Abstract
Objectives
To quantify the prognostic impact of coronary artery disease (CAD) on patients with acute heart failure (HF).
Design
Prospective cohort study of 217 consecutive patients presenting with acute HF to the emergency department. Treatment, hospitalisation, the use of revascularisation procedures, and survival were observed during follow up of up to three years.
Results
CAD was present in 153 patients (71%). Patients with and without CAD were similar with respect to age and sex. Although adequate HF treatment was initiated more rapidly among patients with CAD, their initial outcomes including hospitalisation rate, time to discharge, and total treatment cost were significantly worse. Moreover, despite higher use of angiotensin converting enzyme inhibitors and β blockers during follow up, patients with CAD had a significantly lower survival rate. Cumulative survival at 720 days was 48.7% of patients with CAD as compared with 76.4% of patients without CAD (p = 0.0004). In Cox regression analysis the presence of CAD increased the risk of death by more than 250% (hazard ratio 2.57, 95% confidence interval 1.50 to 4.39, p = 0.001). This strong association persisted after multivariate adjustments. The use of coronary angiography and coronary revascularisation procedures was low, both at initial presentation and during follow up.
Conclusion
CAD is a strong and independent predictor of mortality among patients with acute HF. Whether, for example, less restrictive use of revascularisation procedures in this elderly HF population can improve the outcome for patients with CAD warrants further study.
Keywords: coronary artery disease, acute heart failure, mortality, revascularisation
Coronary artery disease (CAD) is a major cause of heart failure (HF).1,2,3 Previous studies of patients with chronic stable cardiomyopathy have shown the importance of distinguishing between ischaemic and non‐ischaemic cardiomyopathy, because the diagnosis influences management. Smoking cessation, lipid lowering treatment, and revascularisation are important interventions in patients with left ventricular dysfunction due to CAD.1,2,3,4,5 Moreover, the diagnosis of CAD seems to influence prognosis, as CAD is an independent predictor of long term mortality for patients with chronic cardiomyopathy.4,5,6,7,8 The role of CAD as a prognostic factor in patients with acute HF is poorly defined. Therefore, this study sought to evaluate the impact of CAD on short and long term outcome of patients presenting with acute congestive HF to the emergency department.
METHODS
Setting and study population
This study specifically evaluated the impact of CAD on short and long term outcome of patients with acute congestive HF recruited in the BASEL (B‐type natriuretic peptide for the acute shortness of breath evaluation) study.9 The BASEL study was a prospective, randomised, single blind study conducted in the emergency department of the University Hospital Basel, Switzerland, from May 2001 to April 2002. The study was carried out according to the principles of the Declaration of Helsinki and approved by our local ethics committee. Written informed consent was obtained from all participating patients.
A total of 452 consecutive patients presenting with acute dyspnoea as the primary complaint and no obvious traumatic cause of dyspnoea were enrolled. Among these, 217 patients had acute congestive HF. Patients with severe renal disease (serum creatinine > 250 μmol/l) and patients in cardiogenic shock were excluded. No other exclusion criteria in the BASEL trial may have excluded patients with CAD or biased their enrolment into that trial. CAD was defined as the presence of at least one of the following criteria: a significant (> 70% diameter stenosis) lesion on coronary angiography, a history of myocardial infarction, percutaneous coronary intervention or coronary artery bypass grafting, and documented myocardial ischaemia during exercise testing.
Routine clinical assessment
All patients underwent an initial clinical assessment that in general included clinical history, physical examination, electrocardiography, pulse oximetry, blood tests including arterial blood gas analysis (when indicated), and chest radiography. Echocardiography and pulmonary function tests were strongly recommended in the emergency department on an outpatient basis or in the hospital if the patient was admitted. The final discharge diagnosis of acute congestive HF was based on all information available after discharge of the patient including response to treatment and necropsy data for those patients who died in hospital. B‐type natriuretic peptide (BNP) concentrations were available for the diagnosis for half the patients and were determined in a blinded fashion for the other half.
End points
Time to treatment was defined as the time interval from presentation to the emergency department to the initiation of treatment specific for acute congestive HF. This included the administration of diuretics, morphine, glyceryl trinitrate, or angiotensin converting enzyme (ACE) inhibitors. Total days in hospital, total treatment cost, and all cause mortality were prospectively assessed during follow up. The calculation of total days in hospital and total cost of hospitalisations summed up all hospitalisations after the initial presentation to the emergency department. Since ratios of costs to charges have not been defined for the majority of services and departments at our institution, nor that of other hospitals in the area, hospital charges were used as the most appropriate estimate of the true costs.10,11 To avoid an imbalance owing to differences in reimbursement or charges associated with different types or classes of insurance, charges were standardised according to the actual rates for patients with general insurance who were living in Basel. Total treatment cost did include total cost of hospitalisations and medication but not regular outpatient visits to a private doctor.
A single trained researcher contacted patients six, 12, and 24 months after the initial presentation for a telephone interview. In addition, referring physicians were contacted in case of uncertainties regarding health status or hospitalisations. The administrative databases of the patients' hometowns were assessed to ascertain the vital status of those patients who could not be contacted by telephone. All information derived from contingent hospital readmission records or provided by the referring physician or by the outpatient clinic was reviewed and entered on to the computer database.
Statistical analysis
Data were statistically analysed with the SPSS/PC version 12.0 (SPSS Inc, Chicago, Illinois, USA) software package. A significance level of 0.05 was used. Data were compared by the t test, Mann‐Whitney U test, Fisher's exact test, and χ2 test as appropriate. All hypothesis testing was two tailed. Cox regression analysis was used to identify predictors of death in univariate and multivariate analyses.
RESULTS
Baseline characteristics
A total of 153 patients (71%) had CAD. Patients with and without CAD were similar with respect to age and sex (table 1). Differences in baseline characteristics were a higher incidence of diabetes mellitus, stroke or peripheral vascular disease, and pulmonary disease in patients with CAD. Symptoms, signs, and vital status were also similar with the exception of nocturia and rales, both being more common among patients with CAD. Laboratory tests showed important differences between the groups. Haemoglobin and glomerular filtration rate were significantly lower, whereas troponin I and BNP were significantly higher in patients with CAD.
Table 1 Baseline characteristics of patients with acute congestive heart failure.
| Variable | All patients (n = 217) | CAD (n = 153) | No CAD (n = 64) | p Value* |
|---|---|---|---|---|
| Age (years) | 75 (11) | 75 (11) | 73 (13) | 0.226 |
| Female sex | 93 (43%) | 66 (43%) | 27 (42%) | 0.897 |
| History | ||||
| Systemic hypertension | 138 (64%) | 103 (67%) | 35 (55%) | 0.078 |
| Diabetes mellitus | 67 (31%) | 56 (37%) | 11 (17%) | 0.005 |
| Chronic obstructive pulmonary disease | 52 (24%) | 41 (27%) | 11 (17%) | 0.130 |
| Asthma | 4 (2%) | 1 (1%) | 3 (5%) | 0.078 |
| Pulmonary embolism | 6 (3%) | 5 (3%) | 1 (2%) | 0.673 |
| Pneumonia | 27 (12%) | 22 (14%) | 5 (8%) | 0.259 |
| Other pulmonary disease | 15 (7%) | 14 (9%) | 1 (2%) | 0.073 |
| Any pulmonary disease | 88 (41%) | 69 (45%) | 19 (30%) | 0.035 |
| Depressive disorder | 14 (7%) | 10 (7%) | 4 (6%) | 1.000 |
| Stroke or peripheral vascular disease | 60 (28%) | 50 (33%) | 10 (16%) | 0.010 |
| Chronic kidney disease | 85 (39%) | 66 (43%) | 19 (30%) | 0.064 |
| Deep vein thrombosis | 15 (7%) | 10 (7%) | 5 (8%) | 0.772 |
| Symptoms | ||||
| Dyspnoea† | 0.465 | |||
| Slight hill | 26 (12%) | 20 (13%) | 6 (9%) | |
| Level ground | 122 (56%) | 89 (58%) | 33 (52%) | |
| At rest | 67 (31%) | 43 (28%) | 24 (38%) | |
| Paroxysmal nocturnal dyspnoea | 102 (47%) | 76 (50%) | 26 (41%) | 0.223 |
| Nocturia | 86 (40%) | 68 (44%) | 18 (28%) | 0.025 |
| Weight gain | 38 (18%) | 27 (18%) | 11 (17%) | 0.935 |
| Weight loss | 21 (10%) | 16 (11%) | 5 (8%) | 0.624 |
| Chest pain | 85 (39%) | 63 (41%) | 22 (34%) | 0.349 |
| Nausea | 32 (15%) | 22 (14%) | 10 (16%) | 0.813 |
| Coughing | 93 (43%) | 64 (42%) | 29 (45%) | 0.636 |
| Expectoration | 59 (27%) | 44 (29%) | 15 (23%) | 0.422 |
| Fever | 32 (15%) | 27 (18%) | 5 (8%) | 0.091 |
| Vital status | ||||
| Systolic blood pressure (mm Hg) | 147 (31) | 147 (32) | 146 (31) | 0.813 |
| Diastolic blood pressure (mm Hg) | 88 (21) | 88 (19) | 88 (24) | 0.897 |
| Heart rate (beats/min) | 97 (26) | 95 (25) | 101 (29) | 0.161 |
| Temperature (°C) | 37.2 (0.9) | 37.2 (0.9) | 37.3 (0.8) | 0.558 |
| Signs | ||||
| Tachypnoea (>20 breaths/min) | 96 (44%) | 68 (44%) | 28 (44%) | 0.925 |
| Increased jugular venous pressure | 49 (23%) | 35 (23%) | 14 (22%) | 0.872 |
| Hepatojugular reflux | 34 (16%) | 21 (14%) | 13 (20%) | 0.223 |
| Rales | 130 (60%) | 99 (65%) | 31 (48%) | 0.026 |
| Wheezing | 30 (14%) | 21 (14%) | 9 (14%) | 0.948 |
| Hyperresonant percussion | 16 (7%) | 12 (8%) | 4 (6%) | 0.783 |
| Dullness | 24 (11%) | 20 (13%) | 4 (6%) | 0.163 |
| Lower extremity oedema | 101 (47%) | 72 (47%) | 29 (45%) | 0.814 |
| Cyanosis | 17 (8%) | 10 (7%) | 7 (11%) | 0.271 |
| Laboratory tests | ||||
| Ejection fraction (%)‡ | 40 (30–55) | 40 (25–50) | 40 (35–60) | 0.168 |
| GFR (ml/min/1.73 m2) | 53 (28) | 50 (28) | 61 (27) | 0.009 |
| Haemoglobin (g/l) | 130 (24) | 127 (24) | 135 (22) | 0.013 |
| Serum albumin (g/l) | 33 (6) | 33 (5) | 34 (7) | 0.284 |
| Troponin I (μg/l) | 0.5 (0.3–2.1) | 0.6 (0.3–2.7) | 0.3 (0.3–1.1) | 0.003 |
| B‐type natriuretic peptide (pg/ml) | 822 (410–1300) | 888 (474–1300) | 682 (222–1300) | 0.048 |
Data are presented as mean (SD), median (interquartile range), or number of patients (%).
*Patients with coronary artery disease (CAD) versus patients without CAD; †one patient in both groups had dyspnoea only while walking up a steep incline; ‡available for 148 patients.
GFR, glomerular filtration rate.
Initial outcome
Although adequate treatment was initiated more rapidly in patients with CAD, initial outcome was worse. Hospitalisation rate, time to discharge, and total treatment cost were significantly higher in patients with CAD (table 2). Discharge medication in patients with CAD more often included ACE inhibitors or angiotensin receptor blockers, β blockers, diuretics, nitrates, and aspirin as compared with patients without CAD.
Table 2 Impact of CAD on outcome.
| Variable | CAD (n = 153) | No CAD (n = 64) | p Value |
|---|---|---|---|
| Initial outcome | |||
| Time to treatment (min) | 49 (19–163) | 105 (24–195) | 0.051 |
| Hospital admission | 140 (92%) | 51 (80%) | 0.015 |
| Admission to intensive care | 45 (29%) | 13 (20%) | 0.167 |
| Time to discharge (days) | 13 (6–20) | 9 (1–17) | 0.042 |
| 30 day mortality | 21 (14%) | 6 (9%) | 0.377 |
| Initial total treatment cost ($) | 6 374 (3 732–9 606) | 4 432 (756–8 670) | 0.006 |
| Discharge medication | |||
| ACE inhibitor or ARB | 116 (84%) | 41 (70%) | 0.027 |
| β Blocker | 90 (65%) | 27 (46%) | 0.013 |
| Diuretic | 127 (92%) | 45 (76%) | 0.002 |
| Glyceryl trinitrate | 61 (44%) | 9 (15%) | <0.001 |
| Aspirin | 75 (54%) | 17 (29%) | 0.001 |
| Anticoagulation | 66 (48%) | 36 (61%) | 0.081 |
| Long term outcome | |||
| Total days in hospital | |||
| At 90 days | 14 (7–25) | 11 (2–21) | 0.047 |
| At 180 days | 16 (8–29) | 14 (3–22) | 0.095 |
| At 360 days | 18 (8–36) | 17 (5–30) | 0.250 |
| Treatment cost ($) | |||
| A 90 days | 7 413 (4 435–12 364) | 5 082 (1 606–9 472) | 0.005 |
| At 180 days | 7 981 (4 755–14 033) | 6 109 (2 511–10 328) | 0.016 |
| At 360 days | 10 415 (5 384–18 365) | 8 122 (3 376–14 558) | 0.078 |
| Cumulative survival (%) | |||
| At 360 days | 64.1 (3.9) | 80.0 (5.0) | |
| At 720 days | 48.7 (4.1) | 76.4 (5.3) | 0.0004* |
Data are presented as median (interquartile range), number of patients (%), or mean (SE).
*By log rank test.
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker.
Long term follow up
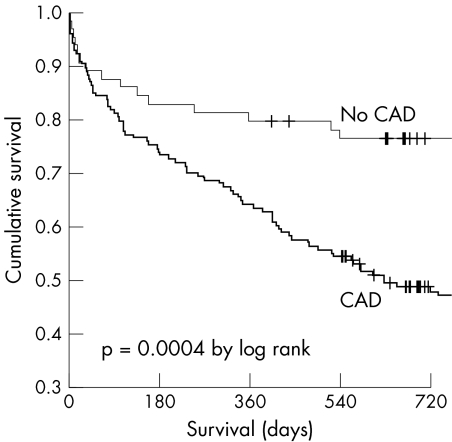
Clinical 360 day follow up data were available for all 217 patients (100%). The median time interval to last patient contact or patient death was 678 days. Patients with CAD had a significantly lower survival rate. Cumulative survival at 720 days was 48.7% of patients with CAD as compared with 76.4% of patients without CAD (p = 0.0004; fig 1). Total treatment cost remained significantly higher for patients with CAD at 90 and 180 days and still tended to be much higher at 360 days.
Figure 1 Cumulative survival of patients with and without coronary artery disease (CAD).
CAD as a predictor of death
Cox regression analysis showed that CAD was a powerful predictor of death in this cohort. In univariate analysis, the presence of CAD increased the risk of death by 257% (hazard ratio (HR) 2.57, 95% confidence interval (CI) 1.50 to 4.39; p = 0.001). This strong association persisted after adjustment for age and sex (HR 2.41, 95% CI 1.41 to 4.13; p = 0.001). When diabetes mellitus, stroke or peripheral vascular disease, and pulmonary disease were entered as additional variables in the model, CAD remained a strong predictor of death (HR 2.24, 95% CI 1.28 to 3.89; p = 0.001). Additionally including those laboratory test that differed between the groups in the model (haemoglobin, glomerular filtration rate, troponin I, and BNP) resulted in a hazard ratio for CAD of 2.10 (95% CI 1.07 to 4.13: p = 0.032). Interestingly, left ventricular ejection fraction, which was available for 148 patients, was not a significant predictor of death (p = 0.259).
Use of coronary revascularisation procedures
As table 3 shows, the use of coronary angiography and coronary revascularisation procedures was low for patients with CAD. At initial presentation, only 12% of patients with CAD received either percutaneous or surgical revascularisation.
Table 3 Use of procedures related to coronary revascularisation.
| Variable | CAD (n = 153) | No CAD (n = 64) | p Value |
|---|---|---|---|
| At initial presentation | |||
| Coronary angiography | 27 (18%) | 4 (6%) | 0.033 |
| PCI | 8 (5%) | 0 | 0.109 |
| CABG | 10 (7%) | 0 | 0.036 |
| Myocardial perfusion SPECT | 13 (9%) | 0 | 0.012 |
| Exercise ECG | 12 (8%) | 0 | 0.020 |
| During follow up | |||
| Coronary angiography | 19 (12%) | 3 (5%) | 0.136 |
| PCI | 10 (7%) | 1 (2%) | 0.181 |
| CABG | 2 (1%) | 1 (2%) | 1.000 |
| Myocardial perfusion SPECT | 31 (20%) | 5 (8%) | 0.025 |
Data are presented as number of patients (%).
CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; SPECT, single photon emission computed tomography.
DISCUSSION
This study showed the impact of CAD on outcome of patients presenting with acute congestive HF to the emergency department. Most important, more than 70% of patients had CAD, and these patients with CAD had a significantly lower survival rate. Moreover, CAD remained an independent predictor of death in multivariate analysis. Assessment of cardiac markers at presentation showed that patients with CAD had more extensive myocardial necrosis (troponin) and haemodynamic stress (BNP) than did patients without CAD. In addition, although the groups were of similar age, we noted higher co‐morbidity including diabetes mellitus, diffuse vascular disease, and resulting end organ damage (glomerular filtration rate) in patients with CAD. These differences may in part explain the dismal outcome of patients with CAD.
Current American College of Cardiology/American Heart Association and European Society of Cardiology management guidelines for patients with acute congestive HF recommend searching for and eventually treating myocardial ischaemia as the cause of decompensation.1,2 However, coronary revascularisation procedures are notoriously underused in this elderly patient cohort in clinical practice.1,2,12 In our study, only 12% of patients with CAD and acute congestive HF received a coronary revascularisation procedure during initial presentation. This finding is in complete agreement with data from the EuroHeart survey.12 We hypothesise that CAD would have a less negative impact on outcome of patients with acute congestive HF if myocardial ischaemia were treated less restrictively with coronary revascularisation in these patients. Early revascularisation for acute congestive HF due to CAD and myocardial ischaemia may be as beneficial as early revascularisation for acute coronary syndromes.13,14 Obviously, elderly patients with HF do have significant co‐morbidity and a higher risk of periprocedural complications than do patients with acute coronary syndromes.15 However, they also seem to have more to gain from revascularisation. In fact, subgroup analysis from the TACTICS‐TIMI 18 (treat angina with Aggrastat and determine cost of therapy with an invasive or conservative strategy‐thrombolysis in myocardial infarction 18) study showed that almost all of the benefit from early revascularisation was seen in the elderly high risk patients.16 Moreover, elderly patients with chronic symptomatic CAD receive long term benefit from an invasive versus optimised medical treatment strategy.17,18
Our results extend the results of previous studies of patients with chronic and stable cardiomyopathy. To determine which of the many clinical parameters routinely collected influence mortality in patients with chronic HF, Likoff and colleagues5 followed up 201 patients with idiopathic or ischaemic dilated cardiomyopathy for 28 months. Three characteristics at the study entry independently predicted an increased mortality risk: left ventricular ejection fraction, maximum oxygen uptake, and ischaemic cardiomyopathy. The increased mortality risk among patients with cardiomyopathy due to CAD was later confirmed by several groups.4,6,7 Overall, these studies suggest that CAD increases mortality by 50% (hazard ratio 1.50) in patients with chronic cardiomyopathy.4,5,6,7 Interestingly, the prognostic impact of CAD in acute HF even exceeds that in chronic cardiomyopathy. The hazard ratio for CAD was 2.57 in this study.
Limitation
CAD was defined according to established criteria.19 As coronary angiography was not performed in all patients, those with previously undiagnosed CAD may have been incorrectly classified as not having CAD.20 However, as the standard criteria showed an incidence of CAD of 71% in this study, the number of missed cases most likely was small. As any misclassification would only reduce the difference between groups, the prognostic impact of CAD may even be greater than calculated in our study.
Conclusion
CAD is a strong and independent predictor of mortality in patients with acute HF. Whether, for example, less restrictive use of revascularisation procedures in this elderly HF population can improve the outcome of patients with CAD warrants further study.
Abbreviations
ACE - angiotensin converting enzyme
BASEL - B‐type natriuretic peptide for the acute shortness of breath evaluation
BNP - B‐type natriuretic peptide
CAD - coronary artery disease
HF - heart failure
TACTICS‐TIMI 18 - treat angina with Aggrastat and determine cost of therapy with an invasive or conservative strategy‐thrombolysis in myocardial infarction 18
Footnotes
This study was supported by research grants from the Swiss National Science Foundation, the Swiss Heart Foundation, and the Novartis Foundation (to Dr Mueller).
References
- 1.Hunt S A, Baker D W, Chin M H.et al ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 20011042996–3007. [DOI] [PubMed] [Google Scholar]
- 2.Nieminen M S, Böhm M, Cowie M R.et al The task force on acute heart failure of the European Society of Cardiology. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure. Eur Heart J 200526384–416. [DOI] [PubMed] [Google Scholar]
- 3.Alderman E L, Fisher L D, Litwin P.et al Results of coronary artery surgery in patients with poor left ventricular function (CASS). Circulation 198368785–795. [DOI] [PubMed] [Google Scholar]
- 4.Bart B A, Shaw L K, McCants C B., Jret al Clinical determinants of mortality in patients with angiographically diagnosed ischemic or nonischemic cardiomyopathy. J Am Coll Cardiol 1997301002–1008. [DOI] [PubMed] [Google Scholar]
- 5.Likoff M J, Chandler S L, Kay H R. Clinical determinants of mortality in chronic congestive heart failure secondary to idiopathic dilated or to ischemic cardiomyopathy. Am J Cardiol 198759634–638. [DOI] [PubMed] [Google Scholar]
- 6.Adams K F, Jr, Dunlap S H, Sueta C A.et al Relation between gender, etiology and survival in patients with symptomatic heart failure. J Am Coll Cardiol 1996281781–1788. [DOI] [PubMed] [Google Scholar]
- 7.Felker G M, Thompson R E, Hare J M.et al Underlying causes and long‐term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 20003421077–1084. [DOI] [PubMed] [Google Scholar]
- 8.Gersh B J, Kronmal R A, Schaff H V.et al Comparison of coronary artery bypass surgery and medical therapy in patients 65 years of age or older: a nonrandomized study from the coronary artery surgery study (CASS) registry. N Engl J Med 1985313217–224. [DOI] [PubMed] [Google Scholar]
- 9.Mueller C, Scholer A, Laule Kilian K.et al Use of B‐type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med 2004350647–654. [DOI] [PubMed] [Google Scholar]
- 10.Weinstein M C, Siegel J E, Gold M R.et al Recommendations of the panel on cost‐effectiveness in health and medicine. JAMA 19962761253–1258. [PubMed] [Google Scholar]
- 11.Siegel J E, Weinstein M C, Russell L B.et al Recommendations for reporting cost‐effectiveness analysis. JAMA 19962761339–1341. [DOI] [PubMed] [Google Scholar]
- 12.Cleland J G, Swedberg K, Follath F.et al Study group on diagnosis of the working group on heart failure of the European Society of Cardiology. The EuroHeart failure survey programme: a survey on the quality of care among patients with heart failure in Europe. Part 1. Patient characteristics and diagnosis. Eur Heart J 200324442–463. [DOI] [PubMed] [Google Scholar]
- 13.Cannon C P, Weintraub W S, Demopoulos L A.et al Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med 20013441879–1887. [DOI] [PubMed] [Google Scholar]
- 14.Neumann F J, Kastrati A, Pogatsa‐Murray G.et al Evaluation of prolonged antithrombotic pretreatment (“cooling‐off strategy”) before intervention in patients with unstable coronary syndromes: a randomized controlled trial. JAMA 20032901593–1599. [DOI] [PubMed] [Google Scholar]
- 15.TIME Investigators Trial of invasive versus medical therapy in elderly patients with chronic symptomatic coronary‐artery disease (TIME): a randomised trial. Lancet 2001358951–957. [DOI] [PubMed] [Google Scholar]
- 16.Bach R G, Cannon C P, Weintraub W S.et al The effect of routine, early invasive management on outcome for elderly patients with non‐ST‐segment elevation acute coronary syndromes. Arch Intern Med 2004141186–195. [DOI] [PubMed] [Google Scholar]
- 17.Pfisterer M, Buser P, Osswald S.et al Outcome of elderly patients with chronic symptomatic coronary artery disease with an invasive vs optimized medical treatment strategy: one‐year results of the randomized TIME trial. JAMA 20032891117–1123. [DOI] [PubMed] [Google Scholar]
- 18.Pfisterer M, for the TIME Investigators Long‐term outcome in elderly patients with chronic angina managed invasively versus by optimized medical therapy: four‐year follow‐up of the randomized trial of invasive versus medical therapy in elderly patients (TIME). Circulation 20041101213–1218. [DOI] [PubMed] [Google Scholar]
- 19.The IONA Study Group Effect of nicorandil on coronary events in patients with stable angina: the impact of nicorandil in angina (IONA) randomised trial. Lancet 20023591269–1275. [DOI] [PubMed] [Google Scholar]
- 20.Fox K F, Cowie, Wood D A.et al Coronary artery disease as the cause of incident heart failure in the population. Eur Heart J 200122228–236. [DOI] [PubMed] [Google Scholar]