Abstract
Objective
To determine whether concentrations of heart‐type fatty acid binding protein (H‐FABP) measured before hospital discharge predict critical cardiac events in patients with idiopathic dilated cardiomyopathy (DCM).
Patients
92 consecutive patients with DCM were enrolled and followed up for four years.
Main outcome measures
Serum concentrations of H‐FABP, brain natriuretic peptide (BNP), cardiac troponin T before hospital discharge and survival rate.
Results
23 patients died of cardiac causes, received a left ventricular assist device or underwent heart transplantation during the four‐year follow up. Univariate analyses showed that New York Heart Association functional class, heart rate, ejection fraction, serum H‐FABP and plasma BNP were significant variables. According to multivariate analysis, serum H‐FABP and plasma BNP concentrations were independent predictors of critical cardiac events. Cardiac troponin T before hospital discharge was not a predictor. The area under the receiver operating characteristic curve for death from critical cardiac events was similar between H‐FABP and BNP. Patients with an H‐FABP concentration at or above the median (⩾ 5.4 ng/ml) had a significantly lower survival rate than those below the median, according to analysis by log rank test (p < 0.0001). When combined with BNP concentration at or above the median (⩾ 138 pg/ml), H‐FABP below the median predicted the worst prognosis among the combinations.
Conclusions
The concentration of serum H‐FABP before discharge from hospital may be an independent predictor for critical cardiac events in DCM.
Keywords: brain natriuretic peptide, cardiac events, cardiomyopathy, fatty acid binding protein
Idiopathic non‐ischaemic dilated cardiomyopathy (DCM) has wide range of phenotypes and variable clinical outcomes.1 Identification of patients with DCM at higher risk for adverse outcomes in its earlier stage may optimise the use of limited health care resources. Brain natriuretic peptide (BNP) is now widely recognised as the most powerful prognostic marker for heart failure,2 and BNP‐guided tailored treatment3 is advocated. Raised BNP in heart failure is mainly attributable to its gene expression in stretched cardiomyocytes undergoing raised ventricular pressure.4 The regulation of BNP secretion, however, is complex. BNP is also raised in patients with cardiac hypertrophy,5 renal failure6 or acute coronary syndrome.7 β blocker directly enhances expression and release of BNP from cardiomyocytes.8
Fatty acid binding protein is extremely abundant in cytoplasm, has low molecular weight and is considered to be one of the key fatty acid carrier proteins.9 Thus, fatty acid binding proteins are rapidly released into the circulation shortly after cell damage. Heart‐type fatty acid binding protein (H‐FABP) is immunologically specific to cardiomyocytes and is used as an early diagnostic marker for acute myocardial infarction.10 In the present study, we tested whether the concentrations of H‐FABP measured before discharge predict critical cardiac events for patients with DCM.
METHODS
Of 97 consecutive patients with DCM admitted to our institute for diagnosis or treatment between January 1997 and December 2000, five patients were excluded from this study because of renal dysfunction (serum creatinine concentration ⩾ 177 μmol/l). The remaining 92 patients (66 men and 26 women; mean age 49 years, range 16–76 years) were enrolled in the study. The study procedures were in accordance with the guidelines of our institute, and informed consent was obtained from each patient. The diagnosis of DCM was based on the definition of the World Health Organization/International Society and Federation of Cardiology Task Force.11 No patient had a history of myocardial infarction, infective myocarditis, metabolic disease or systemic illness. All patients underwent coronary angiography and endomyocardial biopsy for differential diagnosis of DCM. No significant coronary stenosis was found in any patient. Myocarditis was excluded on the basis of the Dallas criteria12 as well as the method of Edwards et al13 and Katsuragi et al.14 Immunohistochemical analysis with CD45RO was conducted to clarify T lymphocyte infiltration. In a quiescent condition with optimal medical treatment, patients underwent electrocardiography, echocardiography and blood sampling for standard laboratory chemical analysis, myocardial markers and complete blood count just before discharge from hospital. Myocardial markers measured in the present study were as follows: plasma BNP (Shionogi Co, Osaka, Japan), serum H‐FABP (MARKIT‐M, Dainippon Pharmaceutical Company, Osaka, Japan) and cardiac troponin T (cTnT) (Boehringer Mannheim, Mannheim, Germany). The analytical range, intra‐assay and interassay coefficients of variation, and normal reference range of the assays were 4.0–4000 pg/ml, 4.94% and 2.22%, and < 18.4 pg/ml, respectively, for BNP; 1.25–250 ng/ml, 5.8% and 1.7%, and < 5.25 ng/ml for H‐FABP; and 0.01–25 ng/ml, 1.1% and 1.5%, and < 0.01 ng/ml for cTnT.
Results are presented as mean (SD) for continuous variables. Data were statistically analysed with JMP statistical software (JMP version 5.1, SAS Institute). Differences between groups were estimated by the unpaired t test or Mann–Whitney U test, as appropriate for continuous variables, and by Fisher's exact test or χ2 test, as appropriate for categorical variables. The risk ratio with the 95% confidence interval for progression to cardiac death, left ventricular assist device or heart transplantation was estimated by univariate and multivariate Cox proportional hazards models. Variables that were significant in univariate analyses were entered into the multivariate analysis. Biochemical values such as BNP and H‐FABP were log transformed (ln) to remove skewness of data distribution. Survival curves were constructed by the Kaplan–Meier method and compared by the log rank test. Receiver operating characteristic curves were generated from multiple sensitivity–specificity pairs. A value of p <0.05 was considered significant.
RESULTS
Patient characteristics
During 48 months of follow up, 23 patients had critical cardiac events. Thirteen patients died of left ventricular failure, three patients received a left ventricular assist device and were added to the waiting list for transplantation, and seven patients received a heart transplant. Table 1 compares the characteristics of patients who had critical cardiac events (non‐survivors) and the remaining patients (survivors). New York Heart Association functional class, heart rate, BNP and H‐FABP concentrations before hospital discharge were significantly higher among non‐survivors than among survivors. Left ventricular ejection fraction was significantly lower in non‐survivors than in survivors. The two groups did not differ significantly in other variables including drug treatment at discharge.
Table 1 Patients' characteristics.
| Variable | Non‐survivors (n = 23) | Survivors (n = 69) | p Value |
|---|---|---|---|
| Age (years) | 50 (13) | 49 (11) | 0.5519 |
| Men/women | 16/7 (70%/30%) | 50/19 (72%/28%) | 0.7892 |
| NYHA functional class | 0.0132 | ||
| I | 1 (4%) | 21 (30%) | |
| II | 8 (35%) | 26 (38%) | |
| III | 14 (61%) | 22 (32%) | |
| IV | 0 | 0 | |
| Atrial fibrillation | 4 (17%) | 10 (14%) | 0.7375 |
| Duration of CHF (years) | 3.6 (2.7) | 4.0 (2.4) | 0.4979 |
| Body mass index (kg/m2) | 21 (3) | 22 (3) | 0.2342 |
| Heart rate (beats/min) | 81 (13) | 73 (12) | 0.0176 |
| Mean arterial BP (mm Hg) | 81 (10) | 82 (11) | 0.7447 |
| LVEF (%) | 30 (8) | 37 (9) | 0.0020 |
| LVEDD (mm) | 61 (9) | 60 (10) | 0.4521 |
| QTc (ms) | 419 (26) | 411 (25) | 0.1780 |
| Packed cell volume | 0.38 (0.02) | 0.38 (0.02) | 0.8495 |
| Sodium (mmol/l) | 136 (3) | 137 (3) | 0.5980 |
| Creatinine (μmol/l) | 97 (35) | 88 (35) | 0.8523 |
| Uric acid (μmol/) | 488 (184) | 428 (143) | 0.1816 |
| CK‐MB (ng/ml) | 5.4 (2.2) | 4.7 (2.0) | 0.2389 |
| cTnT (ng/ml) | 0.02 (0.01) | 0.02 (0.01) | 0.1155 |
| BNP (pg/ml) | 267 (141) | 108 (81) | <0.0001 |
| H‐FABP (ng/ml) | 9.3 (3.5) | 5.1 (2.6) | <0.0001 |
| Drugs | |||
| Oral inotropics | 3 (13%) | 6 (9%) | 0.5433 |
| Digitalis | 13 (57%) | 30 (43%) | 0.2776 |
| Nitrates | 3 (13%) | 12 (17%) | 0.6250 |
| Diuretics | 22 (96%) | 68 (99%) | 0.4091 |
| ACE inhibitors | 16 (70%) | 55 (80%) | 0.3154 |
| β blockers | 18 (78%) | 52 (75%) | 0.7778 |
ACE, angiotensin converting enzyme; BNP, brain natriuretic peptide; BP, blood pressure; CHF, congestive heart failure; CK, creatine kinase; cTnT, cardiac troponin T; H‐FABP, heart‐type fatty acid binding protein; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Event analyses
By univariate analyses, functional class (p = 0.0035), heart rate (p = 0.0021) and left ventricular ejection fraction (p = 0.0018) were related to critical cardiac events in DCM. Concentrations of H‐FABP(ln) (p < 0.0001) and BNP(ln) (p < 0.0001) before discharge were also associated with critical cardiac events in DCM. Among five significant variables in univariate analysis, H‐FABP(ln) and BNP(ln) concentrations were the sole independent predictors of critical cardiac events in patients with DCM (table 2). Repeating the analysis with these two independent variables showed that H‐FABP(ln) (p = 0.0068 and BNP(ln) (p = 0.0001) had significant effects on critical cardiac events. Risk ratios of H‐FABP(ln) and BNP(ln) were 7.450 and 10.87, respectively, in this reanalysis. Thus, patients had a 10.9 times higher risk of events with each increase of BNP(ln) by one unit. Likewise, patients had a 7.5 times higher risk of events with each increase of H‐FABP(ln) by one unit.
Table 2 Multivariate proportional hazards analysis.
| Variable | RR | 95% CI | p Value |
|---|---|---|---|
| NYHA class II v I | 1.971 | 0.421 to 5.825 | 0.3190 |
| NYHA class III v I | 3.051 | 0.636 to 9.736 | 0.1344 |
| Heart rate | 1.025 | 0.978 to 1.076 | 0.3022 |
| LVEF | 0.957 | 0.898 to 1.017 | 0.1601 |
| BNP(ln) | 10.87 | 3.527 to 35.32 | <0.0001 |
| H‐FABP(ln) | 7.450 | 1.722 to 36.12 | 0.0068 |
BNP, brain natriuretic peptide; CI, confidence interval; H‐FABP, heart‐type fatty acid binding protein; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; RR, risk ratio.
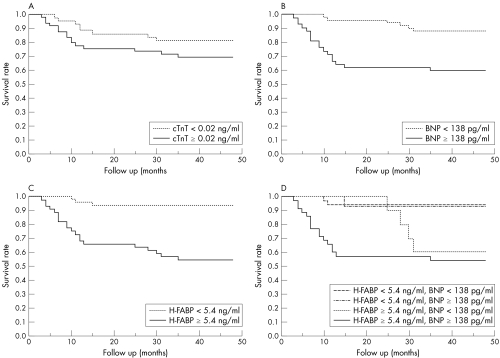
Figure 1 shows Kaplan–Meier event curves according to the median concentrations of cTnT (0.02 ng/ml), BNP (138 pg/ml) and H‐FABP (5.4 ng/ml). Patients with a concentration of cTnT ⩾ 0.02 ng/ml had a similar survival rate to those with cTnT < 0.02 ng/ml (log rank test, p = 0.1585). Patients with BNP ⩾ 138 pg/ml had a significantly lower survival rate than those with BNP < 138 pg/ml (log rank test, p = 0.0008). Patients with H‐FABP ⩾ 5.4 ng/ml had a significantly lower survival rate than those with H‐FABP < 5.4 ng/ml (log rank test, p < 0.0001). The area under the receiver operating characteristic curve for critical cardiac events was similar between H‐FABP and BNP (0.853 v 0.848, p = 0.9322). Thus, the prognostic value of the H‐FABP concentration was comparable to that of the BNP concentration. When the H‐FABP and BNP concentrations were combined to produce four segments (H‐FABP ⩾ 5.4 ng/ml and BNP < 138 pg/ml; H‐FABP ⩾ 5.4 ng/ml and BNP ⩾ 138 pg/ml; H‐FABP < 5.4 ng/ml and BNP < 138 pg/ml; H‐FABP < 5.4 ng/ml and BNP ⩾ 138 pg/ml) in the study population, patients with H‐FABP ⩾ 5.4 ng/ml and BNP ⩾ 138 pg/ml had a lower survival rate (log rank test, p = 0.0002) (fig 1D).
Figure 1 Kaplan–Meier event curves according to the median concentrations of (A) cardiac troponin T (cTnT), (B) brain natriuretic peptide (BNP), (C) heart‐type fatty acid binding protein (H‐FABP) and (D) H‐FABP and BNP combined.
DISCUSSION
In the present study, we showed that a serum concentration of H‐FABP before discharge independently predicted the long‐term risk of critical cardiac events in non‐ischaemic DCM. The predictive power of H‐FABP was comparable to that of BNP. Furthermore, a combination of high‐concentration BNP and high‐concentration H‐FABP yielded a worse prognosis.
cTnT concentrations were reported to rise in DCM15 as well as in acute myocardial infarction.16 The cut off value of 0.02 ng/ml in the present study was the same as that in a previous report on DCM.15 cTnT is located in myofilaments, and its molecular weight (37.0 kDa) is greater than that of H‐FABP (14.9 kDa), found in cytosol, which makes cTnT harder to detect than H‐FABP. In fact, cTnT was detected in 36–46% of patients with acute myocardial infarction,16,17 whereas H‐FABP was detected in 93%.10 In the present study, the concentrations of cTnT were similar between survivors and non‐survivors. Two Kaplan–Meier event curves for patients over and under the cut off did not differ significantly. A sustained rise of cTnT for 16 months significantly and independently predicted adverse outcomes in DCM.15 We assume that a point‐of‐care measurement of cTnT at a single time point may not closely reflect the severity of non‐ischaemic DCM. A previous report on the predictability of cTnT for cardiac events in heart failure may be attributable to the ischaemic aetiology of heart failure.18
Ongoing myocardial damage in DCM may be one of the plausible mechanisms for the release of H‐FABP.19 The correlation between H‐FABP concentration and heart failure severity, and the correlation between H‐FABP concentration and BNP concentration were reported in a previous study.19 Although that previous study19 suggested that the prognostic power of H‐FABP for cardiac events in DCM is comparable to that of BNP, we confirmed the role of H‐FABP as a predictor in our four‐year follow up. In the present study, an endomyocardial biopsy did not provide evidence of overt active myocarditis in all of the patients. Our method did not thoroughly exclude the possibility of inactive and chronic inflammatory or viral cardiomyopathy causing non‐ischaemic cardiomyopathy.20 For any reason, a transient loss of cell membrane integrity may cause cytoplasmic molecules to leak into the bloodstream. These events may yield detectable biomarkers even in the absence of myocyte death. Although the present study did not identify known possible causes of non‐ischaemic heart failure, such as chronic myocardial inflammation or chronic viral infection,1,21 increased serum concentrations of H‐FABP were shown to predict the long‐term risk of critical cardiac events with a predictive power comparable to that of BNP, independently of the underlying causes. In this view, H‐FABP may provide additional information for risk stratification and management of these patients with DCM. Whereas raised H‐FABP concentrations reflect myocardial membrane damage, raised BNP concentrations reflect increased ventricular filling pressure. The combination of these two provides an index for a worse prognosis. Thus, H‐FABP concentration may provide a novel estimate of the clinical outcome in DCM. Caution is needed in interpreting the present small study, which may have confounding associations of other variables. Thus, larger clinical trials would help to clarify the potential role of H‐FABP in determining the prognosis of patients with DCM.
ACKNOWLEDGEMENTS
This study was partly supported by the Program for Promotion of Fundamental Studies in Health Sciences of the Pharmaceuticals and Medical Devices Agency (PMDA), Japan.
Abbreviations
BNP - brain natriuretic peptide
cTnT - cardiac troponin T
DCM - dilated cardiomyopathy
H‐FABP - heart‐type fatty acid binding protein
References
- 1.Dec G W, Fuster V. Idiopathic dilated cardiomyopathy. N Engl J Med 19943311564–1575. [DOI] [PubMed] [Google Scholar]
- 2.Remme W J, Swedberg K. Task Force for the Diagnosis and Treatment of Chronic Heart Failure, European Society of Cardiology. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J 2001221527–1560. [DOI] [PubMed] [Google Scholar]
- 3.Troughton R W, Frampton C M, Yandle T G.et al Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N‐BNP) concentrations. Lancet 20003551126–1130. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa O, Ogawa Y, Itoh H.et al Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy: evidence for brain natriuretic peptide as an “emergency” cardiac hormone against ventricular overload. J Clin Invest 1995961280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schirmer H, Omland T. Circulating N‐terminal pro‐atrial natriuretic peptide is an independent predictor of left ventricular hypertrophy in the general population. The Tromso study. Eur Heart J 199920755–763. [DOI] [PubMed] [Google Scholar]
- 6.Buckley M G, Sethi D, Markandu N D.et al Plasma concentrations and comparisons of brain natriuretic peptide and atrial natriuretic peptide in normal subjects, cardiac transplant recipients and patients with dialysis‐independent or dialysis‐dependent chronic renal failure. Clin Sci (Lond) 199283437–444. [DOI] [PubMed] [Google Scholar]
- 7.Sabatine M S, Morrow D A, de Lemos J A.et al Multimarker approach to risk stratification in non‐ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C‐reactive protein, and B‐type natriuretic peptide. Circulation 20021051760–1763. [DOI] [PubMed] [Google Scholar]
- 8.Ohta Y, Watanabe K, Nakazawa M.et al Carvedilol enhances atrial and brain natriuretic peptide mRNA expression and release in rat heart. J Cardiovasc Pharmacol 200036S19–S23. [DOI] [PubMed] [Google Scholar]
- 9.Schaap F G, van der Vusse G J, Glatz J F. Fatty acid‐binding proteins in the heart. Mol Cell Biochem 199818043–51. [PubMed] [Google Scholar]
- 10.Okamoto F, Sohmiya K, Ohkaru Y.et al Human heart‐type cytoplasmic fatty acid‐binding protein (H‐FABP) for the diagnosis of acute myocardial infarction: clinical evaluation of H‐FABP in comparison with myoglobin and creatine kinase isoenzyme MB. Clin Chem Lab Med 200038231–238. [DOI] [PubMed] [Google Scholar]
- 11.Richardson P, McKenna W, Bristow M.et al Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 199693841–842. [DOI] [PubMed] [Google Scholar]
- 12.Aretz H T, Billingham M E, Edwards W D.et al Myocarditis: a histopathologic definition and classification. Am J Cardiovasc Pathol 198713–14. [PubMed] [Google Scholar]
- 13.Edwards W D, Holmes D R, Jr, Reeder G S. Diagnosis of active lymphocytic myocarditis by endomyocardial biopsy: quantitative criteria for light microscopy. Mayo Clin Proc 198257419–425. [PubMed] [Google Scholar]
- 14.Katsuragi M, Yutani C, Imakita M.et al Cell infiltration caused deterioration in the prognosis of patients with clinical diagnosis of dilated cardiomyopathy (DCM): application of biopsy criteria of myocarditis to 42 autopsy cases. Heart Vessels 1993842–47. [PubMed] [Google Scholar]
- 15.Sato Y, Yamada T, Taniguchi R.et al Persistently increased serum concentrations of cardiac troponin T in patients with idiopathic dilated cardiomyopathy are predictive of adverse outcomes. Circulation 2001103369–374. [DOI] [PubMed] [Google Scholar]
- 16.Giannitsis E, Muller‐Bardorff M, Lehrke S.et al Admission troponin T level predicts clinical outcomes, TIMI flow, and myocardial tissue perfusion after primary percutaneous intervention for acute ST‐segment elevation myocardial infarction. Circulation 2001104630–635. [DOI] [PubMed] [Google Scholar]
- 17.Ohman E M, Armstrong P W, Christenson R H.et al Cardiac troponin T levels for risk stratification in acute myocardial ischemia. GUSTO IIA Investigators. N Engl J Med 19963351333–1341. [DOI] [PubMed] [Google Scholar]
- 18.Ishii J, Nomura M, Nakamura Y.et al Risk stratification using a combination of cardiac troponin T and brain natriuretic peptide in patients hospitalized for worsening chronic heart failure. Am J Cardiol 200289691–695. [DOI] [PubMed] [Google Scholar]
- 19.Goto T, Takase H, Toriyama T.et al Circulating concentrations of cardiac proteins indicate the severity of congestive heart failure. Heart 2003891303–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maisch B, Ristic A D, Hufnagel G.et al Dilated cardiomyopathies as a cause of congestive heart failure. Herz 200227113–134. [DOI] [PubMed] [Google Scholar]
- 21.Frustaci A, Chimenti C, Calabrese F.et al Immunosuppressive therapy for active lymphocytic myocarditis: virological and immunologic profile of responders versus nonresponders. Circulation 2003107857–863. [DOI] [PubMed] [Google Scholar]