerve activity, although a previous report had indicated that this treatment did result in an improvement.15 However, that study included patients with almost non‐ischaemic cardiomyopathy. In contrast, 36% of the patients in the enalapril group in our study had CHF due to old myocardial infarction. Moreover, in the patients included in our study, cardiac function was relatively low and left ventricular volume was relatively high compared with previously reported patients.15 Therefore, enalapril did not cause improvement in 123I‐MIBG scintigraphic parameters. On the basis of the results of our study and previous reports, we hypothesise that valsartan treatment probably blocks angiotensin more effectively in patients with severe CHF.
In a clinical report, the combination of ARB and an ACE inhibitor was more beneficial for preventing left ventricular remodelling and suppressing neurohormonal activation than either ARB or an ACE inhibitor alone.37 However, in that report, ARB alone was more effective in patients with CHF than the ACE inhibitor alone.37 Therefore, valsartan ARB treatment may improve cardiac sympathetic nerve activity and left ventricular performance in patients with CHF compared with enalapril treatment.
In general, patients with high blood pressure have impaired cardiac sympathetic nerve activity, and the use of agents has proved beneficial as shown by 123I‐MIBG scintigraphy.38 Moreover, patients with hypertensive heart disease have an increased plasma BNP concentration, and antihypertensive treatment decreases this parameter.39 In the present study, systolic and diastolic blood pressure did not differ significantly after six months of treatment in either group (in patients receiving valsartan, 132 (16) mm Hg v 129 (14) mm Hg and 74 (8) mm Hg v 72 (10) mm Hg, respectively; in patients receiving enalapril, 131 (14) mm Hg v 128 (13) mm Hg and 72 (8) mm Hg v 71 (9) mm Hg, respectively). Therefore, we believe that valsartan can improve cardiac sympathetic nerve activity and left ventricular performance in patients with CHF and that this effect is independent of a blood pressure lowing effect.
The small number of patients included in this study was a major limitation. In addition, the doses of valsartan and enalapril given to our patients were lower than those used in previously reported trials.18,23,24 However, the doses of these agents used in Japan are generally lower than those used in other countries. Valsartan at a dose of 80 mg once daily and enalapril of 5 mg are considered to be effective and safe for the treatment of Japanese patients with heart failure.11,14 Therefore, the doses in this study were not too low for Japanese patients with heart failure. Moreover, Shimizu et al40 reported that combination treatment with enalapril and valsartan had an additive effect at lower doses compared with enalapril alone in hamsters with heart failure. On the other hand, our study design did not include a combination treatment group. In the future, we need to study the effects of valsartan alone, enalapril alone, and their combination at half the dose of each agent on cardiac sympathetic nerve activity and left ventricular parameters in a larger number of patients.
Conclusion
TDS, H/M ratio, and WR determined by 123I‐MIBG scintigraphy were significantly improved after six months of valsartan treatment. In addition, plasma BNP concentration and echocardiographic parameters improved with this treatment. In contrast, these parameters did not change significantly with enalapril treatment. These findings indicate that valsartan treatment can improve cardiac sympathetic nerve activity and left ventricular performance in patients with CHF compared with enalapril treatment.
ACKNOWLEDGEMENTS
The authors thank Takayoshi Honjo, Akira Nakaya, Hiromitsu Takahashi, Hiroyuki Takada, and Takehiro Ishikawa for their technical assistance.
Abbreviations
ACE - angiotensin converting enzyme
ARB - angiotensin receptor blocker
BNP - brain natriuretic peptide
CHF - congestive heart failure
H/M - heart to mediastinum count
123I‐MIBG - iodine‐123 labelled meta‐iodobenzylguanidine
LVEDV - left ventricular end diastolic volume
LVEF - left ventricular ejection fraction
NYHA - New York Heart Association
RAAS - renin‐angiotensin‐aldosterone system
SPECT - single photon emission computed tomographic
TDS - total defect score
Val‐HeFT - valsartan heart failure trial
WR - washout rate
Footnotes
The authors have indicated they have no financial conflicts of interest.
References
- 1.Henderson E B, Kahn J K, Corbett J R.et al Abnormal I‐123 metaiodobenzylguanidine myocardial washout and distribution may reflect myocardial adrenergic derangement in patients with congestive cardiomyopathy. Circulation 1988781192–1199. [DOI] [PubMed] [Google Scholar]
- 2.Ogita H, Shimonagata T, Fukunami M.et al Prognostic significance of cardiac (123)I metaiodobenzylguanidine imaging for mortality and morbidity in patients with chronic heart failure: a prospective study. Heart 200186656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merlet P, Valette H, Dubois‐Rande J L.et al Prognostic value of cardiac metaiodobenzylguanidine imaging in patients with heart failure. J Nucl Med 199233471–477. [PubMed] [Google Scholar]
- 4.Yamazaki J, Muto H, Kabano T.et al Evaluation of beta‐blocker therapy in patients with dilated cardiomyopathy: clinical meaning of iodine 123‐metaiodobenzylguanidine myocardial single‐photon emission computed tomography. Am Heart J 2001141645–652. [DOI] [PubMed] [Google Scholar]
- 5.Yasue H, Yoshimura M, Sumida H.et al Localization and mechanism of secretion of B‐type natriuretic peptide in comparison with those of A‐type natriuretic peptide in normal subjects and patients with heart failure. Circulation 199490195–203. [DOI] [PubMed] [Google Scholar]
- 6.Tsutamoto T, Wada A, Maeda K.et al Attenuation of compensation of endogenous cardiac natriuretic peptide system in chronic heart failure: prognostic role of plasma brain natriuretic peptide concentration in patients with chronic symptomatic left ventricular dysfunction. Circulation 199796509–516. [DOI] [PubMed] [Google Scholar]
- 7.Cohn J N, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an international forum on cardiac remodeling. J Am Coll Cardiol 200035569–582. [DOI] [PubMed] [Google Scholar]
- 8.Harrap S B, Dominiczak A F, Fraser R.et al Plasma angiotensin II, predisposition to hypertension, and left ventricular size in healthy young adults. Circulation 1996931148–1154. [DOI] [PubMed] [Google Scholar]
- 9.Kasama S, Toyama T, Kumakura H.et al Spironolactone improves cardiac sympathetic nerve activity and symptoms in patients with congestive heart failure. J Nucl Med 2002431279–1285. [PubMed] [Google Scholar]
- 10.Kasama S, Toyama T, Kumakura H.et al Effect of spironolactone on cardiac sympathetic nerve activity and left ventricular remodeling in patients with dilated cardiomyopathy. J Am Coll Cardiol 200341574–581. [DOI] [PubMed] [Google Scholar]
- 11.Kasama S, Toyama T, Kumakura H.et al Addition of valsartan to an angiotensin‐converting enzyme inhibitor improves cardiac sympathetic nerve activity and left ventricular function in patients with congestive heart failure. J Nucl Med 200344884–890. [PubMed] [Google Scholar]
- 12.Kasama S, Toyama T, Kumakura H.et al Effects of intravenous atrial natriuretic peptide on cardiac sympathetic nerve activity in patients with decompensated congestive heart failure. J Nucl Med 2004451108–1113. [PubMed] [Google Scholar]
- 13.Kasama S, Toyama T, Kumakura H.et al Effects of candesartan on cardiac sympathetic nerve activity in patients with congestive heart failure and preserved left ventricular ejection fraction. J Am Coll Cardiol 200545661–667. [DOI] [PubMed] [Google Scholar]
- 14.Kasama S, Toyama T, Kumakura H.et al Effects of perindopril on cardiac sympathetic nerve activity in patients with congestive heart failure: comparison with enalapril. Eur J Nucl Med Mol Imaging 200532964–971. [DOI] [PubMed] [Google Scholar]
- 15.Somsen G A, van Vlies B, de Milliano P A.et al Increased myocardial [123I]‐metaiodobenzylguanidine uptake after enalapril treatment in patients with chronic heart failure. Heart 199676218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baruch L, Anand I, Cohen I S.et al Vasodilator Heart Failure Trial (V‐HeFT) Study Group. Augmented short‐ and long‐term hemodynamic and hormonal effects of an angiotensin receptor blocker added to angiotensin converting enzyme inhibitor therapy in patients with heart failure. Circulation 1999992658–2664. [DOI] [PubMed] [Google Scholar]
- 17.De Tommasi E, Iacoviello M, Romito R.et al Comparison of the effect of valsartan and lisinopril on autonomic nervous system activity in chronic heart failure. Am Heart J 2003146E17. [DOI] [PubMed] [Google Scholar]
- 18.Cohn J N, Tognoni G. Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin‐receptor blocker valsartan in chronic heart failure. N Engl J Med 20013451667–1675. [DOI] [PubMed] [Google Scholar]
- 19.Kasama S, Toyama T, Hoshizaki H.et al Dobutamine gated blood pool scintigraphy predicts the improvement of cardiac sympathetic nerve activity, cardiac function, and symptoms after treatment in patients with dilated cardiomyopathy. Chest 2002122542–548. [DOI] [PubMed] [Google Scholar]
- 20.Kasama S, Toyama T, Kumakura H.et al Effects of nicorandil on cardiac sympathetic nerve activity after reperfusion therapy in patients with first anterior acute myocardial infarction. Eur J Nucl Med Mol Imaging 200532322–328. [DOI] [PubMed] [Google Scholar]
- 21.Cerqueira M D, Weissman N J, Dilsizian V.et al Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002105539–542. [DOI] [PubMed] [Google Scholar]
- 22.Kasama S, Toyama T, Kumakura H.et al Dobutamine stress 99mTc‐tetrofosmin quantitative gated SPECT predicts improvement of cardiac function after carvedilol treatment in patients with dilated cardiomyopathy. J Nucl Med 2004451878–1884. [PubMed] [Google Scholar]
- 23.The CONSENSUS Trial Study Group Effects of enalapril on mortality in severe congestive heart failure. Results of the cooperative north scandinavian enalapril survival study (CONSENSUS). N Engl J Med 19873161429–1435. [DOI] [PubMed] [Google Scholar]
- 24.The SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991325293–302. [DOI] [PubMed] [Google Scholar]
- 25.Curtiss C, Cohn J N, Vrobel T.et al Role of the renin‐angiotensin system in the systemic vasoconstriction of chronic congestive heart failure. Circulation 197858763–770. [DOI] [PubMed] [Google Scholar]
- 26.Urata H, Healy B, Stewart R W.et al Angiotensin II‐forming pathways in normal and failing human hearts. Circ Res 199066883–890. [DOI] [PubMed] [Google Scholar]
- 27.Balcells E, Meng Q C, Johnson W H., Jret al Angiotensin II formation from ACE and chymase in human and animal hearts: methods and species considerations. Am J Physiol 1997273H1769–H1774. [DOI] [PubMed] [Google Scholar]
- 28.Bumpus F M, Catt K J, Chiu A T.et al Nomenclature for angiotensin receptors: a report of the Nomenclature Committee of the Council for High Blood Pressure Research. Hypertension 199117720–721. [DOI] [PubMed] [Google Scholar]
- 29.Leri A, Liu Y, Li B.et al Up‐regulation of AT(1) and AT(2) receptors in postinfarcted hypertrophied myocytes and stretch‐mediated apoptotic cell death. Am J Pathol 20001561663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Francis G S, Cohn J N. Heart failure: mechanisms of cardiac and vascular dysfunction and the rationale for pharmacologic intervention. FASEB J 199043068–3075. [DOI] [PubMed] [Google Scholar]
- 31.Patten R D, Udelson J E, Konstam M A. Ventricular remodeling and its prevention in the treatment of heart failure. Curr Opin Cardiol 199813162–167. [PubMed] [Google Scholar]
- 32.Kohno M, Horio T, Yokokawa K.et al Brain natriuretic peptide as a cardiac hormone in essential hypertension. Am J Med 19929229–34. [DOI] [PubMed] [Google Scholar]
- 33.Troughton R W, Frampton C M, Yandle T G.et al Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N‐BNP) concentrations. Lancet 20003551126–1130. [DOI] [PubMed] [Google Scholar]
- 34.Latini R, Masson S, Anand I.et al The comparative prognostic value of plasma neurohormones at baseline in patients with heart failure enrolled in Val‐HeFT. Eur Heart J 200425292–299. [DOI] [PubMed] [Google Scholar]
- 35.Hughes J, Roth R H. Evidence that angiotensin enhances transmitter release during sympathetic nerve stimulation. Br J Pharmacol 197141239–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antonaccio M J, Kerwin L. Evidence for prejunctional inhibition of norepinephrine release by captopril in spontaneously hypertensive rats. Eur J Pharmacol 198068209–212. [DOI] [PubMed] [Google Scholar]
- 37.McKelvie R S, Yusuf S, Pericak D.et al Comparison of candesartan, enalapril, and their combination in congestive heart failure: randomized evaluation of strategies for left ventricular dysfunction (RESOLVD) pilot study. The RESOLVD Pilot Study Investigators. Circulation 19991001056–1064. [DOI] [PubMed] [Google Scholar]
- 38.Sakata K, Shirotani M, Yoshida H.et al Comparison of effects of enalapril and nitrendipine on cardiac sympathetic nervous system in essential hypertension. J Am Coll Cardiol 199832438–443. [DOI] [PubMed] [Google Scholar]
- 39.Kohno M, Horio T, Yokokawa K.et al Brain natriuretic peptide as a marker for hypertensive left ventricular hypertrophy: changes during 1‐year antihypertensive therapy with angiotensin‐converting enzyme inhibitor. Am J Med 199598257–265. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu T, Okamoto H, Chiba S.et al Long‐term combined therapy with an angiotensin type I receptor blocker and an angiotensin converting enzyme inhibitor prolongs survival in dilated cardiomyopathy. Jpn Heart J 200243531–543. [DOI] [PubMed] [Google Scholar]