Abstract
Objective
To determine in an observational study whether N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) is raised in patients with an atrial septal defect (ASD) and whether concentrations change after interventional closure.
Methods
12 patients (6 men, mean (SD) age 44.4 (18.6) years) with a moderate sized ASD type II (23.3 (4.5) mm, pulmonary to systemic flow ratio 2.1 (0.68)) were investigated. In all patients a magnetic resonance imaging (MRI) study was performed and NT‐proBNP was assessed at baseline and early (9 (13) days) and late (138 (64) days) after intervention.
Results
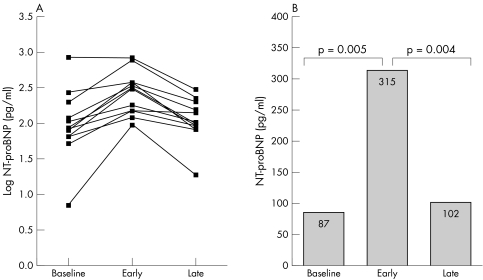
Concentrations of NT‐proBNP were found to be within the normal range at baseline (median 87 pg/ml, interquartile range 65–181 pg/ml) but increased early after the interventional closure (315 pg/ml, 133–384 pg/ml, p = 0.005 versus baseline). The increase of NT‐proBNP was associated with an increase in left ventricular dimensions as assessed by MRI (left ventricular end diastolic volume 104 (27) ml to 118 (27) ml, p = 0.003). Late after ASD closure NT‐proBNP returned to baseline concentrations (102 pg/ml, 82–188 pg/ml, p = 0.004 versus early follow up).
Conclusion
These findings suggest the presence of transitory haemodynamic stress during adaptation of the left ventricle after ASD closure, which may contribute to the understanding of the pathological mechanism of acute heart failure and delayed improvement of exercise capacity after ASD closure.
Keywords: natriuretic peptides, NT‐proBNP, cardiac MRI, atrial septal defect, interventional ASD closure
An atrial septal defect (ASD) of the secundum type is a common form of congenital heart disease in adults.1,2 For symptomatic patients or patients with a relevant shunt volume (pulmonary to systemic flow ratio > 1.5) ASD closure is generally recommended. Standard therapy used to be surgical closure, which has been practised for over 45 years with good results and a low risk.3,4 However, interventional ASD closure has become a frequently used alternative to surgery in recent years with a high success rate and low complication rate. Nevertheless, there are several reports on the incidence of acute heart failure after surgical closure, requiring partial reopening of the defect in some patients, and case reports of patients developing acute heart failure after interventional closure.5,6,7,8,9,10 In keeping with these results an increase in cardiopulmonary exercise capacity has been found not within the first two months after ASD closure but after 6–12 months.11,12,13 The underlying pathological mechanism for acute heart failure and the delayed improvement after ASD closure are not fully understood. Although data derived from several studies are consistent, showing haemodynamic and functional improvement of the right ventricle, only sparse data exist on the left ventricular function of patients with an ASD, and haemodynamic and morphological changes of the left ventricle after ASD closure showing an increase in left ventricular end diastolic volume (LVEDV) and ejection fraction.14,15,16,17,18,19,20
B‐type natriuretic peptide (BNP) is synthesised by the myocardium as a prohormone (preproBNP). After cleavage, BNP and its N‐terminal fragment (NT‐proBNP) are released into the circulation in equal proportion. The stimulus for their release is an increase of ventricular wall stress caused by either pressure or volume overload of the cardiac ventricles. In several studies both markers have proved to be highly sensitive for myocardial stress in various cardiovascular disorders.21
The goal of this observational study was to determine whether NT‐proBNP is increased in patients with an ASD and whether concentrations change after interventional closure.
METHODS
In this analysis we enrolled 12 adult patients who underwent interventional ASD closure. For these patients serum specimens before ASD closure and during follow up were available and they underwent transthoracic echocardiography study and magnetic resonance imaging (MRI) at baseline and during follow up. All patients were clinically assessed for functional status according to the New York Heart Association (NYHA) criteria.
Haemodynamic assessment
Right heart catheterisation was performed by a common technique and in all patients pulmonary capillary wedge pressure, pulmonary artery pressure, and right atrial pressure were measured. Shunt volume was calculated by oximetry according to the Fick principle and expressed as the pulmonary to systemic flow ratio. Defect size was measured as stretched balloon diameter.
Echocardiography
Transthoracic echocardiography was performed with an Agilent Sonos 1.75–3.5 MHz scanner (Phillips Medical Ultrasound) with the use of harmonic imaging. A complete examination according to current guidelines was performed.22 Left ventricular end diastolic diameter (LVEDD) was assessed by M mode in the left parasternal view. Left ventricular function was quantitatively assessed and calculated according to the modified formula of Quinones et al.23
Magnetic resonance imaging
MRIs were recorded on a 1.5 T Sonata scanner (Siemens, Erlangen, Germany). Standard fast precession steady state (repetition time 48 ms, echo time 1.6 ms, slice thickness 5 mm, flip angle 60°, single acquisition) sequences were used in the breath hold technique. Pre‐ and post‐interventional studies were performed on the same scanner in each case and identical image planes were chosen. A short axis stack (slice thickness 10 mm, no gap) spanning the entire left ventricle was acquired. The most basal end diastolic and end systolic frames were carefully chosen to avoid inclusion of atrial or left ventricular outflow tract areas. Endocardial and epicardial contours were drawn in each slice by using commercially available software (Argus; Siemens, Erlangen, Germany). Papillary muscles were included in blood pool volumes. Left ventricular functional parameters such as ejection fraction, end diastolic volume, end systolic volume, and stroke volume were calculated by multiplying area with slice thickness according to Simpson's method. End diastolic and end systolic diameters were measured in short axis views at the level of the tip of the papillary muscles. Two experienced and independent observers analysed the magnetic resonance and echocardiographic data. The observers were blinded to the results of clinical examination and to NT‐proBNP concentrations.
NT‐proBNP measurement
Blood was taken from an antecubital vein into gel filled tubes containing no anticoagulant (S‐Monovette 4.9 ml Z‐Gel; Sarstedt, Nürnbrecht, Germany) from all patients. The specimens were centrifuged within one hour and serum was frozen at −80°C until analysis. NT‐proBNP was measured by an electrochemiluminescence immunoassay (Elecsys proBNP; Roche Diagnostics, Mannheim, Germany). The upper reference limit was defined as the 97.5th centile of healthy blood donors according to the manufacturer's information.
Statistical analysis
Concentrations for NT‐proBNP are given as median and interquartile range and all other data as mean (SD). For statistical analysis the Mann‐Whitney test for unpaired non‐parametric data, the Wilcoxon test for paired non‐parametric data, and the Friedman test for n samples of paired non‐parametric data were performed. For all statistical analysis the statistical software SPSS 10.0 for Windows was used (SPSS Inc, Chicago, Illinois, USA).
RESULTS
Twelve patients (six men) clinically stable in NYHA class I–III with an indication for percutaneous ASD closure were studied. Table 1 shows the detailed baseline characteristics of the patients. All patients had preserved left ventricular function with a mean ejection fraction of 60.5 (5.1)%. Examinations with blood drawing, echocardiography, and MRI took place at baseline before ASD closure, early during follow up with a median duration of 9 (13) days after ASD closure, and late after intervention with a median duration of 138 (64) days.
Table 1 Baseline characteristics of the patients.
| Women | 6 (50%) |
| Age (years) | 44.4 (18.6) (22–71) |
| Body surface area (m2) | 1.8 (0.2) (1.5–2.1) |
| Creatinine (μmol/l) | 77.8 (32.7) (48.6–157) |
| Ejection fraction (%) | 60.5 (5.1) (51–67) |
| Mean pulmonary artery pressure (mm Hg) | 16.3 (6.3) (9–26) |
| Pulmonary to systemic flow ratio | 2.1 (0.68) (1.3–3.3) |
| ASD size (stretched diameter) (mm) | 23.3 (4.5) (16–30) |
Values are given as frequency (%) or mean (SD) and range.
ASD, atrial septal defect.
Three patients had NT‐proBNP concentrations at baseline increased above the upper reference limit. This rise of NT‐proBNP was only slight in two patients with a 4% (201 pg/ml (upper reference limit 194 pg/ml)) and 21% and (269 pg/ml (upper reference limit 222 pg/ml)) increase above the upper reference limit. We found a large increase of 336% above the upper reference limit (845 pg/ml (upper reference limit 194 pg/ml)) in only one patient. NT‐proBNP concentrations increased after ASD closure in 11 patients from baseline to early follow up and returned to baseline concentrations from early to late follow up (fig 1A). In only one patient with an already greatly increased concentration of NT‐proBNP on admission, we observed no further increase after ASD closure. Table 2 gives the median concentrations and interquartile ranges of NT‐proBNP (fig 1B).
Figure 1 N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) concentrations at baseline, and early (9 (13) days) and late (138 (64) days) after interventional atrial septal defect (ASD) closure. (A) Values for each patient. (B) Median values.
Table 2 Results of assessments at baseline, and early and late after ASD closure.
| Baseline | Early | Late | p Value (Friedman test) | |
|---|---|---|---|---|
| NT‐proBNP (pg/ml) | 87 (65–181) | 315 (133–384) | 102 (82–188) | <0.001 |
| Magnetic resonance imaging | ||||
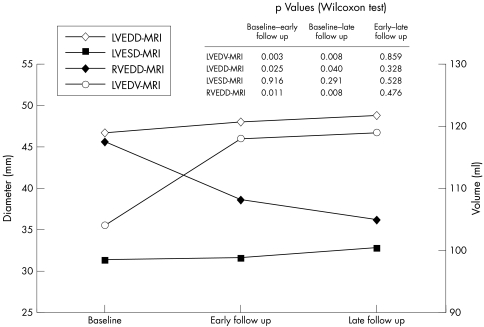
| LVEDV (ml) | 104 (27) | 118 (27) | 119 (22) | 0.001 |
| LVEDV/BSA (ml/m2) | 58 (14) | 66 (13) | 66 (12) | 0.001 |
| LVEDD (mm) | 46.7 (5.1) | 48.0 (4.2) | 48.8 (3.9) | 0.016 |
| LVEDD/BSA (mm/m2) | 26.2 (3.3) | 26.9 (2.9) | 27.4 (3.3) | 0.016 |
| LVESD (mm) | 31.3 (4.7) | 31.6 (4.5) | 32.8 (3.2) | 0.67 |
| RVEDD (mm) | 45.6 (7.0) | 38.6 (4.3) | 36.2 (7.9) | 0.001 |
| RVEDV (ml) | 211 (70) | 129 (37) | 139 (42) | 0.015 |
| RVESV (ml) | 110 (37) | 72 (21) | 73 (43) | 0.015 |
| RVEF (%) | 47.0 (6.1) | 43.6 (6.3) | 50.8 (13.8) | 0.165 |
| Echocardiography | ||||
| LVEDD (mm) | 43.4 (3.3) | 47.9 (2.7) | 46.1 (5.2) | 0.016 |
| EF (%) | 59.2 (4.9) | 63.4 (3.6) | 62.8 (4.2) | 0.067 |
Data are median (interquartile range) or mean (SD).
EF, ejection fraction; LVEDD, left ventricular end diastolic diameter; LVEDD/BSA, left ventricular end diastolic diameter adjusted to body surface area; LVEDV, left ventricular end diastolic volume; LVEDV/BSA, left ventricular end diastolic volume adjusted to body surface area; LVESD, left ventricular end systolic diameter; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; RVEDD, right ventricular end diastolic diameter; RVEDV, right ventricular end diastolic volume; RVEF, right ventricular ejection fraction; RVESV, right ventricular end systolic volume.
LVEDV unadjusted and adjusted for body surface area (BSA) at baseline assessed by MRI were reduced as compared with established normal values (LVEDV: 104 (27) ml v 121 (34) ml; LVEDV/BSA: 58 (14) ml/m2v 66 (12) ml/m2, respectively).24 After ASD closure we found an increase of LVEDV and LVEDD and of the respective values adjusted for BSA from baseline to early follow up. Values remained unchanged, however, from early to late follow up (fig 2). Similar results were found for left ventricular end diastolic diameter assessed by echocardiography with an increase from baseline to early follow up but no further change from early to late follow up (43.4 (3.3) mm, 47.9 (2.7) mm, and 46.1 (5.2) mm, p = 0.011 for baseline versus early follow up and p = 0.623 for early versus late follow up). Left ventricular end systolic diameter assessed by MRI remained unchanged from baseline to early and late follow up (table 1). In contrast right ventricular end diastolic diameter (fig 2), right ventricular end diastolic volume, and right ventricular end systolic volume assessed by MRI decreased from baseline to early follow (table 2).
Figure 2 Mean (SD) left ventricular end diastolic volume (LVEDV) assessed by magnetic resonance imaging (MRI), left ventricular end diastolic diameter (LVEDD) assessed by MRI, left ventricular end systolic diameter (LVESD), and right ventricular end diastolic diameter (RVEDD).
Left ventricular function assessed as the calculated ejection fraction by echocardiography increased from baseline to early follow up after ASD closure (59.2 (4.9)% v 63.4 (3.6)%, p = 0.046), but remained unchanged from early to late follow up (62.8 (4.2)%, p = 0.887).
DISCUSSION
In this observational study we investigated NT‐proBNP, a biomarker of neurohormonal activation in patients with an ASD of the secundum. We showed that NT‐proBNP is not increased in patients with a moderate sized ASD but increases early after interventional closure with a return to normal values during further follow up. The increase of NT‐proBNP was associated with an increase in left ventricular dimensions and an improvement in function as shown by MRI and echocardiography.
The left to right shunt in patients with an ASD leads primarily to a volume overload and increased myocardial stress of the right ventricle. Consequently right ventricular dimensions are increased in these patients. In contrast left ventricular geometry is altered with a reduction of left ventricular dimensions and an impairment of left ventricular filling.19,25,26 Since the myocardium of the left ventricle is the major source of BNP and NT‐proBNP synthesis, and in ASD the underlying pathological mechanism is volume overload of the right ventricle, our observation of normal and only moderately increased values in patients with an ASD and normal ejection fraction can be well explained. Thus, our data suggest that NT‐proBNP is probably not of diagnostic use in moderate sized ASD of adults. However, the prognostic concentration of NT‐proBNP in patients with an ASD is unknown and needs to be addressed for the evaluation of its diagnostic utility. But this was not the aim of the present study. In contrast to our findings a BNP rise in paediatric patients with an ASD has been described.27 However, these conflicting findings can result from differences of haemodynamic adaptation between children and adults. Furthermore, differences of the diagnostic value of BNP and NT‐proBNP between children and adults have been described. In a study on neurohormonal activation in adults with congenital heart disease, Bolger et al28 described an increase of BNP concentrations. In contrast to our study, their study population consisted mainly of patients with highly complex congenital heart disease and not all of those patients had undergone corrective surgery. Only four of 53 patients had either an ASD or a ventricular septal defect, of whom three patients had undergone defect closure. Therefore, these results cannot be compared with the results of our study.
Left ventricular dimensions of patients with an ASD assessed by MRI were reduced as compared with established normal values and increased early after interventional closure associated with an improvement in left ventricular function—for example, left ventricular ejection fraction. Similar results were obtained by recently published studies in which left ventricular dimensions were assessed by echocardiography.12,20,26 Our findings, together with the results of the above mentioned studies, suggest that the abolition of the left to right shunt after ASD closure leads to an increase in left ventricular filling and thus to an increase in left ventricular dimensions and an enhancement of left ventricular function. Together with these haemodynamic changes, we did find an increase of NT‐proBNP concentrations, a biomarker for myocardial stress, early after ASD closure with a return to normal concentrations late after ASD closure. Muta et al27 reported similar results when examining the time kinetics of BNP in children after ASD closure. They found an increase of BNP immediately and 24 hours after ASD closure with a return to normal concentrations after one month. Considering the differences in the half life of BNP and of NT‐proBNP the findings are comparable. These results lead to the hypothesis that the increase in left ventricular filling after ASD closure causes a volume overload of the left ventricle with consecutive haemodynamic myocardial stress. These findings can also serve as an explanation for the observation by Kudo et al29 that in 33% of patients aged over 40 years heart failure develops after ASD closure. Similar reports have been presented by other authors, although the incidences of heart failure after ASD closure were lower in these reports.7,8 The underlying pathological mechanism has not been fully elucidated and is controversial.
The normalisation of NT‐proBNP concentrations during a follow up period of four months and the absence of a further increase of left ventricular dimensions indicate complete adaptation of the left ventricle to the haemodynamic changes occurring after ASD closure. The prolonged adaptation can also explain the delayed improvement of exercise capacity after ASD closure assessed by ergospirometry, which we did not find one month after ASD closure but rather 6–12 months after closure and which we have reported previously.13 These results are consistent with results of Brochu et al,30 who found an improvement of exercise capacity six months after ASD closure, and of Rhodes et al,11 who found no improvement of exercise capacity two months after ASD closure. These findings may also explain a common observation in daily routine—that some patients do not improve clinically immediately after ASD closure.
Conclusion
In patients with a moderate sized ASD, NT‐proBNP as a neurohormonal marker reflecting myocardial stress is not increased. However, NT‐proBNP concentrations increase notably within days after interventional ASD closure associated with an increase in left ventricular dimensions and a return to baseline concentrations within four months. These findings suggest that the left ventricle is haemodynamically stressed in response to volume overload after the abolition of the left to right shunt. This may explain the acute heart failure and delayed improvement of exercise capacity after ASD closure.
Although this study is limited by the small number of patients included, it provides insights into the understanding of haemodynamic adaptation after ASD closure.
Abbreviations
ASD - atrial septal defect
BNP - B‐type natriuretic peptide
BSA - body surface area
LVEDV - left ventricular end diastolic volume
MRI - magnetic resonance imaging
NT‐proBNP - N‐terminal pro‐brain natriuretic peptide
NYHA - New York Heart Association
References
- 1.Dave K S, Pakrashi B C, Wooler G H.et al Atrial septal defect in adults: clinical and hemodynamic results of surgery. Am J Cardiol 1973317–13. [DOI] [PubMed] [Google Scholar]
- 2.Brest A N. Congenital heart disease in adults. Cardiovasc Clin 19702257–265. [PubMed] [Google Scholar]
- 3.Donti A, Bonvicini M, Placci A.et al Surgical treatment of secundum atrial septal defect in patients older than 50 years. Ital Heart J 20012428–432. [PubMed] [Google Scholar]
- 4.Konstantinides S, Geibel A, Olschewski M.et al A comparison of surgical and medical therapy for atrial septal defect in adults. N Engl J Med 1995333469–473. [DOI] [PubMed] [Google Scholar]
- 5.Beyer J. Atrial septal defect: acute left heart failure after surgical closure. Ann Thorac Surg 19782536–43. [DOI] [PubMed] [Google Scholar]
- 6.Beyer J, Brunner L, Hugel W.et al [Acute left heart failure following repair of atrial septal defects: its treatment by reopening]. Thoraxchir Vask Chir 197523346–349. [DOI] [PubMed] [Google Scholar]
- 7.Davies H, Oliver G C, Rappoport W J.et al Abnormal left heart function after operation for atrial septal defect. Br Heart J 197032747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tikoff G, Keith T B, Nelson R M.et al Clinical and hemodynamic observations after surgical closure of large atrial septal defect complicated by heart failure. Am J Cardiol 196923810–817. [DOI] [PubMed] [Google Scholar]
- 9.Ewert P, Berger F, Nagdyman N.et al [Acute left heart failure after interventional occlusion of an atrial septal defect]. Z Kardiol 200190362–366. [DOI] [PubMed] [Google Scholar]
- 10.Tomai F, Gaspardone A, Papa M.et al Acute left ventricular failure after transcatheter closure of a secundum atrial septal defect in a patient with coronary artery disease: a critical reappraisal. Catheter Cardiovasc Interv 20025597–99. [DOI] [PubMed] [Google Scholar]
- 11.Rhodes J, Patel H, Hijazi Z M. Effect of transcatheter closure of atrial septal defect on the cardiopulmonary response to exercise. Am J Cardiol 200290803–806. [DOI] [PubMed] [Google Scholar]
- 12.Giardini A, Donti A, Formigari R.et al Determinants of cardiopulmonary functional improvement after transcatheter atrial septal defect closure in asymptomatic adults. J Am Coll Cardiol 2004431886–1891. [DOI] [PubMed] [Google Scholar]
- 13.Weber M, Neumann T, Rau M.et al [Cardiopulmonary exercise capacity increases after interventional ASD‐closure]. Z Kardiol 200493209–215. [DOI] [PubMed] [Google Scholar]
- 14.Shaheen J, Alper L, Rosenmann D.et al Effect of surgical repair of secundum‐type atrial septal defect on right atrial, right ventricular, and left ventricular volumes in adults. Am J Cardiol. 2000;86: 1395–7, A6, [DOI] [PubMed]
- 15.Dhillon R, Josen M, Henein M.et al Transcatheter closure of atrial septal defect preserves right ventricular function. Heart 200287461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St John Sutton M G, Tajik A J, Mercier L A.et al Assessment of left ventricular function in secundum atrial septal defect by computer analysis of the M‐mode echocardiogram. Circulation 1979601082–1090. [DOI] [PubMed] [Google Scholar]
- 17.Booth D C, Wisenbaugh T, Smith M.et al Left ventricular distensibility and passive elastic stiffness in atrial septal defect. J Am Coll Cardiol 1988121231–1236. [DOI] [PubMed] [Google Scholar]
- 18.Carabello B A, Gash A, Mayers D.et al Normal left ventricular systolic function in adults with atrial septal defect and left heart failure. Am J Cardiol 1982491868–1873. [DOI] [PubMed] [Google Scholar]
- 19.Walker R E, Moran A M, Gauvreau K.et al Evidence of adverse ventricular interdependence in patients with atrial septal defects. Am J Cardiol. 2004;93: 1374–7, A6, [DOI] [PubMed]
- 20.Salehian O, Horlick E, Schwerzmann M.et al Improvements in cardiac form and function after transcatheter closure of secundum atrial septal defects. J Am Coll Cardiol 200545499–504. [DOI] [PubMed] [Google Scholar]
- 21.De Lemos J A, McGuire D K, Drazner M H. B‐type natriuretic peptide in cardiovascular disease. Lancet 2003362316–322. [DOI] [PubMed] [Google Scholar]
- 22.Cheitlin M D, Alpert J S, Armstrong W F.et al ACC/AHA guidelines for the clinical application of echocardiography: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Clinical Application of Echocardiography) developed in collaboration with the American Society of Echocardiography. J Am Coll Cardiol 199729862–879. [DOI] [PubMed] [Google Scholar]
- 23.Quinones M A, Waggoner A D, Reduto L A.et al A new, simplified and accurate method for determining ejection fraction with two‐dimensional echocardiography. Circulation 198164744–753. [DOI] [PubMed] [Google Scholar]
- 24.Lorenz C H, Walker E S, Morgan V L.et al Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson 199917–21. [DOI] [PubMed] [Google Scholar]
- 25.Popio K A, Gorlin R, Teichholz L E.et al Abnormalities of left ventricular function and geometry in adults with an atrial septal defect: ventriculographic, hemodynamic and echocardiographic studies. Am J Cardiol 197536302–308. [DOI] [PubMed] [Google Scholar]
- 26.Feneley M, Gavaghan T. Paradoxical and pseudoparadoxical interventricular septal motion in patients with right ventricular volume overload. Circulation 198674230–238. [DOI] [PubMed] [Google Scholar]
- 27.Muta H, Ishii M, Maeno Y.et al Quantitative evaluation of the changes in plasma concentrations of cardiac natriuretic peptide before and after transcatheter closure of atrial septal defect. Acta Paediatr 200291649–652. [DOI] [PubMed] [Google Scholar]
- 28.Bolger A P, Sharma R, Li W.et al Neurohormonal activation and the chronic heart failure syndrome in adults with congenital heart disease. Circulation 200210692–99. [DOI] [PubMed] [Google Scholar]
- 29.Kudo T, Hashimoto M, Uchino T.et al [Surgery of atrial septal defect in patients aged over 40 years: comparative study of direct suture and patch closure]. Kyobu Geka 199144387–390. [PubMed] [Google Scholar]
- 30.Brochu M C, Baril J F, Dore A.et al Improvement in exercise capacity in asymptomatic and mildly symptomatic adults after atrial septal defect percutaneous closure. Circulation 20021061821–1826. [DOI] [PubMed] [Google Scholar]