Abstract
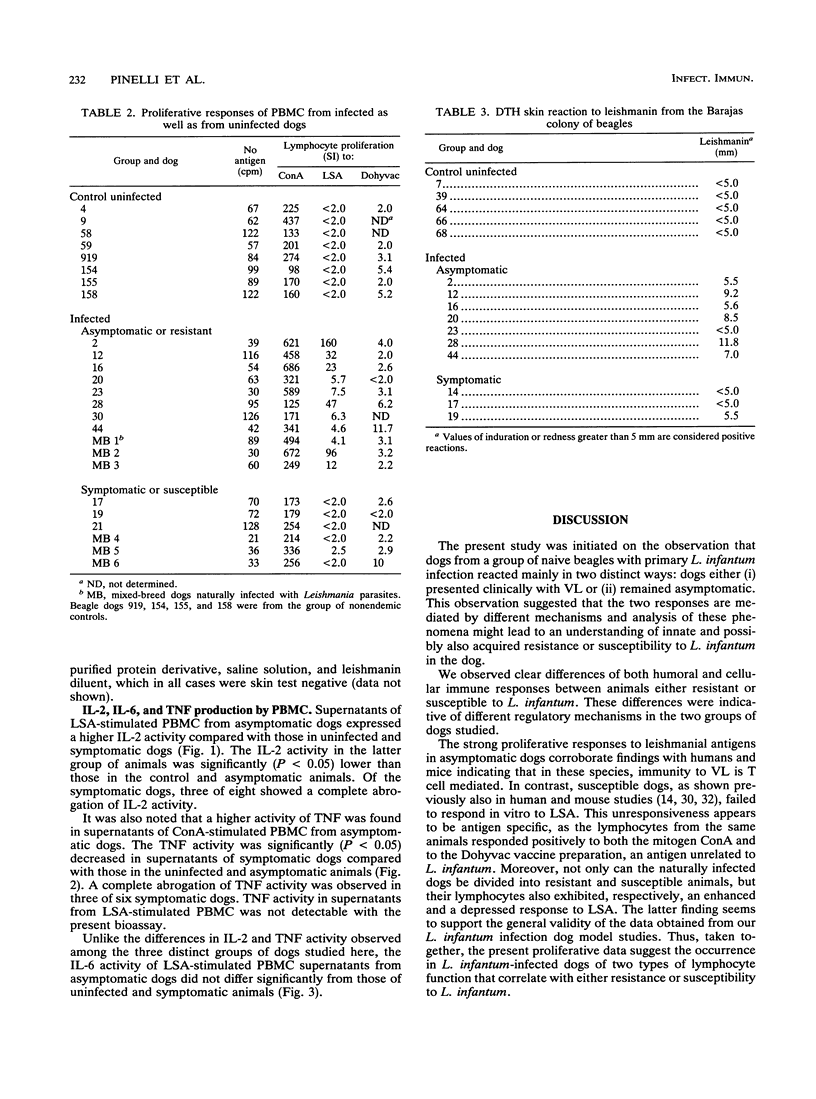
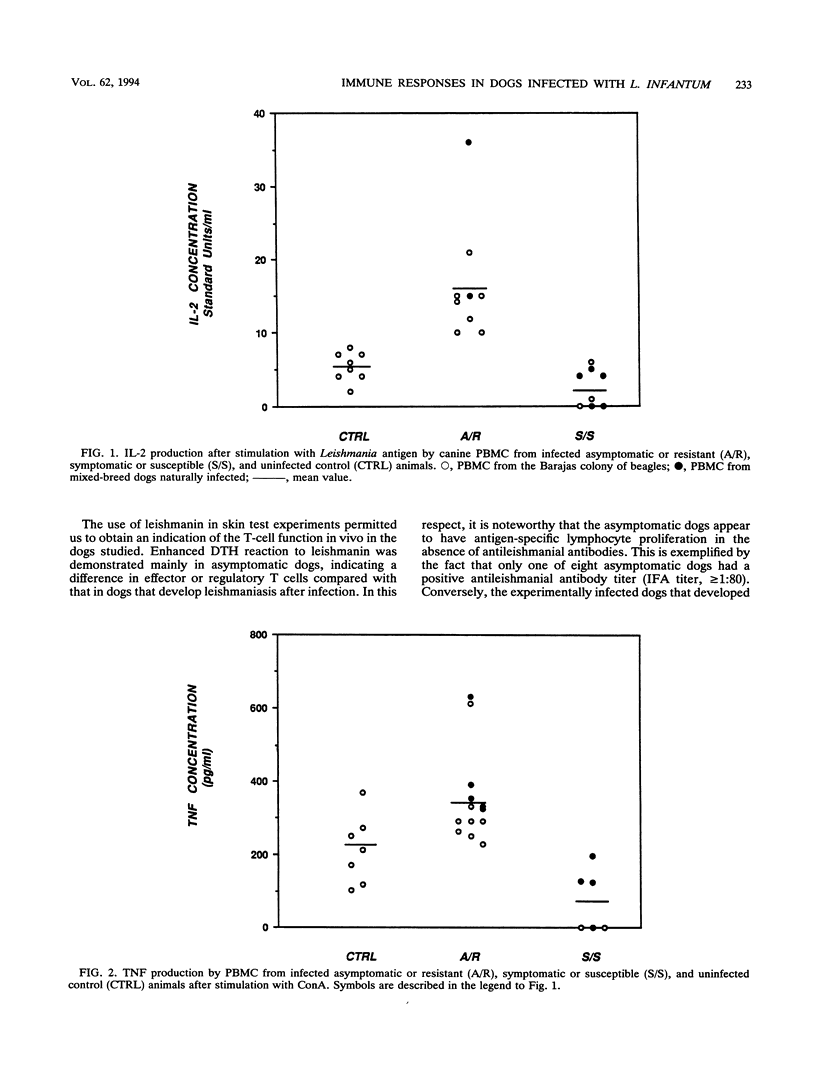
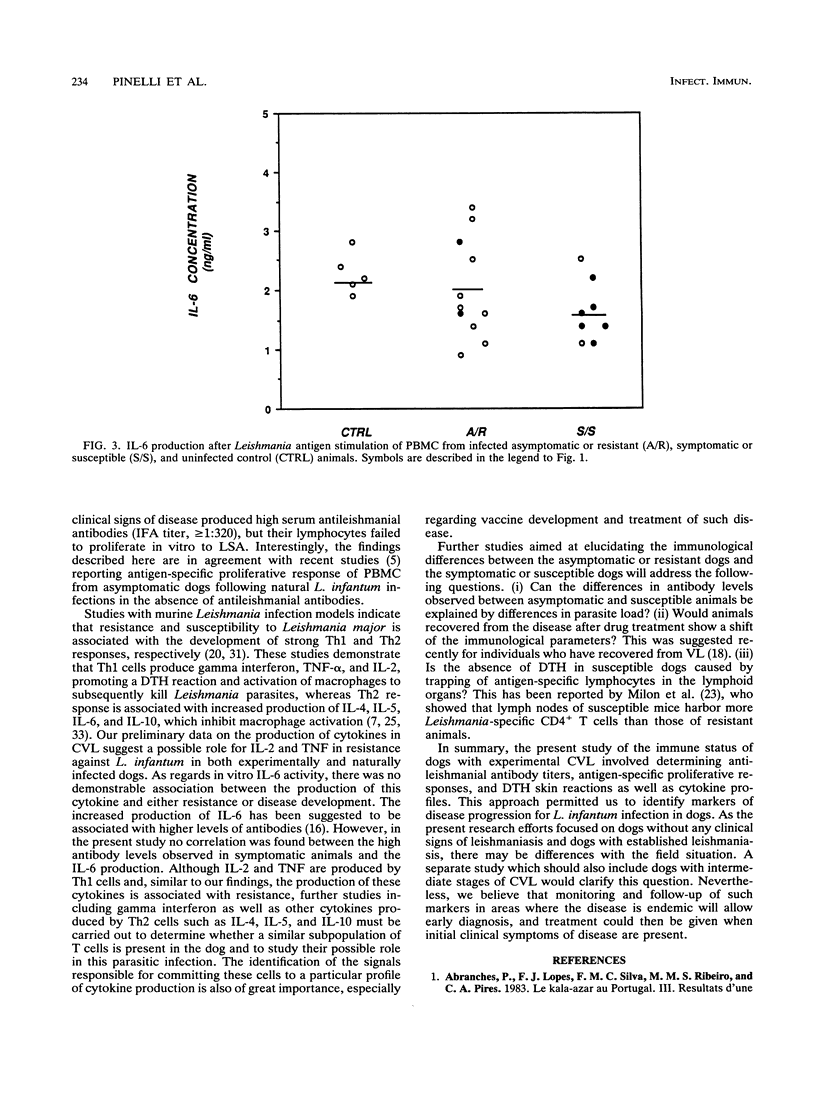
In this paper we describe a number of immunological parameters for dogs with a chronic Leishmania infantum infection which exhibit patterns of progressive disease or apparent resistance. The outcome of infection was assessed by isolation of parasites, serum antibody titers to Leishmania antigen, and development of clinical signs of leishmaniasis. Our studies show that 3 years after experimental infection, asymptomatic or resistant dogs responded to L. infantum antigen both in lymphocyte proliferation assays in vitro and in delayed-type hypersensitivity reaction, whereas no serum antibodies to parasite antigen were shown. In contrast, symptomatic or susceptible animals failed to respond to parasite antigen in cell-mediated assays both in vitro and in vivo and showed considerably higher serum antibodies to leishmanial antigens. In addition, significantly higher level of interleukin 2 (IL-2) and tumor necrosis factor were found in supernatants from stimulated peripheral mononuclear cells from asymptomatic dogs compared with those from symptomatic and control uninfected dogs. IL-6 production did not vary significantly among the groups studied. Finally, we observed similar results with a group of mixed-breed dogs with natural Leishmania infections also grouped as asymptomatic or symptomatic on the basis of clinical signs of canine visceral leishmaniasis. These results demonstrate that serum antibody titers, antigen-specific proliferative responses, delayed-type hypersensitivity skin reactions, and IL-2 and tumor necrosis factor production by peripheral mononuclear cells can be used as markers of disease progression.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abranches P., Lopes F. J., Silva F. M., Ribeiro M. M., Pires C. A. Le kala-azar au Portugal. III. Résultats d'une enquête sur la leishmaniose canine réalisée dans les environs de Lisbonne. Comparaison des zones urbaines et rurales. Ann Parasitol Hum Comp. 1983;58(4):307–315. [PubMed] [Google Scholar]
- Abranches P., Santos-Gomes G., Rachamim N., Campino L., Schnur L. F., Jaffe C. L. An experimental model for canine visceral leishmaniasis. Parasite Immunol. 1991 Sep;13(5):537–550. doi: 10.1111/j.1365-3024.1991.tb00550.x. [DOI] [PubMed] [Google Scholar]
- Cabral M., O'Grady J., Alexander J. Demonstration of Leishmania specific cell mediated and humoral immunity in asymptomatic dogs. Parasite Immunol. 1992 Sep;14(5):531–539. doi: 10.1111/j.1365-3024.1992.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Carvalho E. M., Bacellar O., Barral A., Badaro R., Johnson W. D., Jr Antigen-specific immunosuppression in visceral leishmaniasis is cell mediated. J Clin Invest. 1989 Mar;83(3):860–864. doi: 10.1172/JCI113969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox F. E., Liew F. Y. T-cell subsets and cytokines in parasitic infections. Immunol Today. 1992 Nov;13(11):445–448. doi: 10.1016/0167-5699(92)90072-F. [DOI] [PubMed] [Google Scholar]
- Dunan S., Frommel D., Monjour L., Ogunkolade B. W., Cruz A., Quilici M. Vaccination trial against canine visceral leishmaniasis. Phocean Veterinary Study Group on Visceral Leishmaniasis. Parasite Immunol. 1989 Jul;11(4):397–402. doi: 10.1111/j.1365-3024.1989.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Dye C., Killick-Kendrick R., Vitutia M. M., Walton R., Killick-Kendrick M., Harith A. E., Guy M. W., Cañavate M. C., Hasibeder G. Epidemiology of canine leishmaniasis: prevalence, incidence and basic reproduction number calculated from a cross-sectional serological survey on the island of Gozo, Malta. Parasitology. 1992 Aug;105(Pt 1):35–41. doi: 10.1017/s0031182000073662. [DOI] [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Federico G., Damiano F., Caldarola G., Fantini C., Fiocchi V., Ortona L. A seroepidemiological survey on Leishmania infantum infection. Eur J Epidemiol. 1991 Jul;7(4):380–383. doi: 10.1007/BF00145003. [DOI] [PubMed] [Google Scholar]
- Haldar J. P., Ghose S., Saha K. C., Ghose A. C. Cell-mediated immune response in Indian kala-azar and post-kala-azar dermal leishmaniasis. Infect Immun. 1983 Nov;42(2):702–707. doi: 10.1128/iai.42.2.702-707.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helle M., Boeije L., Aarden L. A. Functional discrimination between interleukin 6 and interleukin 1. Eur J Immunol. 1988 Oct;18(10):1535–1540. doi: 10.1002/eji.1830181010. [DOI] [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Jaffe C. L., Keren E., Nahary O., Rachamim N., Schnur L. Canine visceral leishmaniasis at Wadi Hamam, in Israel. Trans R Soc Trop Med Hyg. 1988;82(6):852–853. doi: 10.1016/0035-9203(88)90015-6. [DOI] [PubMed] [Google Scholar]
- Kemp M., Kurtzhals J. A., Bendtzen K., Poulsen L. K., Hansen M. B., Koech D. K., Kharazmi A., Theander T. G. Leishmania donovani-reactive Th1- and Th2-like T-cell clones from individuals who have recovered from visceral leishmaniasis. Infect Immun. 1993 Mar;61(3):1069–1073. doi: 10.1128/iai.61.3.1069-1073.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liew F. Y. Regulation of cell-mediated immunity in leishmaniasis. Curr Top Microbiol Immunol. 1990;155:53–64. doi: 10.1007/978-3-642-74983-4_4. [DOI] [PubMed] [Google Scholar]
- Locksley R. M., Scott P. Helper T-cell subsets in mouse leishmaniasis: induction, expansion and effector function. Immunol Today. 1991 Mar;12(3):A58–A61. doi: 10.1016/S0167-5699(05)80017-9. [DOI] [PubMed] [Google Scholar]
- Meller-Melloul C., Farnarier C., Dunan S., Faugere B., Franck J., Mary C., Bongrand P., Quilici M., Kaplanski S. Evidence of subjects sensitized to Leishmania infantum on the French Mediterranean coast: differences in gamma interferon production between this population and visceral leishmaniasis patients. Parasite Immunol. 1991 Sep;13(5):531–536. doi: 10.1111/j.1365-3024.1991.tb00549.x. [DOI] [PubMed] [Google Scholar]
- Milon G., Titus R. G., Cerottini J. C., Marchal G., Louis J. A. Higher frequency of Leishmania major-specific L3T4+ T cells in susceptible BALB/c as compared with resistant CBA mice. J Immunol. 1986 Feb 15;136(4):1467–1471. [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Musumeci S., Schilirò G., Li Volti S., Sciotto A. Lymphocyte changes in Mediterranean kala-azar. Trans R Soc Trop Med Hyg. 1981;75(2):304–305. doi: 10.1016/0035-9203(81)90342-4. [DOI] [PubMed] [Google Scholar]
- Oldham G., Williams L. Interleukin 2 (IL-2) production by mitogen stimulated bovine peripheral blood lymphocytes and its assay. Vet Immunol Immunopathol. 1984 Oct;7(3-4):201–212. doi: 10.1016/0165-2427(84)90079-5. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Lal S. L., Shrivastava S. N., Blackwell J., Neva F. A. An analysis of T cell responsiveness in Indian kala-azar. J Immunol. 1987 Feb 1;138(3):908–913. [PubMed] [Google Scholar]
- Scott P. A., Farrell J. P. Experimental cutaneous leishmaniasis. I. Nonspecific immunodepression in BALB/c mice infected with Leishmania tropica. J Immunol. 1981 Dec;127(6):2395–2400. [PubMed] [Google Scholar]
- Scott P., Pearce E., Cheever A. W., Coffman R. L., Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989 Dec;112:161–182. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- Scott P. The role of TH1 and TH2 cells in experimental cutaneous leishmaniasis. Exp Parasitol. 1989 Apr;68(3):369–372. doi: 10.1016/0014-4894(89)90120-3. [DOI] [PubMed] [Google Scholar]
- Segovia M., Martin-Luengo F. Leishmaniasis in the south-east of Spain: preliminary results of a serological and parasitological study in dogs. Ann Trop Med Parasitol. 1985 Jun;79(3):337–338. doi: 10.1080/00034983.1985.11811928. [DOI] [PubMed] [Google Scholar]
- Sheppard H. W., Scott P. A., Dwyer D. M. Recognition of Leishmania donovani antigens by murine T lymphocyte lines and clones. Species cross-reactivity, functional correlates of cell-mediated immunity, and antigen characterization. J Immunol. 1983 Sep;131(3):1496–1503. [PubMed] [Google Scholar]



